Translate this page into:
Remedial effects of casticin as an antioxidant on cisplatin induced oxidative damage in rat liver
⁎Corresponding author. shahidmahboob60@hotmail.com (Shahid Mahboob) asmaashrafgcuf@gmail.com (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cisplatin (CP) is an active cytotoxic agent, which has been verified to be effective in multiple cancer regimen. In this study, potential antioxidant effects of casticin (CAS) were assessed against CP generated oxidative stress in rat liver. “Twenty-four male Sprague Dawley rats were divided into four experimental groups. Group-1 (control group) received only normal saline”. Group-2 was intraperitoneally injected with CP (10 mg/kg). Group-3 was orally provided by CAS (50 mg/kg) along with CP (10 mg/kg) injection at first day. Group-4 group was orally administered with CAS (50 mg/kg) throughout the experiment. The rats of group-1 indicated “increase in serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP), while a significant decrease in the activities of catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD)” was noticed, which revealed that CP generated oxidative stress in rat liver. Moreover, administration of CP increased the hydrogen peroxide (H2O2) concentration and level of “thiobarbituric acid reactive substances (TBARS)”, while reducing the % DNA head and head length in liver cell nuclei. CP disturbs the lobular structure of the liver and increased the sinusoidal dilation in rat liver. However, co-treatment with CAS successfully mitigated the CP generated DNA disruption, biochemical and pathological changes in rat liver. These findings revealed that an effective antioxidant, CAS, alleviated the CP generated hepatotoxicity and oxidative stress in rats.
Keywords
Cisplatin
Oxidative stress
DNA damage
Liver damage
Antioxidant
Casticin
1 Introduction
The platinum-derived antineoplastic, DNA alkylating agent Cisplatin (CP) has a broad spectrum of clinical applications, such as sarcoma, lung cancer, testicular, ovarian, bladder, germ cell tumor, lymphoma and head and neck neoplasms (Wilson and Lippard, 2013). The chemotherapeutic effect of CP is associated with its ability to crosslink with DNA strands which may lead to DNA disruption. These events interrupt the DNA replication and alter gene expression, eventually inducing apoptosis (Melnikov et al., 2016). However, regardless of having extensive clinical efficacy, it has a narrow therapeutic index owing to undesirable serious injuries, particularly hepatotoxicity (Niu et al., 2017).
The actual mechanism behind the CP stimulated toxicity is not thoroughly understood yet. ROS as highly reactive intermediates may induce oxidative disruption to DNA, lipids and nitrogenous compounds which ultimately impair the cell integrity (Casares et al., 2012). CP generated liver toxicity is a multifaceted phenomenon as evident by significant escalation in serum levels of ALT, AST and ALP (Ghazal et al., 2016). Histopathological studies have indicated that CP generates hepatic injuries such as deterioration of hepatocytes and is capable of sinusoids dilation (Miyamoto et al., 2007).
“Hepatotoxicity is the dose limiting factor for the clinical practice” of CP (Sudhakar et al., 2010). For that reason, it is mandatory to find an approach to ameliorate the CP induced toxicity without minimizing its healing effects. Antioxidant agents have a remarkable contribution in averting the oxidative stress (Goudarzi et al., 2017). Numerous antioxidant compounds such as propofol, hyperin and thymoquinone have been used for the inhibition of CP induced hepatotoxicity (Al-Malki and Sayed, 2014; Cagin et al., 2015; Ghazal et al., 2016).
Casticin “(3′,5-dihydroxy-3,4′,6,7-tetramethoxyflavone) is a flavonoid”, extracted from a variety of plant species, which is extensively used for the treatment of various ailments like respiratory infections, diarrhea, migraine, rheumatic pain and headaches (Kobayakawa et al., 2004). CAS shows a “broad spectrum of pharmacological activities such as antioxidant, anti-cancer and anti-inflammatory effects” (Lee et al., 2017).
Even with the auspicious curative benefits of CAS, its defensive role in hepatotoxicity and oxidative damage generated by CP is yet to be reported. The objective of this study was to monitor the protective aptitude of CAS on CP persuaded hepatotoxicity and oxidative damage in rats.
2 Materials and methods
2.1 Chemicals
CAS and CP were provided by Sigma-Aldrich (Germany).
CAS solution was prepared by dissolving it 0.9% normal saline.
