Translate this page into:
Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs
⁎Corresponding author. miss.bagheri40@yahoo.com (Marzieh Bagheri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present work, the performance of photocatalytic removal of reactive blue 203 (RB203) using three different additive as photocatalyst: ZnO nanoparticles (ZnO (NPs)), multi wall carbon nanotubes and ZnO (NPs) stabilized on Functionalized multi wall carbon nanotubes (ZnO (NPs)/MWCNTs) was investigated. For this purpose, firstly the surface of multi wall carbon nanotubes (MWCNTs) were acidified using sulfuric acid and nitric oxide and then ZnO (NPs) was stabilized on the MWCNTs surface to form ZnO (NPs)/MWCNTs. The structural properties of the ZnO (NPs)/MWCNTs were studied by using the results of Scanning Electron Microscope analysis (SEM). In addition, the effects of various influencing factors such as pH, temperature, dye concentration, UV radiation time, UV intensity and photocatalysts dosage were investigated on photocatalytic removal of RB203. The maximum dye removal was obtained at optimum condition which was pH = 10, 20 min radiation time, 50 mm lamp distance from catalyst level, and the UV radiation intensity of 23 mW/cm2. This condition was created in 100 ml of synthetic wastewater having dye concentration of 20 mg/L and catalyst mass of 5 mg/L. This experiment was carried out for all three additives and the removal efficiencies of 19%, 85.4% and 99.1% were attained for the additives of MWCNTs, ZnO (NPs) and ZnO (NPs)/MWCNT, respectively.
Keywords
Removal dye
Reactive blue 203
Zinc oxide nanoparticles
Stabilized photocatalyst
Functionalized MWCNTs
Aqueous solutions
1 Introduction
Synthetic dyes are a group of organic pollutants that are widely used in the textile, paper, printing, leather, cosmetic, plastic and food industries (Talaiekhozani et al., 2020). The wastewater of the textile and dyeing industries has a lot of colored materials (Eskandari et al., 2019). The reactive and acid dyes are the most important dyes used in industries. Therefore, removing them is essential. Reactive dyes are soluble in water and it is very difficult to remove them by flocculation and biological decomposition (Colak et al., 2009). Dyes can reduce light penetration into aquatic environment; therefore, it has a negative effect on the plants (abnormality in photosynthesis function) (Kim et al., 2015). In addition, removing these types of dyes and combinations is important because of their toxicity, mutagenicity, and carcinogenicity resulting from their decomposition, which may be due to their aromatic structure that can affect the life of all creatures. Thus, removing dyes from wastewater and colored sewage is indispensable and inevitable (Xuejing et al., 2009; Daneshvar et al., 2008).
Recently, researchers have studied different methods for dye removal, including adsorption (Yu and Fugetsu, 2010), ozonization (Souza et al., 2010), reverse osmosis (Bhattacharya et al., 2013) and chemical oxidation (Torrades and Garcia-Montao, 2014). Conventional processes such as absorption or coagulation are not effective enough to purify these effluents. Because it firstly causes incomplete degradation of organic compounds, and secondly, only the compounds are transferred from one phase to another. Other processes, such as sedimentation, filtration, and membrane use, have high operating costs and can also create secondary pollutants (Chong et al., 2010). Another method is the advanced oxidation processes by ultraviolet radiation in the presence of catalysts such as TiO2 and ZnO. This method has some advantages such as non-toxicity and high efficiency compared to other methods (Kamat and Huehn, 2008). Today, ZnO as a semiconductor material, for its energy of the straight-line gap, the binding energy of 60 mega-electron volts, the stability of these particles against optical and chemical corrosion, lack of toxicity, insolubility, photocatalytic ability and the band gap energy has the ability to decompose many toxic compounds in the presence of ultraviolet light (Xie et al., 2010).
