Translate this page into:
Remodulatory effects of coral fossil on hematobiochemical parameters and histoaccumulation of specific organs in lead intoxicated broilers
⁎Corresponding author. afrina.mustari@bau.edu.bd (Afrina Mustari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The research aims to assess the impact of coral fossil (CF) on hematobiochemical parameters, organ’s accumulation, and remodulation in lead (Pb) intoxicated broiler birds.
Methods
45 broilers were used in the experiment, which was divided into 03 groups, each of which contained 15 broilers. The control group was Group A. Pb (280 mg/kg b. w.) was administered orally to the birds in group B. Group C received Pb (280 mg/kg b.w.) and CF (1 gm/kg b.w.) oral supplements. The research was carried out for 30 days. Blood samples were collected, and sera were prepared for biochemical analysis. Pb accumulation concentration was assessed in muscle, liver and kidney. Moreover, samples of the brain, liver, and kidneys were obtained and prepared for histological analysis.
Results
Pb affected hematological markers (Total RBC, Hb conc., hematocrit value, differential leukocytes and blood indices), which were restored with CF supplementation. In Pb-treated broilers, hepatic enzymes (AST, ALT), renal biomarkers (creatinine, total protein and albumin) and lipid profile (TC, TG, HDL and LDL) increased substantially (p < 0.05), but dropped significantly (p < 0.05) after CF supplementation. Pb accumulation was higher in liver, kidney and muscle tissue that was reduced by CF treatment. The histopathological study demonstrated that there was accumulation of fluid around in brain, hyperplasia of bile duct and glomerular epithelium in Pb intoxicated broilers and these were restored after CF supplementation.
Conclusions
Therefore, it can be concluded that CF supplementation restored the lead induced detrimental effects on broilers. So, CF has remodulatory effects on Pb toxicity.
Keywords
Lead
Coral fossil
Hematobiochemical
Accumulation and histotexture
1 Introduction
Poultry meat is a significant source of protein because it is affordable in Bangladesh and around the world. Since they biomagnify and bioconcentrate harmful metals along the chain of food, polluted broiler’s feeds (Levengood, 2003) cause a risk to both human and animal wellbeing (Mottalib et al., 2018). In Bangladesh, industrial facilities that produce sulfuric acid, ceramics, dyes for textiles, and tanneries are particularly linked to the discharge of cadmium, lead, manganese, nickel, copper and zinc in excessive amounts, raising more concerns about the contamination of vegetation, soils and water bodies (Kashem and Singh, 1999). Among them, Pb is the most dangerous one, capable of poisoning at very small doses and harming a variety of organs (Amadi et al., 2019). Lead (Pb) is a dangerous heavy metal that makes up 0.002 % of the Earth's crust and exists in a divalent condition (Arias et al., 2010). Due to its sluggish rate of elimination, lead is typically a chronic or cumulative toxin that is hazardous to both humans and animals (Jiao et al., 2017). The primary major routes by which Pb enters the human body are through direct inhalation of dust containing lead, cutaneous exposure to dust and soils having Pb, drinking of Pb exposed water, and food produced in Pb-contaminated areas (Kumar et al., 2020). By raising the degree of lipid peroxidation and disrupting the action of numerous antioxidant enzymes, lead induces oxidative stress, which has a negative impact on erythropoiesis, kidney function, the immunological system, and the central nervous system (Sadhana et al., 2011). The time and dosage of lead acetate consumption influence the decline in total RBC count. Some adverse consequences include disturbances in the morphology and chemistry of several organs, especially the renal and hepatic systems (Muhammad et al., 2017). Muscles typically have the lowest Pb deposition, although the kidney and liver are usually the organs with the highest Pb levels (Wang et al., 2021).
Different materials have different levels of binding ability. Bhatti et al. (2018) mentioned that the commonly used binding materials are silicates, charcoal and bentonite. ULKAL is made from natural coral fossil without any chemicals. It can remove mycotoxin, able to remove toxic substances. It includes certain calcium carbonate which has the highest absorption ratio. Fossil shells are mostly composed of silicates of incorporeal shape which is readily dissolved in the body of animals and can be employed to alleviate the problem (Adeyemo, 2013).
The study's objective is to assess how coral fossil works as a toxin binder to eliminate Pb's negative effects and ensure the safety of broiler products. There is limited or virtually no existing research with a similar focus. So, this investigation stands out in its novelty of assessing the capacity of coral fossils to alleviate the detrimental impact of Pb on broilers.
2 Materials and Methods
2.1 Declaration of ethics
The Guidelines for animal care, experimentation, and administration were approved by Bangladesh Agricultural University's Committee on the Welfare of Animals and Research Morality. (The ref code is AWEEC/BAU/2021–41).
2.2 Experimental birds
In order to examine the effects of lead exposure and coral fossil supplementation, a set of 45-day-old chicks, or DOCs, was procured. These chicks were given timely vaccines while being reared in an open house setting for thirty days.
2.3 Experimental design
Forty-five broilers were used in the experiment, and they were randomly divided into three groups of fifteen broilers each to continue the process. Group A received regular feed of poultry and acted as the vehicle control. Pb (280 mg/kg BW) was given to the Group B broilers on a regular basis. Group C got Pb (280 mg/kg BW) as well as a coral fossil (1 gm/kg BW). Both the Pb and the coral fossil were delivered with water at the dosage rates recommended by Suleman et al. (2011) and Mustari et al. (2023), respectively. The experiment lasted thirty days.