2.2 Animals
Twenty-four male Sprague-Dawley rats (190–220 g) were obtained from Animal house from Pharmacology Department, University of Agriculture, Faisalabad. Rats were accommodated in an animal care facility, at 25 ± 1 °C temperature with 12 h. light/dark cycles. Moreover, all of the rats had open access to tap water ad libitum and standard food throughout the experimental period. Prior to the treatment, the rats were acclimatized to the laboratory environment for one week. The experimental design was approved by the Department Ethical Committee, University of Agriculture, Faisalabad.
2.3 Experimental design
To examine the protective effects of CAS on CP induced oxidative stress and inflammatory reactions in the liver, four groups, each of six male rats were kept in separate cages. The rats of group I (control group) orally received normal saline during the whole experiment. Group II was injected intraperitoneally with CP at the dose of (10 mg/kg) once at the start of the experiment. Group III was provided by single injection of CP (10 mg/kg) intraperitoneally at the start of the experiment, followed by regular doses of CAS (50 mg/kg) orally for seven repeated days. Group IV received a dose of CAS (50 mg/kg) orally once daily until the end of the experiment. Each rat was given anesthesia and slaughtered by decapitation.
2.4 Biochemical analysis
CAT and POD activity was assessed in accordance with the standard method illustrated by Chance and Maehly (1955). SOD activity of the hepatic tissue homogenate was evaluated according to the procedure of Kakkar et al. (1984). The activity of GSR was assessed by the method of Carlberg and Mannervik (1975). Assessment of glutathione (GSH) content in liver tissue homogenate was executed with spectrophotometric protocol described by Jollow et al. (1974). TBARS level was measured according to the procedure demonstrated by Iqbal et al. (1996). The Hydrogen peroxide (H2O2) concentration was measured in accordance with the procedure of Pick and Keisari (1981). According to the protocol of Lowry et al. (1951) the assessment of total liver protein was accomplished.
2.5 Assay of ALT, AST, ALP levels
Serum levels of ALT, ALP and AST were measured in accordance with the procedure provided by the commercial kits, purchased from Wiesbaden (Germany).
2.6 Histopathological examination
Histopathological studies were executed for the evaluation of CP induced Liver impairments, according to the procedure of Fukuzawa et al. (1996). Firstly, hepatic tissue samples were rinsed gently in 0.9% chilled saline, kept in 10% formalin solution for 24hrs, dehydrated in alcohol then fixed in paraffin wax. Thin segments of paraffin embedded tissues (5-µm thickness) were sectioned using microtome then stained with H & E stains and finally observed under a light microscope (Nikon Labophot, Japan) at 40X.
2.7 Estimation of DNA damage
The protective effect of CAS on CP generated DNA damage was assessed by means of modified neutral SCGE /comet assay according to the protocol described by Dhawan et al. (2009). First of all, hepatic tissues were rinsed with phosphate buffer saline (PBS) then dried instantly at 37 °C. Previously sterilized slides were coated with 100 ml of 1% regular melting point agarose, covered with a coverslip and placed at 4 °C for solidification. A small portion of hepatic tissue was kept in 1 ml cold lysis buffer and homogenized with 85 μl of low melting point agarose. This suspension was coated over the previously coated slides, covered with a coverslip and placed in a freezer up to solidify. Subsequent to the third coating of agarose, slides were again placed in lysis buffer for almost 10 min then shifted to freezer for 2 h. The slides were then positioned in the electrophoresis columns holding neutral buffer. After execution of electrophoresis at 25 V for 20 min, dried the slides at 5 °C, rehydrated and stained using 1% ethidium bromide then studied under an epifluorescent microscope (400X, Nikon AFX-1 Optiphot). Comet readings were accomplished with the help of TRITEK software. 50–100 cells were observed from each slide for determining the number of comets, comet length, % DNA head, tail length, head length, % DNA tail, olive-tail moment and tail moment were accounted in this research.
2.8 Statistical analyses
The data was expressed as means ± SEM. “One-way ANOVA” was applied to values and “Tukey’s test” was used for comparative estimation between treatments. Entire data were estimated by Graph Pad prism software. “The significance level was adjusted at p < 0.05”.
3 Results
3.1 Protective role of CAS on serum enzymes
ALT, AST and ALP are indicators of liver dysfunction. A significant (p < 0.05) elevation was observed in levels of ALT, AST and ALP in CP group in comparison to control group. While, comparison between treated groups revealed that the increase in levels of serum enzymes due to CP was signed (p < 0.05) minimized with CAS therapy (Table 1). Means that do not share a letter are significantly different.