Advanced oxidation processes are based on the production of high oxidation hydroxyl radicals when in semiconducting materials such as TiO2 and ZnO, the energy of a photon is equal to or greater than the energy gap of a semiconductor. It causes an electron to be excited from the valence band to the conduction band, producing a hole in the valence band. In neutral and alkaline pH, holes can, directly and indirectly produce radical hydroxyls and these radicals are capable of converting organic materials into minerals (Ren et al., 2010; Akyol and Bayramoglu, 2010). The neutral form of the hydroxide ion (OH−) is known as the hydroxyl radical (OH). This chemical is highly reactive; therefore, it can be used to oxidize recalcitrant chemicals in wastewater. Organic compounds are converted to carbon dioxide and water when they are oxidized by radical hydroxyls. This process is called mineralization. Hydroxyl radicals attack organic pollutants through four basic pathways: radical addition, hydrogen abstraction, electron transfer, and radical combination (Deng and Zhao, 2015). Radical hydroxyl production equations by photocatalytic ZnO process in the presence of ultraviolet light were shown in the Eqs. (1)–(8) (Abdollahi, et al., 2012). A greater amount of hydroxyl can be produced by ZnO (NPs) since its surface is very higher than common form of ZnO. Also Fig. 1 shows the schematic diagram of a photocatalytic mechanism for ZnO (NPs)/MWCNTs.
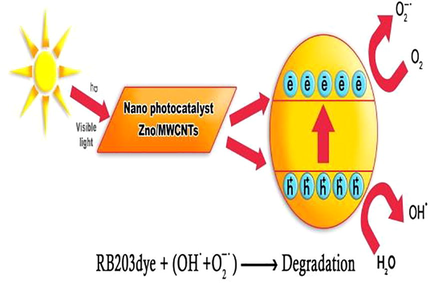
The schematic diagram of a photocatalytic mechanism for ZnO NPs/MWCNTs.
Wang et al. were Used modified zinc oxide nanoparticles with Au and ZIF-8 to detect and oxidize formaldehyde (Wang et al., 2018). They could found that the modified zinc oxide nanoparticles with Au and ZIF-8 are able to oxidize formaldehyde into non-toxic formic acid. The main disadvantage of the photocatalytic decomposition of semiconductor materials such as zinc oxide is the fast recombination of produced Electron–hole pairs which slows down their efficiency in removing and destroying organic pollutants (Liu et al., 2012). To solve this problem there are several different ways, such as decorated semiconductor photocatalysts with materials which have wider band gaps (Wang et al., 2009) using different materials such as silver, which can act as an electron sink (Kuriakose et al., 2014; Zhang et al., 2012), or stabilizing semiconductor catalysts on materials that have a very large surface. The use of stabilized catalysts in the photocatalytic process is more economical on a larger scale owing to the lack of separation of the catalyst after the process (Bouazza et al., 2009). For example, a study conducted by Azarang et al., zinc oxide nanoparticles fixed on graphene sheets were used for photocatalytic removal of methylene blue dye (Azarang et al., 2014). In another study, Zhang et al., were used to ZnO nanowire/reduced graphene oxide nanocomposites for photocatalytic removal of Rhodamine 6G (Zhang et al., 2014). They could remove nearly 98% of Rhodamine 6G during 10 min. In recent years, due to the unique properties of multi-wall carbon nanotubes, they have been widely used as a base for catalytic materials (Byrappa et al., 2008). MWCNTs have unique properties such as High electrical conductivity, high mechanical strength, thermal conductivity, elasticity, a good catalytic activity and high aspect ratio (Bouazza et al., 2009). In a study by Zeo et al., it was found that the photocatalytic activity of carbon nanotubes coated by ZnO composites is more effective than the ZnO microsphere alone (Zhu et al., 2015; Chakrabarti and Dutta, 2004). Using functionalized MWCNTs to modify ZnO (NPs) can increase the photocatalyst removal process by increasing the surface area, increasing absorption of light by photocatalyst and improving charge transport (inhibiting the recombination of electron-hole pairs). Despite the progress made so far in recognizing hybrid materials for photocatalysts, very little research has been done on ZnO (NPs)/MWCNts composites. In this research, for the first time, the focus was on the process of photocatalytic removal of RB203 dye by MWCNTs modified with acid, ZnO (NPs) and ZnO(NPs)/MWCNTs in the presence of UV light. Besides, the effective parameters on the photocatalytic efficiency of the mentioned compounds were examined and the optimal conditions were experimentally determined for each of the effective parameters.