2.4 Hematobiochemical studies
5 ml of blood were collected in a falcon tube for the purpose of conducting hematology analysis, while an additional 5 ml of blood were allowed to coagulate. Following the coagulation, the serum was carefully extracted from the clot and subsequently subjected to centrifugation in order to obtain a clear serum. Subsequently, within the physiology department, a comprehensive evaluation of various hematological parameters took place, including the examination of the total RBC count, Hb contents, hematocrit value, blood indices (MCV, MCH and MCHC) as well as the differential leukocyte count (DLC). The following biochemical measurements were made at Professor Dr. Mohammad Hussain Central Laboratory in Bangladesh Agricultural University, Mymensingh-2200: serum AST (aspartate aminotransferase), ALT (alanine transaminase), creatinine, TP (total protein), albumin and lipid profile.
2.5 Histopathology
Organs (brain, liver, and kidney) were removed and stored in 10 % neutral buffered formalin for fifteen consecutive days. Following that, the correctly preserved organs were processed, sectioned off, and stained in the Pathology Department at BAU, Mymensingh-2202. To enhance comprehension of slides having histotexture, a photomicroscope (Model: CX43, Olympus Corporation, Tokyo, Japan) was used to view the histological results at magnifications of 100x and 400x.
2.6 Determination of Pb contents
At necropsy, some specific organs were collected from every broiler. Subsequently, inductively coupled plasma-mass spectrometry, or ICP-MS, was used to evaluate the accumulation of Pb in muscle, liver, and kidney (Agilent 7500c, Japan).
2.7 Statistical analysis
The research data were collected and organized in Microsoft Excel 2016, then these data were imported into GraphPad Prism 5.0 for analysis. The one-way ANOVA with Bonferroni multiple comparison test was used (p ≤ 0.05) with 95 % confidence intervals for analysis.
3 Results
3.1 Blood parameters (Total RBC, Hb, hematocrit, MCV, MCH, MCHC and DLC)
The hematological examination showed that broilers treated with Pb had significantly (p < 0.05) lower TEC, hemoglobin content, and hematocrit than control broilers. CF supplementation broilers along with Pb raised these parameters (Table. 1). As compared to control groups, the MCV and MCH values considerably raised in the Pb-treated broilers (Table. 1). However, adding coral fossil to the diet significantly (p < 0.05) reduced MCV and MCH. Pb and coral fossil treatment had no significant impact on MCHC. By comparing the group exposed to Pb with the control groups, we observed a significant decrease in the number of heterophils, while there was a notable increase in the number of lymphocytes (Table 1) with statistical significance (p < 0.05). The decline in heterophil (15.50 ± 0.50 %) and the ascent in lymphocytes (66.50 ± 2.50 %) in Pb treated group were restored by CF. After Pb and coral fossil supplementation, there were no significant changes in the percentage of monocyte, eosinophil, and basophil (Table. 1). Data indicate mean ± SEM. Values with different superscript letters in a same column differ significantly.