Groups
Control
CP
CP + CAS
CAS
ALT (U/I)
43.67 ± 5.70c
430.67 ± 12.34a
84.67 ± 8.38b
65.67 ± 6.36bc
AST (U/I)
50.67 ± 2.33c
311.92 ± 6.50a
112.00 ± 6.08b
97.00 ± 5.29b
ALP (U/I)
62.67 ± 3.84 a
185.00 ± 7.21b
104.33 ± 2.90 a
91.33 ± 2.96 a
3.2 Protective potential of CAS on oxidative stress markers
The antioxidant aptitude of CAS on CP stimulated diminution in the activity of antioxidants such as, CAT, SOD, POD, GSH and GSR are given in (Table 2). The CP treatment showed significant (p < 0.05) reduction in activity of oxidation inhibiting enzymes as compared with control, while CAS administration resulted in a remarkable increase in the activities of these antioxidant enzymes in comparison to the CP treated animals. Conversely, as illustrated in Table 4 the concentration of H2O2 was signed (p < 0.05) enhanced in hepatic tissues of CP administered rats in comparison to the normal rates. Although, co-treatment with CAS led to significant (p < 0.05) decline in H2O2 concentration in rat liver in comparison to CP intoxicated animals. Means that do not share a letter are significantly different.
Groups
Control
CP
CP + CAS
CAS
CAT (U/mg protein)
8.12 ± 0.23a
3.89 ± 0.35b
7.58 ± 0.19 a
7.98 ± 0.12 a
POD (U/mg protein)
4.28 ± 0.20 a
5.52 ± 0.11b
4.05 ± 0.07 a
4.43 ± 0.14 a
SOD (nanomole)
5.35 ± 0.29 a
3.32 ± 0.10b
5.48 ± 0.12 a
5.59 ± 0.17 a
GSR (Nm NADPH oxidized/min/mg tissues)
3.35 ± 0.27 a
2.24 ± 0.13b
3.03 ± 0.07 a
3.13 ± 0.06 a
GSH (nM/min/mg protein)
15.24 ± 0.25 a
7.97 ± 0.28c
15.46 ± 0.23b
13.85 ± 0.20 a
3.3 Protective potential of CAS on lipid peroxidation
CP treatment markedly magnified the LPO as expressed by increased level of TBARS, demonstrated in (Table 3). The level of TBARS, the end product of LPO, was escalated significantly (p < 0.05) in the CP group in comparison to control group. Although, co-treatment with CAS significantly (p < 0.05) declined the level of TBARS in liver tissue homogenate compared to CP treated group. Means that do not share a letter are significantly different. Means that do not share a letter are significantly different.
Groups
Control
CP
CP + CAS
CAS
TBARS (nM/min/mg protein)
17.40 ± 0.30 b
25.64 ± 0.71a
16.47 ± 0.53bc
14.4 ± 0.67c
H2O2 (nM/min/mg protein)
1.86 ± 0.07 c
6.42 ± 0.22 a
2.03 ± 0.04 b
2.26 ± 0.13 c
Protein content (µg/mg tissues)
4.04 ± 0.07 a
1.88 ± 0.06 b
4.02 ± 0.07 a
3.85 ± 0.03 a
Groups
Control
CP
CP + CAS
CAS
No. of comets
1.91 ± 0.95 a
1.89 ± 0.94 c
1.63 ± 0.81 b
1.29 ± 0.64 a
Comet length
48.00 ± 1.08 b
90.50 ± 2.25 a
52.75 ± 1.54 b
54.25 ± 0.75 b
Tail length
7.83 ± 0.32 b
25.49 ± 1.68 a
9.89 ± 0.23 b
10.12 ± 0.10 b
Head length
31.75 ± 0.85 a
21.02 ± 1.06 b
31.18 ± 0.79 a
32.75 ± 0.94 a
% in tail
3.35 ± 0.19 b
26.47 ± 1.60 a
4.22 ± 0.33 b
2.21 ± 0.09 b
% in head
96.64 ± 0.19 a
73.52 ± 1.59b
95.77 ± 0.33 a
97.78 ± 0.09 a
Olive movement
2.55 ± 0.004 b
2.92 ± 0.213 a
2.46 ± 0.013 c
2.53 ± 0.008 b
Tail movement
1.05 ± 0.03b
1.90 ± 0.04 a
1.10 ± 0.04b
1.07 ± 0.04b
3.4 Protective potential of CAS in liver total protein content
The liver total protein contents were assessed to determine the protective effect of CAS on metabolic function of liver in case of CP toxicity, as shown in (Table 3). The results of this study indicated that the liver protein content was significantly (p < 0.05) decreased with the CP administration in comparison to control group. Whereas, co-treatment with CAS led to significant (p < 0.05) elevation in the protein content as compared with CP group.