2 Experimental
2.1 Reagents and materials
H2SO4, HNO3, NaOH, Zinc acetate (Zn (OAc)2·2H2O) which used in this study were purchased from Merck Company located in Germany. Multi-wall carbon nanotubes (purity greater than 95%, with a diameter about 20–30 nm, from cheap tubes.com, USA). All aqueous solutions were prepared with double distilled water. Reactive blue 203 (RB203) with molecular weight 617.54 g/mole and with chemical formula C28H29N5Na4O21S6 was purchased from Alvan Sabet Corporation, Iran and used without further purification.
2.2 Apparatus
A digital pH-meter (Jenway 3020, UK) was used for pH measurements. A UV/Vis spectrophotometer (UV-2100, JENUS, China) was used for absorbance measurements of samples. In this study a Sigma 101 centrifuge (made in Netherland) with speed of 5000 rpm was used to separate ZnO (NPs) from the solutions. In this study several experiments should be mixed; therefore, in order to mix the solutions a magnetic stirrer (MR3001, Heidolph, Germany) was used. The morphology of fixed ZnO (NPs) and ZnO (NPs)/MWCNTs were evaluated using a Scanning Electron Microscope (SEM) (XLC30, Philips, Netherland). The bath ultrasonic system was used to homogenize mixtures (E30H, Elmasonic, Singapore). In this study the weight of used materials was measured using a laboratory digital scales (3020, Jenway, UK). The electric furnace (Lenton, UK) was used to dry produced nanoparticles. In this study a low pressure mercury Philips UV lamp manufactured by Netherland was applied. The UV radiation intensity was measured by the radiation detector (UV-C254, Lutron). The distance between the surface of synthetics wastewater contaminated with RB203dye and UV lamp was only a few millimeters. In this situation, the amount of UV radiation was measured as much as 110 mW/cm2.
2.3 Methods
Photocatalytic experiments were carried out in a glass reactor 20 cm long, 12 cm wide and 5 cm high. The samples used in each step included 100 ml of a 20 mg/L solution containing a dye in the reactor. An ultra-violet lamp of 8 W UV-C type and 10 cm long was used as a source of radiation. Since UV-C is the most powerful type of UV radiation that has ability of molecular bonding breaking and photo chemical degradation of organic compounds, it was selected in this study. Using an intensifier device (UV-C 254 LOTRON model), the intensity of lamp was estimated at about 110 mW/cm2. In order to be better exposed to ultraviolet radiation, the lamp was placed in the center of the container at a distance of 50 mm from the sample. In all experiments, the glass reactor was well covered by an aluminum foil to minimize UV radiation losses. To prepare a 1000 mg/L stock solution of dye, 0.5 g of RB203 dye was weighed using a digital scale and was dissolved in 500 ml distilled water. In order to prevent the concentration change, stock solution after preparation was kept in the refrigerator. Solutions required for other concentrations were obtained by diluting the stock solution. To adjust the pH of the solution was used from 0.1 M NaOH and HNO3. In all of the experiments, after dye removal by using ZnO (NPs)/MWCNTs, the centrifuge was used to separate the nanoparticles. Then the absorbance of the filtered solution was read by a UV–Vis spectrophotometer. To calculate the maximum wavelength of RB203 dye, the absorbance of a 100 mg/L solution in the range of 200–700 nm was read by UV–Vis spectrophotometer. The maximum absorption of RB203 dye solution was achieved at wavelength of 625 nm. Eq. (9) was used to determine the percentage of removal of dye:
where is the initial absorption of RB203 dye solution at the maximum wavelength and is the absorption of RB203 dye solution at the maximum wavelength after photicatalytic dye removal. To do this research, the photolysis rate at pH (3, 5, 7, 8, 9, 10 and 11), initial dye concentration (5, 10, 15,20, 25, 30 and 40 mg/L), radiation time (5, 10, 15, 20 and 25 min), UV intensity (23, 28, 44 and 51 mW/cm2), modified MWCNTs concentration, ZnO (NPs) and ZnO (NPs)/MWCNTs (1, 3, 5 and 6 mg/L) were examined.