Hematological data (Mean ± SEM)
Treatment
Control
Lead
Lead + Coral fossil
TEC (million/mm3)
2.02 ± 0.09a
0.87 ± 0.04b
2.20 ± 0.16a
Hb (gm%)
8.33 ± 0.43a
5.80 ± 0.34b
7.40 ± 0.23a
PCV (%)
29 ± 0.57a
25 ± 0.57b
28 ± 0.57a
MCV (cubic micron)
144.0 ± 9.55a
287.3 ± 12.66b
128.9 ± 12.11a
MCH (pg)
41.31 ± 2.78a
66.33 ± 0.91b
34.12 ± 3.55a
MCHC (%)
28.81 ± 2.02a
23.19 ± 1.20a
26.42 ± 0.28a
Heterophil (%)
30.00 ± 3.00a
15.50 ± 0.50b
29.50 ± 1.50a
Lymphocyte (%)
56.50 ± 1.50a
66.50 ± 2.50b
53.50 ± 0.50a
Monocyte (%)
5.50 ± 0.50a
4.50 ± 0.50a
7.50 ± 0.50a
Eosinophil (%)
6.50 ± 0.50a
8.50 ± 1.50a
7.00 ± 0.50a
Basophil (%)
1.50 ± 0.50a
5.00 ± 1.00a
2.00 ± 1.00a
3.2 Liver function test
According to the liver function test, serum liver enzymes (AST, ALT) increased significantly in the Pb treatment group, reaching levels of 327 ± 4.11 U/L and 37.20 ± 1.33 U/L, respectively, compared to the control group, which had levels of 238 ± 6.37 U/L and 22.63 ± 0.93 U/L, respectively (Fig. 1a, b). The addition of CF resulted in a significant (p < 0.05) decrease in AST and ALT levels in Pb-treated broilers.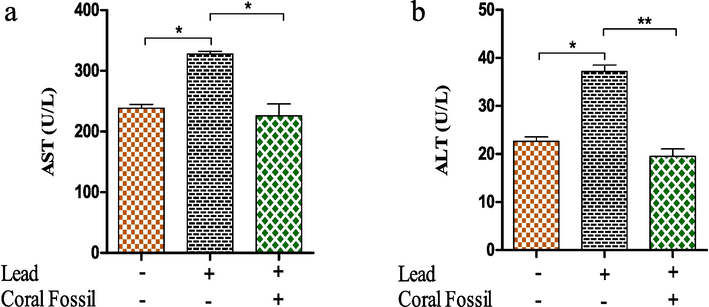
Effect of coral fossil on serum AST and ALT in lead intoxicated broilers. The data represent the mean SEM. One-way ANOVA with the Bonferroni multiple comparison test was used. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3.3 Renal function test
Renal biomarkers demonstrated that creatinine levels (1.07 0.01 mg/dl) were higher in Pb-treated broilers than in controls. In contrast, serum creatinine levels (0.83 ± 0.03 mg/dl) reduced greatly (p < 0.05) in combined Pb and CF exposed broilers (Fig. 2a). Similar to this, broilers treated with Pb had higher blood total protein (TP) and albumin levels (3.59 ± 0.23 mg/dl and 1.81 ± 0.03 mg/dl, respectively) than those in the control group (0.95 ± 0.02 mg/dl and 2.24 ± 0.04 mg/dl, respectively) (Fig. 2b, c). Supplementation of coral fossil with Pb lowered serum total protein and albumin significantly (p < 0.05) with values of 2.24 ± 0.06 mg/dl and 0.94 ± 0.04 mg/dl, respectively.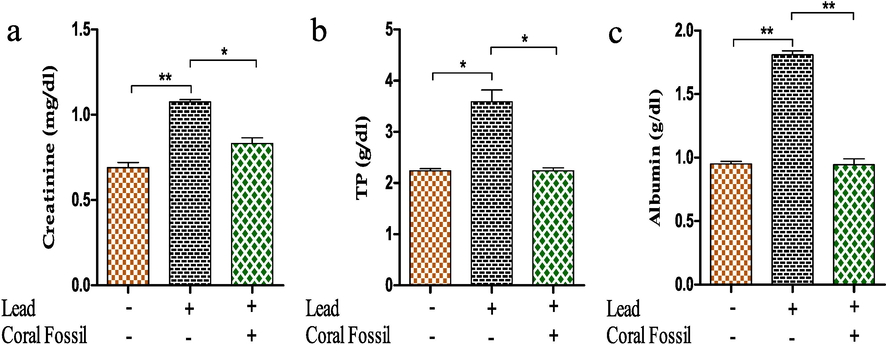
Effect of coral fossil on serum creatinine, total protein (TP) and albumin in lead intoxicated broilers. The data represent the mean SEM. One-way ANOVA with the Bonferroni multiple comparison test was used. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3.4 Lipid profile