3.5 Protective potential of CAS on liver histopathology
The defensive role of CAS on CP induced morphological impairments in rat liver is shown in Fig. 1. The animals of control group displayed livers with standard structural patterns, including normal sinusoids and central veins as illustrated in (Fig. 1a). However, CP intoxication resulted in the serious liver impairments such as a remarkable increase in the fat deposits, degenerated structure of the labels and infiltration of inflammatory cells with dilated sinusoids (Fig. 1b). Although, cotreatment with CAS successfully mitigated the prevalence and acuteness of histological injuries, reduced the dilation of sinusoids with no necrotic cells and hold up the normal liver architecture close to control group (Fig. 1c). Our histopathological studies are supporting the biochemical findings related to oxidative damage.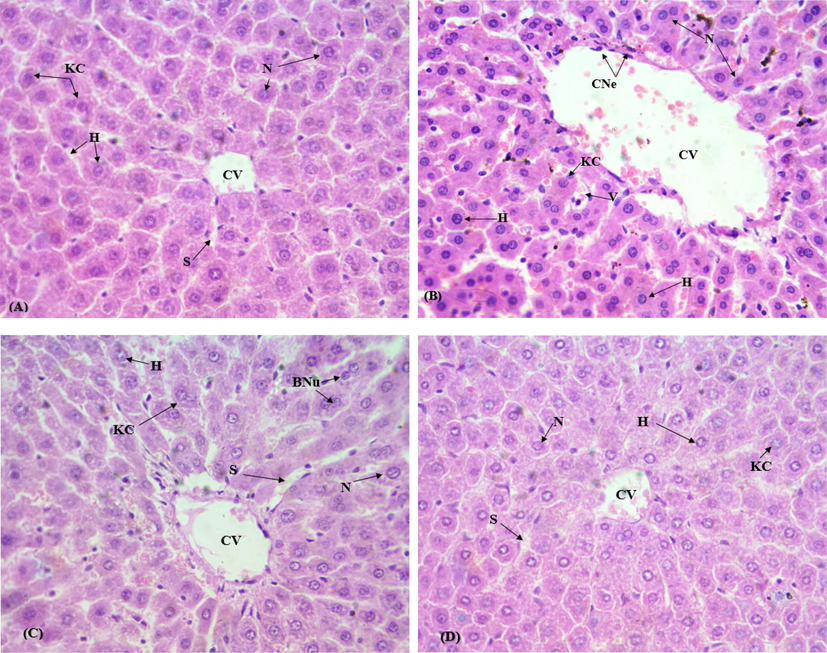
Protective effect of CAS on CIS generated liver histological changes (H & E stain. Mag. 60×). A) Control group B) CIS group (10 mg/kg b.wt.) C) CIS (10 mg/kg b.wt.) + CAS group (50 mg/kg b.wt.) D) CAS group (50 mg/kg b.wt.). CV, Central venule; S, Sinusoids; KC, Kupffer cells; H, Hepatocytes; N, Nucleus; CNe, Centrilobular necro.
3.6 Effects of CAS on comet parameters
DNA damage in the hepatic tissues was determined by comet assay. The therapeutic effects of CAS were studied against CP generated toxicity in hepatocytes of rat. The findings of this research showed that the CP administration brought significant DNA damage in liver cells, as well as, the comet length, olive-tail moment, head length, tail length and % DNA tail was significantly (P < 0.05) increased in comparison to control group. While, a remarkable (P < 0.05) reduction was seen in % DNA head of CP group when compared with the control group. However, CAS treatment attenuated the noxious impacts of CP and recuperated all parameters towards normal.