2.4 The synthesis method of ZnO nanoparticles fixed on MWCNTs
The synthesis of ZnO NPs on FMWCNTs involves two steps. First, MWCNTs must be functionalized with oxygen groups. Then, ZnO (NPs) should be fixed on the outer surface of the functionalized MWCNTs. To functionalized MWCNTs, first, 0.2 g of MWCNTs were mixed in 60 ml of acids (mixing 42 ml H2SO4 and 18 ml HNO3) and the mixture was homogenized for 2 h in the ultrasonic process. Next, it was placed in a stirrer for 2 h. In the end, it was dried at room temperature for 1 day. The dried powder was dissolved into in 23 ml of distilled water. It was then placed in the ultrasonic apparatus for 30 min. Next, the solution was stirred at 90 °C and 2 g of zinc acetate was slowly added. Moreover, 2.66 ml of 5 M NaOH solution was added to the mixture before being centrifuged and washed with distilled water. After that, it was placed at ambient temperature for 24 h to dry. In order to calcine, the resulting material was placed in a 300° C electric furnace for 3 h. In the end, to get uniform powder, the resulting material was completely crushed (Abbasi et al., 2016).
3 Results and discussion
3.1 SEM study
According to the results of (SEM), the average size of synthesized nanoparticles was estimated at 50.1 nm (Fig. 2a). SEM results for zinc oxide nanoparticles indicate that they have a nearly uniform spherical shape and distribution. Fig. 2b shows zinc oxide nanoparticles fixed to functionalized MWCNTs after calcination. MWCNTs were purchased from Cheap Tubes Company, USA. Since the characteristics of the purchased MWCNTs already reported by several researchers as well as the Cheap Tubes Company, the author of this manuscript did not use SEM image of MWCNTs in the manuscript.
(a) SEM image of ZnO (NPs) and (b) SEM image of ZnO (NPs)/MWCNTs.
3.2 Effect dosage of various photocatalyst
The effects of various concentrations of MWCNTs, ZnO (NPs) and ZnO (NPs)/MWCNTs on RB203 dye removal was investigated in the range of 1–6 mg/L. For 100 ml of RB203 dye with a concentration of 20 mg/L, pH = 10, the contact time is 20 min using an 8 W UV lamp at a distance of 50 mm from the catalyst level. To study the effect of concentration, on the removal efficiency of RB203, the absorbance of all solutions after adding the photocatalyst was measured by UV–Vis spectrophotometer and compared with the initial absorption (before the photocatalytic process). According to Fig. 3 it was found that when the MWCNTs were used alone, the removal of the dye was insignificant and increasing the concentration of functionalized MWCNTs did not significantly affect RB203 dye removal. It alone cannot produce radical hydroxyl; as a result, it cannot be used as a photocatalyst. ZnO (NPs) alone had a very good photocatalytic activity. Furthermore, the removal percentage of RB203 dye was raised when the concentration of ZnO (NPs) increased (Fig. 3). This increase in removal can be related to the increase of electron holes pairing. The formed holes can be hydroxyl radicals with water and with anion hydroxyl (Ghaderi et al., 2015). In the case of ZnO (NPs)/MWCNTs, the results (Fig. 4) showed that it exhibits the most photocatalytic activity, because MWCNTS in hybrid photocatalyst acts as a dispersive of nanoparticles and prevents zinc oxide nanoparticles from hunching and agglomeration. As a result, the active surface of ZnO (NPs)/MWCNTs can be increased. Bouazza et al. (2009) reported that decoration of ZnO (NPs) on the outer surface of MWCNTs can increase the activity of the stabilized photocatalyst and increase the absorption of UV light and, consequently, increase the production of radical hydroxyl. According to the results of experiments in the mentioned conditions, the highest dye removal efficiency at an optimal concentration (5 mg/L for each photocatalyst) was obtained as follows: MWCNTs with 19%, ZnO (NPs) with 85.4%, and ZnO (NPs)/MWCNTs with 99.1%.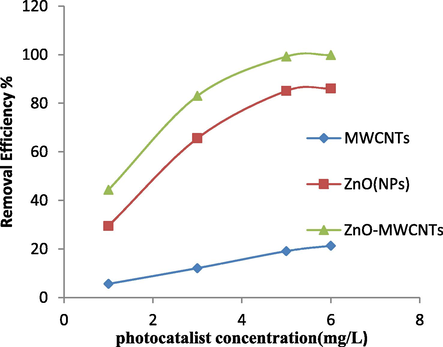
Effect of photocatalyst concentration on the removal of RB203 dye (initial dye concentration: 20 mg/L, pH: 10, time: 20 min, distance from UV lamp: 50 mm).