The serum total cholesterol (TC) level was higher in Pb treated broilers (225 ± 10.74 mg/dl) compared to controls (173.0 ± 2.03 mg/dl). However, it dropped substantially (p < 0.05) after CF treatment (146.2 ± 3.03 mg/dl) (Fig. 3a). However, similar to serum LDL level in Pb and CF supplemented broilers significantly (p < 0.05) decreased serum LDL level (67.55 ± 4.69 mg/dl) (Fig. 3d). There was no significant difference in serum triglyceride and HDL level (Fig. 3 b, c) between treatment and control groups.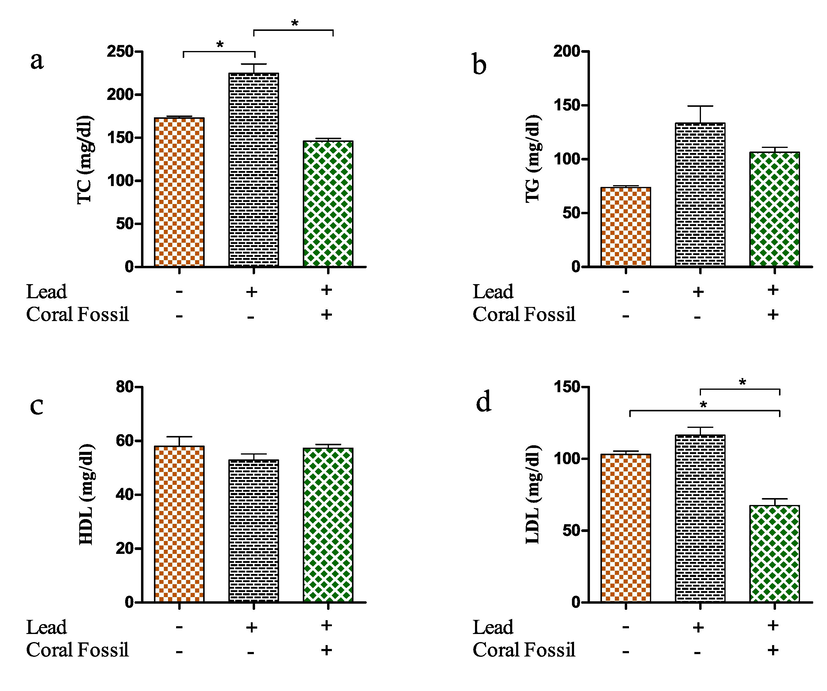
Effect of coral fossil on lipid profile (Serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL)) in lead intoxicated broilers. The data represent the mean SEM. One-way ANOVA with the Bonferroni multiple comparison test was used. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3.5 Accumulation concentration of Pb in muscle, liver and kidney
Muscle’s Pb (Pb) content significantly increased in Pb treated broilers (456 ± 25.00 mg/kg) compared to the control (138.2 ± 10.00 mg/kg). While, Pb content in muscle reduced significantly (p < 0.05) after providing CF (162.9 ± 10.00 mg/kg) (Fig. 4a). Similarly, Pb accumulation in liver (Fig. 4b) and kidney (Fig. 4c) were different among the broilers of control (143.2 ± 5.00 mg/kg and 167.5 ± 15.00 mg/kg respectively) and Pb treated (2660 ± 40.00 mg/kg and 3407 ± 66.50 mg/kg respectively) group. Inclusion of CF along with Pb decreased liver and kidneys Pb contents having the value 1793 ± 115.00 mg/kg and 2070 ± 50.00 mg/dl respectively.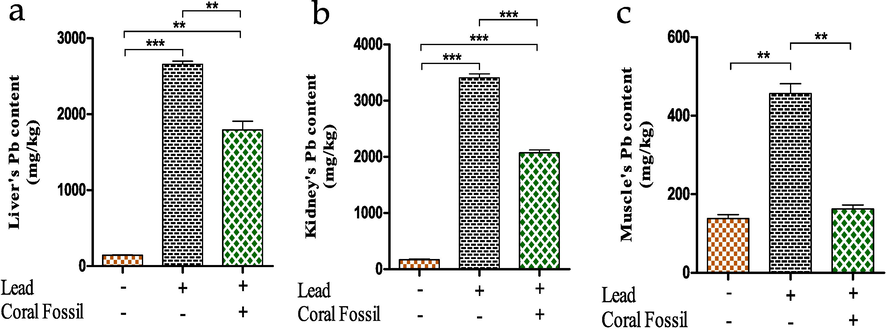
Effect of coral fossil on lead contents of muscle, liver and kidney in lead intoxicated broilers. The data represent the mean SEM. One-way ANOVA with the Bonferroni multiple comparison test was used. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
3.6 Histotexture study
Brain histotexture of non-treated broilers demonstrated normal histological structure whereas Pb treated birds revealed accumulation of fluid around the blood vessel. Addition of CF in Pb treated broilers, brain showed declining edema around blood vessel (Fig. 5). Liver of non-treated broilers showed normal histological structure with normal central vein and hepatocytes. Pb treated broilers showed hyperplasia of bile duct epithelium. Whereas, liver showed less proliferative changes in the bile duct after coral fossil supplementation (Fig. 6). The renal tubule and glomerulus of non-treated broiler kidneys were normal. Whereas Pb treated broilers showed hyperplastic changes in the glomerular epithelium, hyperchromatic nuclei with acidophilic cytoplasm and have degenerative changes. CF supplemented broilers kidney showed less hyperplasia and degenerative changes compared to Pb treated group (Fig. 7).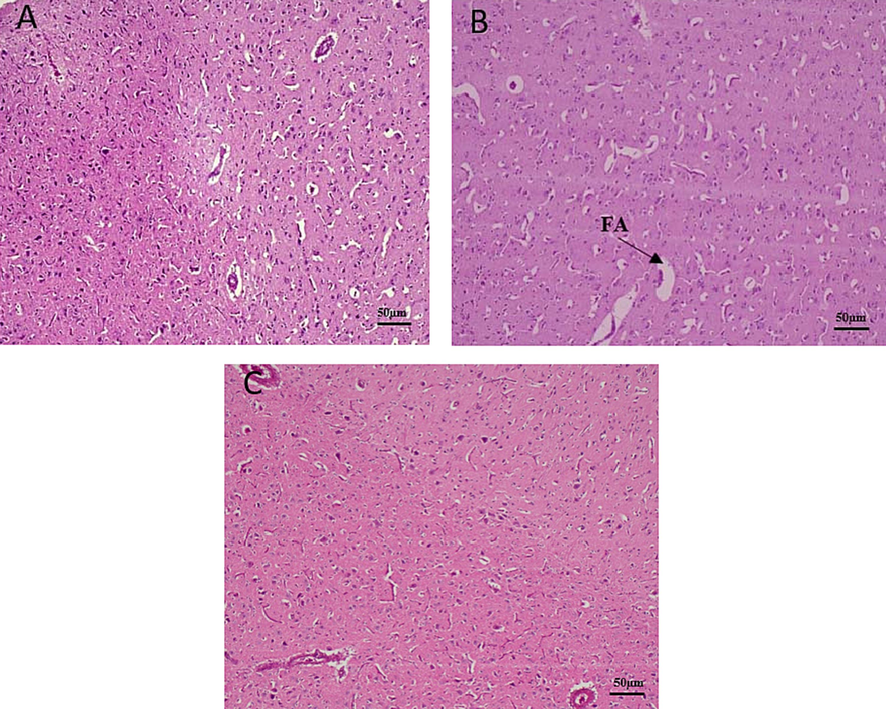
Effects of coral fossil on brain in lead intoxicated broilers. Photomicrograph of brain in broilers of control (A); Lead @ 280 mg/kg BW (B); Lead @ 280 mg/kg BW + Coral Fossil @ 1gm/kg BW (C) at 100x magnification. FA- Fluid Accumulation.