4 Discussion
The therapeutic usefulness of CP in cancer chemotherapy has been comprehensively assessed. In addition to its useful influences, it has been described noxious to the hepatic tissues (Zhu et al., 2014). CP treatment displayed noticeable liver impairments as revealed by histopathological and biochemical modifications with the escalation of liver function enzymes, reduction in antioxidant profile, hepatic oxidative damage and inflammatory reaction (Khan et al., 2012). Hence, boundless efforts have been made to bring a light on hepatoprotective agents from natural products due to their countless medicinal effects and little deleterious influences (Singh et al., 2016). Therefore, this study was undertaken to explore the hepatoprotective effects of CAS, a natural flavonoid, against CP persuaded hepatotoxicity. Our findings indicated that cotreatment with CAS successfully protected the hepatic tissues against the damaging effects of CP and abridged the oxidative stress in tissues. CAS has been reported as a strong antioxidant having antitumor, anti-inflammatory and cytoprotective effects (Chan et al., 2018).
In this study, a remarkable elevation was noticed in levels of serum ALT, ALP and AST followed by CP therapy. Owing to the apoptosis of hepatocytes the liver mitochondria starts liberating these serum enzymes into the blood stream, leading to the liver dysfunction (Nagai et al., 2016). The noxious upshots of CP might be related to its toxic metabolites generated after its execution. Previous studies have proposed that excessive formation of ROS impaired the hepatic structural integrity, as revealed by the abnormal rise in liver serum enzymes (Pratibha et al., 2006). According to our findings, cotreatment with CAS attenuated and upgraded the CP induced liver toxicity by lowering the levels of Hepatic serum markers, ALT, AST and ALP.
The antioxidant defense system is important in defending the cellular components to confront oxidative lesions (Zeeshan et al., 2009). The findings of this research revealed that the activities of CAT, POD and SOD were markedly decreased in hepatocytes of CP intoxicated rats due to persistent and immense production of free radicals such as superoxide and hydroxyl (Bhattacharyya and Mehta, 2012). Superoxide dismutase transforms oxygen free radicals into H2O2 that is subsequently converted by CAT and GPx into H2O and O2 hence, extirpating the lethal impressions of OH radicals to the major body organs (Behndig et al., 1998).
We observed that CP treatment considerably enhanced the TBARS level while decreased the GSH and protein content in hepatocytes. Liver irregularities were affirmed by the estimation of lipid peroxides in tissue homogenates. The excessive production of ROS and oxidative stress has been generally assumed as the most prevalent cause of CP stimulated liver injury. The increased level of TBARS is the result of increased lipid peroxidation, which in turn associated with the reduced content of GSH (Zhao et al., 2014). In this study, co-administration with CAS showed upgrading influences against CP generated liver ailments by improving the TBARS level and total protein content in rats. CP treatment resulted in the upsurge of H2O2 concentration in hepatic tissues. Severe hepatic impairments were observed during this study which indicated that OH radicals produced during the conversion route of H2O2 can remove hydrogen from polyunsaturated fatty acids in the cellular membranes. Previous reports validated that CP produced liver toxicity by elevating the H2O2 concentration which increased the ROS and LPO while decreased the antioxidant index in hepatic tissues (Ahmad et al., 2012). The decline seen in antioxidant enzyme activities and increased production of ROS consequently increased the accumulation of CP toxic metabolites, which ultimately boost up the liver ailments. However, cotreatment with CAS ameliorates the oxidative impairment by wiping out the free oxygen radicals and restoring the activity of protective antioxidant enzymes.
In this study, the level of DNA damage was estimated in hepatocytes of CP inebriated rats via comet assay, which has turned out to be a standard protocol to evaluate DNA disruption. Many cytotoxic agents exert genotoxic effects on DNA via production of ROS, which induce breaks in its helical structure and may also distress the DNA integrity (Baş et al., 2016). In this study, significant variations were noticed in parameters of comet such as, comet length, number of comets, tail length, % DNA head, head length, % DNA tail and olive-tail moment. Our results indicated that CP treatment caused significant reduction in % DNA head in comparison to the untreated group while significantly increased the tail length, tail moment and comet length. In accordance with the aforementioned studies, CP accounted DNA damage in the hepatic tissues, which lead to the DNA movement from head to tail of comet, resulting in the % increase in tail DNA and tail length (Orsolic and Car, 2014), hence tail length is considered as imperative indicator of DNA damage. Our findings presented that CAS is an effective candidate to prevent CP induced DNA disruption by reducing the oxidative stress in the hepatic tissues of the rat, which are in line with the increase in antioxidant enzyme activities followed by CAS treatment.