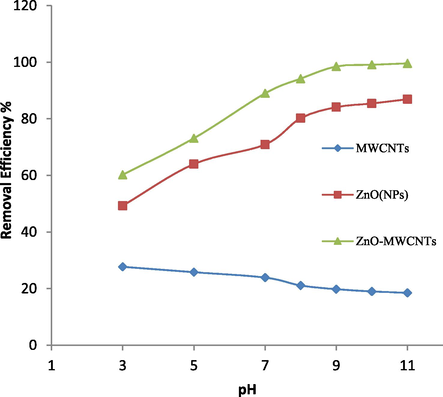
Effect of pH on removal of RB203 dye (photocatalyst concentration: 5 mg/Lg, initial dye concentration: 20 mg/L, pH: 10, time: 20 min, distance from UV lamp: 50 mm).
By comparing the dye removal with ZnO (NPs) and ZnO (NPs)/MWCNTs, it was found that there was little difference in removal efficiency. The reason can be ZnO (NPs) which are not well established on the MWCNTs or so-called “well-dispersed”(Fig. 3). The results showed that the removal efficiency was insignificantly rose when all three photocatalyst concentrations increased more than 5 mg/L. The justification may be that by increasing the photocatalyst concentration, the amount of turbidity of solutions has increased, and thus the amount of light that can reach the catalyst level decreases. Thus, the production of hydroxyl radicals decreases, too. It should be mention that R203 Dye could not be adsorbed on the functionalized MWCNTs because the surface of it was functionalized by acid. Since the reactive blue 203 used in the experiment is an anionic dye, functionalized MWCNTs cannot adsorb onto it, because functionalized MWCNTs surface had a negative charge.
3.3 Effect of pH
One of the most important factors on the dye removal rate is the pH of the test solution. To examine the effect of pH, 100 ml RB 203 solutions (20 mg/L) were used. By adding the appropriate amounts of HNO3 and NaOH 0.1 M solution, the solution pH was adjusted to within the range of 3–12. Next, the amount of 5 mg/L g of each photocatalyst was added to each container separately and each solution was placed in a magnetic stirrer for 20 min at the laboratory temperature. The specimens were then separated by centrifuge and filtered with filter paper. Finally, the absorbance of all solutions was measured by a spectrophotometer and compared with the initial absorption (before the photocatalytic process). The removal percentage was then plotted as a function of pH in the range 3–11 (Fig. 4). Based on experiment results, the most of dye removal has happened in pH = 10. With pay attention to this issue that in alkaline pH the amount of OH− is maximum. Therefore the photocatalyst surface is negatively charged because of the bonding between OH− and metal. So the concentration of dye has been decreased, while the level of
increased. Consequently, attacks by these radicals with a strong oxidation potential are taking place more quickly (Akyol and Bayramoğlu, 2005). It should be noted that the acidic pH of the dye removal percentage showed a significant decrease. Since in the acidic pH of the zinc oxide nanoparticles, in the reaction with the hydrogen ion, they lose their oxygen and become water-soluble in water (Eq. (10)), and lose their photocatalyst property (Ollis et al., 1991).