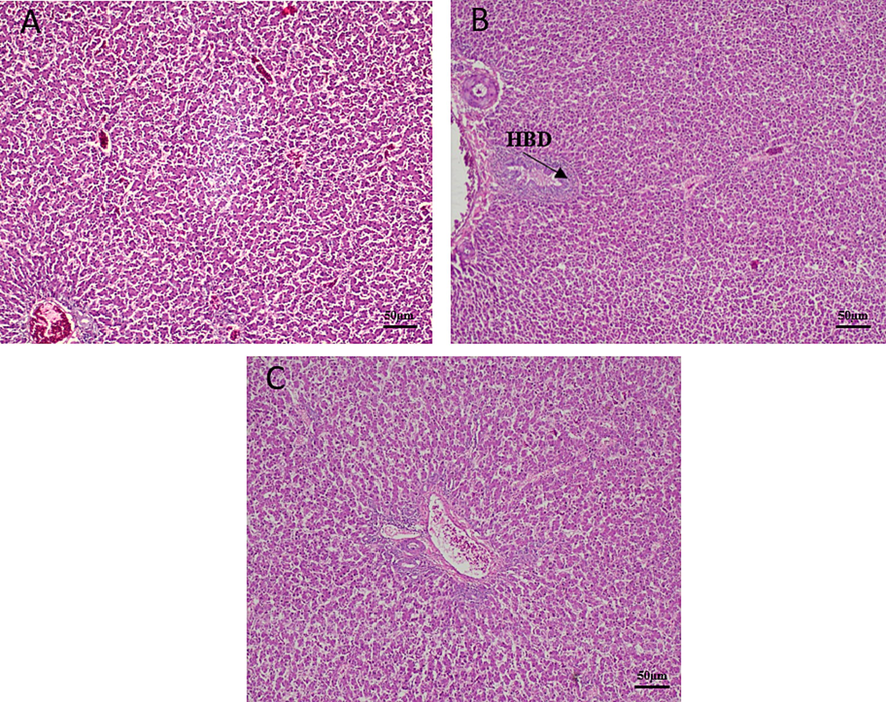
Effects of coral fossil on liver in lead intoxicated broilers. Photomicrograph of liver in broilers of control (A); Lead @ 280 mg/kg BW (B); Lead @ 280 mg/kg BW + Coral Fossil @ 1gm/kg BW (C) at 100x magnification. HBD- Hyperplasia of Bile Duct.
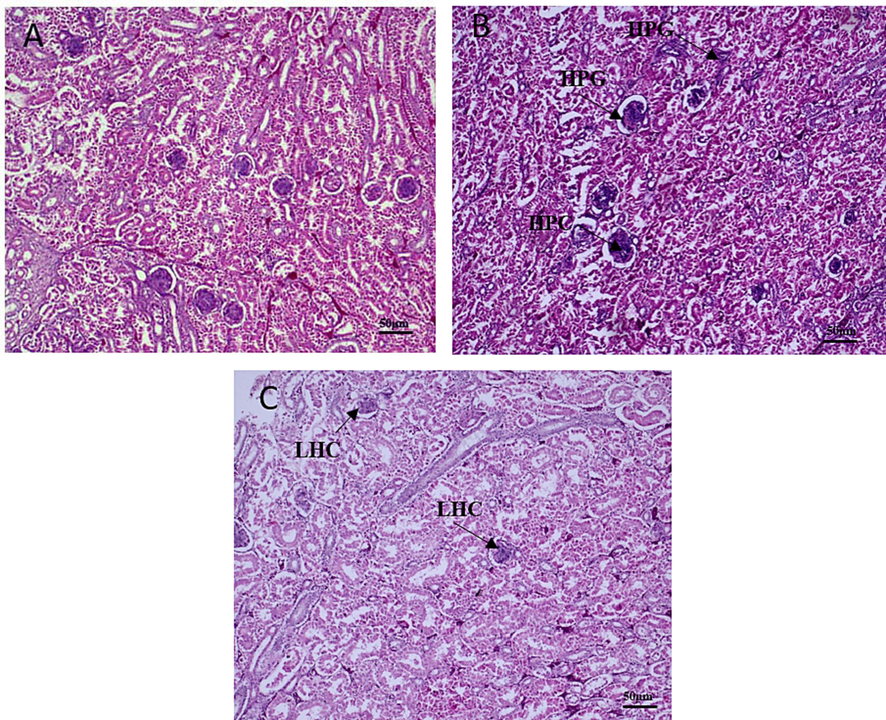
Effects of coral fossil on kidney in lead intoxicated broilers. Photomicrograph of kidney in broilers of control (A); Lead @ 280 mg/kg BW (B); Lead @ 280 mg/kg BW + Coral Fossil @ 1gm/kg BW (C) at 100x magnification. HPC-Hyperplastic Changes, HPC-Hypercellular Glomerulus and LHC- Less Hyperplastic Changes.