3.4 Effect of initial dye concentration
To examine the effect of colored solution concentration on the removal of RB203 dye, the following experiments were carried out using the photocatalyst method. A total of 7 samples of 100 ml solution of colored solution at concentrations of 5, 10, 15, 20, 25, 30 and 40 mg/L, with 5 mg/L of each of the three substances (Functionalized MWCNTs, ZnO (NPs), ZnO (NPs)/MWCNTs) were tested for each sample separately at environment temperature (25 °C) and pH = 10 with constant stirring range for 20 min under UV irradiation with a power of 8 W and a distance of 50 mm from the catalyst level (Fig. 5). According to the results obtained, with increasing dye concentration, the surface of the photocatalyst is obtained less photon. As a result, less radical hydroxyl is produced and oxidized dye molecules are decreased. Furthermore, some researchers believe that decolorization has happened via direct transfer of the electron from ZnO (NPs) surface to dye molecules. As another reason, when dye concentration increases, the production of the hole (h+) and electron (e−) decreases through direct oxidation, which can result in less dye removal (Vinodgopal et al., 1996). Owning to the decomposition of molecules of dye, intermediate products, which will compete with primary molecules for photocatalytic degradation, are produced. For example, a reaction of radical hydroxyl with organic compounds produce carbon-centered radicals (R or R·–OH) (Tang and Chen, 2004).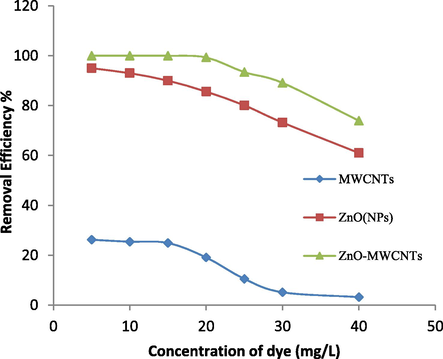
Effect of dye concentration on removal of RB203 dye (photocatalyst concentration: 5 mg/L, pH: 10, time: 20 min, distance from UV lamp: 50 mm).
3.5 Effect of radiation time
To study the effect of light exposure on the amount of dye removal in the photocatalytic process, containers containing 100 ml of the RB203 dye solution at 20 mg/L and pH = 10 were prepared. Then to each container, 5 mg/L of each three photocatalysts was added separately and was placed at a distance of 50 mm from a UV lamp with 8 W power for different durations (5, 10, 15, 20 and 25). According to the results shown in Fig. 6, with increasing the time of UV irradiation, the percentage of removal of RB203 dye was increased. So, the increase in UV radiation time from 5 min to 20 min, the efficiency of removal of RB203 for functionalized MWCNTs from 7.1% to 19%, for ZnO (NPs) from 35.2% to 85.4% and for ZnO (NPs)/MWCNTs from 55.3% to 99.1% was increased. Although this trend is so similar to a first order kinetics, the relative experiments and calculations were not carried out in this study. It is suggested that the kinetics investigation is done in future studies. Increasing UV radiation time increases the sensitivity of photocatalyst nanoparticles. In fact, with the increase in radiation time, the number of electrons transferring from the valence band to the conduction band increases. Hence, the amount of electron-hole pairing increases (Barakat et al., 2005).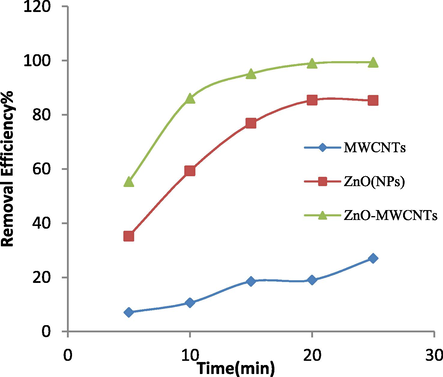
Effect of radiation time on removal of RB203 dye (photocatalyst concentration: 5 mg/L, initial dye concentration: 20 mg/L, pH: 10, time: 20 min, distance from UV lamp: 50 mm).
3.6 Effect of UV lamp intensity
In order to study the effect of UV lamp intensity on dye removal, dishes containing 100 ml of RB203 dye solution with the concentration of 20 mg/L and pH = 10 were prepared. Then, 5 mg/L of mentioned photocatalyst was added to each container separately and placed for 20 min at various UV intensity (51, 44, 28, 23 mW/cm2). According to the results (Fig. 7), it was found that the possibility to produce more radical hydroxyl (the main component of the oxidation of organic compounds) will be provided at maximum UV intensity (51 mW/cm2) due to more catalytic stimulation. The reduction of UV intensity causes fewer electrons to be stimulated from the catalyst and fewer active hydroxyl radicals to be produced, leading to a reduction in catalytic oxidation (Masoumbeigi et al., 2009).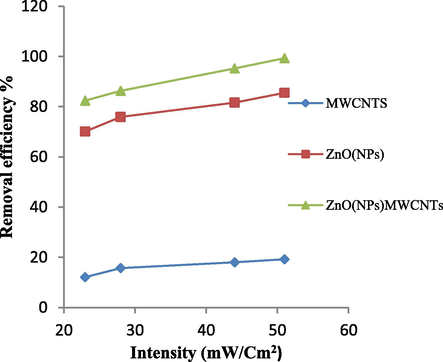
Effect of UV lamp intensity on Removal of RB203 dye (photocatalyst concentration: 5 mg/L, initial dye concentration: 20 mg/L, pH: 10, time: 20 min).
4 Conclusion
In this research, the photocatalytic degradation of RB203 as one of the inflammatory agents was studied using three synthesized photocatalytic materials: functionalized MWCNTs, zinc oxide nanoparticles, and zinc oxide nanoparticles fixed on functionalized MWCNTs. The sequence of synthesized photocatalytic activity according to the percentage of removal percentage of RB203 is as follows: ZnO (NPs)/MWCNTs > ZnO (NPs) > MWCNTs. Besides, factors affecting RB203 decomposition such as dye matter concentration, photocatalyst concentration, solution pH, UV radiation time, and UV intensity were examined in a discontinuous photocatalytic reaction. The optimum conditions to remove RB203 dye with concentration of 20 mg/L using all three photocatalysis were pH of 10, radiation time of 20 min and the UV radiation intensity of 23 mW/cm2. Using photocatalytic reactions to oxidize dyes can be en emerging technology to protect our environment.
References
- Experimental investigation of the rheological behavior and viscosity of decorated multi-walled carbon nanotubeswith TiO2 nanoparticles/water nanofluids. J. Therm. Anal. Calorim.. 2016;123:81-89.
- [Google Scholar]
- Photocatalytic Degradation of p-Cresol by Zinc Oxide under UV Irradiation. Int. J. Mol. Sci... 2012;13:302-315.
- [Google Scholar]
- Photocatalytic degradation of Remazol Red F3B using ZnO catalyst. J. Hazard. Mater.. 2005;124:241-246.
- [Google Scholar]
- Photocatalytic performance of ZnO coated tubular reactor. J. Hazard. Mater.. 2010;180(1):466-473.
- [Google Scholar]
- Synthesis and characterization of ZnO NPs/reduced graphene oxide nanocomposite prepared in gelatin medium as highly efficient photo-degradation of MB. Ceram. Int.. 2014;40:10217-10221.
- [Google Scholar]
- Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl. Catal. B Environ.. 2005;57:23-30.
- [Google Scholar]
- Combination technology of ceramic microfiltration and reverse osmosis for tannery wastewater recovery. Water Resour. Ind.. 2013;3:48-62.
- [Google Scholar]
- TiO2 nanotubes and CNT–TiO2 hybrid materials for the photocatalytic oxidation of propene at low concentration. Appl. Catal. B. 2009;92:377-383.
- [Google Scholar]
- Hydrothermal preparation of ZnO:CNT and TiO2: CNT composites and their photocatalytic applications. J. Mater. Sci.. 2008;43:2348-2355.
- [Google Scholar]
- Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater.. 2004;112(3):269-278.
- [Google Scholar]
- Recent developments in photocatalytic water treatment technology: a review. Water Res.. 2010;44(10):2997-3027.
- [Google Scholar]
- Biosorption of acidic dyes from aqueous solution by Paenibacillus macerans: kinetic, thermodynamic and equilibrium studies. Chem. Eng. J.. 2009;150(1):122-130.
- [Google Scholar]
- Electro-Fenton treatment of dye solution containing Orange II: influence of operational parameters. J. Electroanal. Chem.. 2008;615(2):165-174.
- [Google Scholar]
- Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep.. 2015;1:167-176.