4 Discussion
4.1 Blood parameters (Total RBC, Hb, hematocrit, MCV, MCH, MCHC and DLC)
The findings from our empirical investigation demonstrated notable variations in the blood parameters of broiler chickens administered with Pb, in contrast to the control group. These observations are consistent with the findings reported by Yılmaz et al. (2012), who asserted that exposure to Pb leads to a significant reduction in blood parameters. The findings are consistent with previous research that found a significant decrease in RBC hemoglobin and packed cell capacity in fresh water fish exposed to heavy metals (Vutukuru, 2005). In accordance with Witeska and Kościuk (2003), heavy metals may alter the characteristics of hemoglobin by limiting its affinity for oxygen binding capacities and this might result to erythrocytes being more fragile and permeable, which can cause cell enlargement, distortion, and damage. Pb disrupts calcium homeostasis (Quintanar-Escorza et al., 2010) and changes the composition of cell membranes (Ahyayauch et al., 2013), therefore it is possible that Pb could affect MCV through these processes. It appears that Pb has a stronger impact on MCV and MCH in low Pb exposure than in high Pb exposure. An earlier study found that inhaling Pb acetate increased total leukocyte count in eosinophils, neutrophils, and basophils (Lorenzo and Corfiati, 2006). Our investigation also discovered that feeding coral fossils restored the TEC, Hb conc., and PCV of Pb-exposed broiler chicks. The substances having antioxidant characteristics were reported as efficient in improving TEC and Hb in broilers having Pb exposure (Jaiswal et al., 2017). In the present study, CF have restored hematological values in Pb exposed broilers. These may be due to the protective antioxidative properties of CF but the exact mode of action is not clear.
4.2 Liver function test
In the present study, liver function indicators (AST and ALT) significantly increased in the Pb-treated group. Our results are consistent with Bera et al. (2020), who found that exposure to Pb acetate alters the various liver enzymes, such as ALT, ALP, and AST in plasma, by increasing oxidative stress. Our findings suggest that inclusion of CF considerably declined liver enzymes (AST and ALT) in Pb supplemented broilers. Azadbakht et al. (2017) observed that a toxin binder rich in calcium can revive against Pb toxicities due to the absorption and removal of Pb from the GIT (gastro-intestinal tract). Due to high calcium contents of coral fossil, it may be bonded with these enzymes for their elimination. More investigations are needed to uncover the real cause.
4.3 Kidney function test
Coral fossil supplementation resulted in a substantial decrease in serum renal function markers (albumin, total protein, and creatinine) compared to a considerable increase in the Pb-treated group. Heavy metal exposure has been linked to renal illness in previous investigations (Alissa and Ferns, 2011). Previous study has also connected nephrotoxic heavy metals to the progression of renal diseases or renal damage (Wang et al., 2011). Low Pb levels were linked to changes in specific renal function indicators, but every 2-fold increase in Pb was associated with a decrease in eGFR and an increase in creatinine (Pollack et al., 2015). In autoimmune disorders, the effective glomerular filtration rate (eGFR), a measure of renal function, is elevated by hydrogen-rich coral calcium and plays a role in oxygen transport (Shen et al., 2022). Water supplied with the coral-mine combo can benefit both healthy and damaged nephron tubules as a rehabilitation treatment to enhance kidney function (Zaliavska et al., 2021). Coral fossils aid kidney function because of their potential as a supply of calcium that is high in hydrogen.
4.4 Lipid profile
The blood lipid profile was changed in Pb treated broilers which was recovered after coral fossil supplementation. Increased levels of cholesterol, triglycerides, and lipoproteins are associated with heavy metal exposure (Poręba et al., 2011). Earlier research by Xu et al. (2021) shown that Pb exposure raised TC, Non-HDL-C, and LDL-C levels in the adult population. Although the anti-oxidative activity and de novo lipogenesis of coral hydrate are known to be significantly influenced by redox modulation, this may be connected to the decreases in serum triglycerides (Crewe et al., 2019). Consequently, coral hydrate might influence lipid metabolism indirectly via altering gut flora in addition to directly regulating lipid metabolism in the liver (Wu et al., 2022). Further investigation is needed to clarify the potential mechanisms.
4.5 Accumulation concentration of Pb in muscle, liver and kidney
The current study shows that supplementing coral fossil with Pb reduces Pb absorption in broilers. Metals' chemical characteristics and an organ's physiological state affect the affinity for heavy metals (Storelli et al., 2006). The kidney has the greatest Pb concentration because of glomerular filtration (Hall, 2016). Pb excretion in urine is aided by a number of high-affinity Pb-binding proteins in the kidney (Gonick, 2011). The liver is in charge of detoxifying different compounds and is thought to be the greatest Pb reservoir of soft tissues in mammals (Patrick, 2006). Nonetheless, the Pb content in laying hen muscle was significantly lower than in viscera. Actin and myosin protein filaments in muscle cells have a low affinity for Pb (Tarrago and Brown, 2017). According to Moussa et al. (2001), calcium may react with lead by binding it in the stomach, contending with it for absorption, influencing intestinal cell activity for lead, and affecting the affinity of lead in target tissues. Coral fossil, as a calcium supply, may lower Pb levels in muscle, liver, and kidney.
4.6 Histopathology
Pb treated broilers birds showed accumulation of fluid around the blood vessel in brain, hyperplasia of bile duct epithelium, hyperplastic changes in the glomerular epithelium, hyperchromatic nuclei with acidophilic cytoplasm as well as degenerative changes with focal necrosis in kidney. All these alterations were recovered by coral fossil supplementation. Oladipo et al. (2020) reported that Pb induced hyper eosinophilic cytoplasm with round eosinophilic intranuclear inclusions in kidney. Pb produces perineuronal and perivascular edema, as well as malacia in the brain, and intranuclear inclusion bodies inside hepatocytes along with hyperplastic nodules, which are improved by calcium supplement (Al-Naimi et al., 2011). Because coral fossil is a possible supply of calcium, it may interfere with Pb being absorbed, enhance Pb excretions through urine, and reduce the harmful effects of Pb. So, Coral fossils can be therapeutically employed as a potential remedy for mitigating lead toxicity in broilers. Previous research in broilers lacked comprehensive data on coral fossils, which hindered a clear understanding of their exact mechanism. To determine the precise mode of action, additional research is imperative.
5 Conclusion
Inclusion of CF with water substantially decline the detrimental impacts of Pb on hematological parameters, hepatorenal functions, lipid profile, accumulation concentration in muscle, liver and kidney, and histotexture of brain, liver and kidney of broilers. Therefore, supplementation of CF could be used as an effective approach to combat Pb induced alterations in broilers health. However, further investigation is required to understand the precise mechanism by which coral fossil reduces adverse effects in Pb-exposed broiler.
Funding
The research work was funded by Ministry of Education (MoE), Bangladesh (Project No. LS20201481).
Author contributions
Afrina Mustari, Mahabub Alam: Conceived and designed the experiments; Funding acquisition; Performed the experiments; Wrote the paper.
Shabuj Kumar Pal, Mohammad Alam Miah: Analysed and interpreted the data; Wrote the paper.
Mahmud Hossain Sumon, Emdadul Haque Chowdhury: Contributed reagents, materials, analysis tools; Wrote the paper.
Acknowledgement
The authors are grateful for the research grant from Ministry of Education (MoE), Bangladesh. The authors would also like to thank the Bangladesh Agricultural University Research System (BAURES), BAU, Bangladesh; Department of Pathology and Professor Dr. Mohammad Hussain Central Laboratory at Bangladesh Agricultural University in Mymensingh, Bangladesh, for providing laboratory support, as well as everyone engaged in the research for their enthusiastic involvement in this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Growth performance of broiler chickens fed fossil shell growth promoter. Food Nutr. Sci.. 2013;4:16-19.
- [CrossRef] [Google Scholar]
- Effects of chronic and acute lead treatments on the biophysical properties of erythrocyte membranes, and a comparison with model membranes. Federation of European Biochemical Societies Open Biology.. 2013;15(3):212.
- [CrossRef] [Google Scholar]
- Heavy metal poisoning and cardiovascular disease. Journal of Toxicology. 2011;2011:870125
- [CrossRef] [Google Scholar]
- Toxicopathological study of lead acetate poisoning in growing rats and the protective effect of cysteine or calcium. Al-Anbar J. Vet. Sci. 2011;4:26-39.
- [Google Scholar]
- Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res.. 2019;26(18):18032-18052.
- [CrossRef] [Google Scholar]
- Effects of Glomus deserticola inoculation on Prosopis: enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot.. 2010;68(2):139-148.
- [Google Scholar]
- Assessing the protective effect of bentonite against lead toxicity in growing lambs. Environ. Sci. Pollut. Res.. 2017;24(35):27484-27489.
- [CrossRef] [Google Scholar]
- Liver Function Enzymes are Potential Predictive Markers for Kidney Allograft Dysfunction. Advancements in Journal of Urology and Nephrology. 2020;2(1):27-36.
- [CrossRef] [Google Scholar]
- Comparative efficacy of Bentonite clay, activated charcoal and Trichosporon mycotoxinivorans in regulating the feed-to-tissue transfer of mycotoxins. J. Sci. Food Agric.. 2018;98(3):884-890.
- [CrossRef] [Google Scholar]
- SREBP-regulated adipocyte lipogenesis is dependent on substrate availability and redox modulation of mTORC1. JCI Insight. 2019;4(15)
- [CrossRef] [Google Scholar]
- Hall J.E. Elsevier; Amsterdam, The Netherlands: 2016. Guyton and Hall Textbook of Medical Physiology.
- Jaiswal, R., Ali, S. L., Roy, S., Dinani, O. P., & Jaiswal, S. K. (2017). Effect of dietary lead exposure on hematological parameters and their alleviation by antioxidants in broiler. International Journal of Bio-resource and Stress Management, 8(1), 110-115. DOI: HTTPS://DOI.ORG/10.23910/IJBSM/2017.8.1.1778.
- Alleviation of Pb-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ. Sci. Pollut. Res.. 2017;24(8):7555-7564.