- [Google Scholar]
- Enhancing ferrate (VI) oxidation process to remove blue 203 from wastewater utilizing MgO nanoparticles. J. Environ. Manage.. 2019;231:297-302.
- [Google Scholar]
- Synthesis of SnO2 and ZnO nanoparticles and SnO2–ZnO hybrid for the photocatalytic oxidation of methyl orange. Iran. J. Chem. Eng.. 2015;12:96-105.
- [Google Scholar]
- Semiconductor nanostructures for simultaneous detection and degradation of organic contaminants in water. Photochem Photobiol. Chem.. 2008;42:37-57.
- [Google Scholar]
- Characterization and photocatalytic performance of SnO2–CNT nanocomposites. Appl. Surf. Sci.. 2015;357:302-308.
- [Google Scholar]
- Enhanced photocatalytic activity of Ag–ZnO hybrid plasmonic nanostructures prepared by a facile wet chemical method. Beilstein J. Nanotechnol.. 2014;5:639-650.
- [Google Scholar]
- Microwaveassisted synthesis of CdS-reduced graphene oxide composites for photocatalytic reduction of Cr (VI) Chem. Commun.. 2012;47:11984-11986.
- [Google Scholar]
- Effect of UV radiation intensity on photocatalytic removal of E. coli using immobilized ZnO nanoparticles. Kowsar Med. J.. 2009;14(3):149-156. (In Persian)
- [Google Scholar]
- Photo-catalyzed destruction of watercontaminants. Environ. Sci. Technol.. 1991;25(9):1522-1529.
- [Google Scholar]
- Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater.. 2010;182(1):123-129.
- [Google Scholar]
- Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J. Hazard. Mater.. 2010;179:35-42.
- [Google Scholar]
- Talaiekhozani, A., Banisharif, F., Eskandari, Z., Talaei, M.R., Park, J., Rezania, Sh., 2020. Kinetic investigation of 1, 9-dimethyl-methylene blue zinc chloride double salt removal from wastewater using ferrate (VI) and ultraviolet radiation. J King Saud Uni. Sci. 32, 213–222.
- The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor. J. Water Res.. 2004;38:2775-2781.
- [Google Scholar]
- Using central composite experimental designto optimize the degradation of real dye wastewater by Fenton and photo-Fenton reactions. Dyes Pigm.. 2014;100:184-189.
- [Google Scholar]
- Environmental Photochemistry on Semiconductor Surfaces: photosensitized degradation of a textile azo dye, acid orange 7, on TiO2 particles using visible light. Environ. Sci. Technol.. 1996;30(5):1660-1666.
- [Google Scholar]
- High photocatalytic activity of silver-loaded ZnO–SnO2 coupled catalysts. Chem. Eng. J.. 2009;146:355-361.
- [Google Scholar]
- Simultaneous detection and removal of formaldehyde at room temperature: janus Au@ZnO@ZIF-8 nanoparticles. Nano-Micro Lett.. 2018;10:2-11.
- [Google Scholar]
- Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. Photochem. Photobiol. Chem.. 2010;216:149-155.
- [Google Scholar]
- Sol–gel preparation of CNT/ZnO nanocomposite and its photocatalytic property. Chin. J. Chem.. 2009;27:1317-1320.
- [Google Scholar]
- A novel adsorbent obtained by inserting carbon nanotubes into cavities of diatomite and applications for organic dye elimination from contaminated water. J. Hazard. Mater.. 2010;177(1–3):138-145.
- [Google Scholar]
- Defectmediated formation of Ag cluster-doped TiO2 nanoparticles for efficient photodegradation of pentachlorophenol. Langmuir. 2012;28:3938-3944.
- [Google Scholar]
- ZnO nanowire/reduced grapheme oxide nanocomposite for significantly enhanced photocatalytic degradiation of Rhodamine 6G. Phys. E. 2014;56:251-255.
- [Google Scholar]
- A facile synthesis of ZnO/CNTs hierarchical mircosphere composites with enhanced photocatalytic degradation of methylene blue. R. Soc. Chem.. 2015;5:72476-72481.
- [Google Scholar]