- [CrossRef] [Google Scholar]
- Heavy Metal Contamination of Soil and Vegetation in the Vicinity of Industries in Bangladesh. Water, Air, & Soil Poll.. 1999;155:347-361.
- [CrossRef] [Google Scholar]
- Kumar, A., M M S, C. P., Chaturvedi, A. K., Shabnam, A. A., Subrahmanyam, G., Mondal, R., Gupta, D. K., Malyan, S. K., S Kumar, S., A Khan, S., & Yadav, K. K., 2020. Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health, 17(7), 2179. DOI: 10.3390/ijerph17072179.
- Cadmium and lead in tissues of mallards (Anas platyrhynchos) and wood ducks (Aix sponsa) using the Illinois River (USA) Environ. Pollut.. 2003;122(2):177-181.
- [CrossRef] [Google Scholar]
- Evaluation of peripheral blood neutrophil leucocytes in lead exposed workers. International Archives of Occupational and Environmental Health.. 2006;79:491-498.
- [CrossRef] [Google Scholar]
- Assessment of trace metals in consumer chickens in Bangladesh. Journal of Health & Pollution. 2018;8(20):181208
- [CrossRef] [Google Scholar]
- Influence of dietary calcium on subacute lead toxicity in the rat. J. Pakistan J. of Biol. Sci.. 2001;4(1):77-80.
- [CrossRef] [Google Scholar]
- Effects of experimental lead toxicity on hematology and biochemical parameters in Lohi sheep. Int. J. Agric. Biol.. 2017;19(6):1409-1413.
- [Google Scholar]
- Coral fossil: A potential adsorbent of natural source for cadmium removal in broilers. Saudi Journal of Biological Sciences. 2023;30(9):103742
- [CrossRef] [Google Scholar]
- Lead toxicoses in free-range chickens in artisanal gold-mining communities, Zamfara. Nigeria. Journal of Health and Pollution. 2020;10(26):200606
- [CrossRef] [Google Scholar]
- Pb toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev.. 2006;11:114.
- [Google Scholar]
- Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: a prospective cohort study. J. Toxic. Environ. Health A. 2015;78(2):119-131.
- [CrossRef] [Google Scholar]
- Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environ. Toxicol. Pharmacol.. 2011;31(2):267-277.
- [CrossRef] [Google Scholar]
- Oxidative damage increases intracellular free calcium [Ca2+] concentration in human erythrocytes incubated with lead. Toxicol. In Vitro. 2010;24(5):1338-1346.
- [CrossRef] [Google Scholar]
- Pb toxicity, oxidative damage and health implications. A review. International Journal of Biotechnology and Molecular Biology Research. 2011;2(13):215-221.
- [CrossRef] [Google Scholar]
- Shen, M. C., Chung, J. R. C., Wang, K. Y., Chu, C. F., Tsou, W. H., Chou, H. Y. et al., 2022. Evaluation of the safety and potential therapeutic effects of hydrogen-rich coral calcium on autoimmune diseases. Research Square .4(1),1-22. DOI: https://doi.org/10.21203/rs.3.rs-2018732/v1.
- Trace metals in tissues of Mugilids (Mugil auratus, Mugil capito, and Mugil labrosus) from the Mediterranean Sea. B. Environ. Contam. Tox.. 2006;77:43-50.
- [CrossRef] [Google Scholar]
- Effect of lead acetate administered orally at different dosage levels in broiler chicks. Afr. J. Environ. Sci. Technol.. 2011;5(12):1017-1026.
- [CrossRef] [Google Scholar]
- Case studies in environmental medicine (CSEM) lead toxicity. Agency for Toxic Substances and Disease Registry. 2017;WB2832
- [Google Scholar]
- Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int. J. Environ. Res. Public Health. 2005;2(3):456-462.
- [CrossRef] [Google Scholar]
- Renal impairment caused by chronic occupational chromate exposure. Int. Arch. Occup. Environ. Health. 2011;84(4):393-401.
- [CrossRef] [Google Scholar]
- Influences of lead exposure on its accumulation in organs, meat, eggs and bone during laying period of hens. Poult. Sci.. 2021;100(8):101249
- [CrossRef] [Google Scholar]
- The changes in common carp blood after short-term zinc exposure. Environ. Sci. Pollut. Res.. 2003;10(5):284-286.
- [CrossRef] [Google Scholar]
- Coral Hydrate, a Novel Antioxidant. Improves Alcohol Intoxication in Mice. Antioxidants. 2022;2022(11):1290.
- [CrossRef] [Google Scholar]
- Low-level environmental lead and cadmium exposures and dyslipidemia in adults: Findings from the NHANES 2005–2016. J. Trace Elem. Med Biol.. 2021;63:126651
- [CrossRef] [Google Scholar]
- Analysis of the hematological and biochemical parameters related to lead intoxication. J. Forensic Leg. Med.. 2012;19(8):452-454.
- [CrossRef] [Google Scholar]
- Zaliavska, O. V., Antoniv, A. A., Kaushanska, O. V., Pavlyukovich, N. D., Nika, O.M., 2021. A rehabilitation effect of water with low surface tension on the functional condition of the kidneys. East. Ukrainian Med. J., 9(1), 39-45. DOI: https://doi.org/10.21272/eumj.2021;9(1):39-45.