Our histopathological results showed that CP-based liver dysfunction is a dose-limiting factor mainly verified by the hepatic serum markers e.g. ALT, ALP and AST. CP intoxication promotes the LPO in hepatic tissues which results in the morphological impairments. The obvious hepatic injuries such as sinusoids dilation, formation of lobules, central vein disruption, coagulation and blockage, swelling of connective and supporting tissues, biliary duct propagation and necrosis were documented in the CP treated group (Ez-Din et al., 2011). These severe abnormalities were attenuated by co-treatment with CAS. These defensive impacts exhibited by CAS may be associated with its potential antioxidant property which is indicated by inhibition of LPO in liver.
5 Conclusion
In conclusion, the findings of our study showed that CAS exhibited outstanding protective efficiency against oxidative stress, which is a key aspect of CP generated liver toxicity. CAS treatment efficiently recovered the levels of serum markers, endogenous antioxidant defense mechanism, DNA damage and histological anomalies. This hepatoprotective potential of CAS is associated with its antioxidant and anti-inflammatory properties.
Acknowledgements
“Authors Acknowledge University of Agriculture, Faisalabad for providing financial support to accomplish this research work. The authors (SM and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. RG-1435-012”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Restraint stress-induced central monoaminergic and oxidative changes in rats and their prevention by novel Ocimum sanctum compounds. Indian J. Med. Res.. 2012;135:548-554.
- [Google Scholar]
- Thymoquinone attenuates Cisplatin-induced hepatotoxicity via nuclear factor kappa- β. Complementary Alternative Med.. 2014;14:282.
- [Google Scholar]
- Furan-induced hepatotoxic and hematologic changes in diabetic rats: the protective role of lycopene. Arch. Ind. Hygiene Toxicol.. 2016;67:194-203.
- [Google Scholar]
- Superoxide dismutase isoenzymes in the human eye. Invest. Ophthalmol. Vis. Sci.. 1998;39:471-475.
- [Google Scholar]
- The hepatoprotective potential of Spirulina and vitamin C supplementation in cisplatin toxicity. Food Funct.. 2012;3:164-169.
- [Google Scholar]
- Protective effects of apocynin on cisplatin-induced hepatotoxicity in rats. Arch. Med. Res.. 2015;46:517-526.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:4480-4575.
- [Google Scholar]
- Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur. Arch. Oto-Rhino-Laryngol. 2012;269:2455-2460.
- [Google Scholar]
- Casticin from Vitex species: a short review on its anticancer and anti-inflammatory properties. J. Integrative Med.. 2018;16:147-152.
- [Google Scholar]
- Comet assay: a reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol.. 2009;25:5-32.
- [Google Scholar]
- Physiological and histological impact of Azadirachta indica (neem) leaves extract in a rat model of Cisplatin-induced hepato and nephrotoxicity. J. Med. Plants Res.. 2011;5:5499-5506.
- [Google Scholar]
- Evaluation of glomerular lesion and abnormal urinary findings in OLETF rats resulting from a long-term diabetic state. J. Lab. Clin. Med.. 1996;128:568-578.
- [Google Scholar]
- Hepatoprotective effects of propofol in cisplatin induced rat liver oxidative damage. Pharmacologia. 2016;7:229-233.
- [Google Scholar]
- Pre-treatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam. Clin. Pharmacol.. 2017;31:674-685.
- [Google Scholar]
- Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep.. 1996;2:385-391.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4- bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Chrysin abrogates Cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wister rats. Br. J. Nutr.. 2012;108:1574-1585.
- [Google Scholar]
- G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.) Cancer Lett.. 2004;208:59-64.
- [Google Scholar]
- Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals — A review. Asian-Australas J. Anim. Sci.. 2017;30(3):299-308.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Insights into RNA binding by the anticancer drug cisplatin from the crystal structure of cisplatin-modified ribosome. Nucl. Acids Res.. 2016;44:4978-4987.
- [Google Scholar]
- Cisplatin (CDDP)-induced acute toxicity in an experimental model of hepatic fibrosis. J. Toxicol. Sci.. 2007;32:311-319.
- [Google Scholar]
- Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs. 2016;279:17-23.
- [Google Scholar]
- Hyperin protects against Cisplatin-induced liver injury in mice. Acta Cir. Bras.. 2017;32:633-640.
- [Google Scholar]
- Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumor Biol.. 2014;35:6445-6454.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. Cell Immunol.. 1981;59:301-318.
- [Google Scholar]
- Enzymatic studies of Cisplatin induced oxidative stress in hepatic tissue of rats. Eur. J. Pharmacol.. 2006;532:290-293.
- [Google Scholar]
- Hepatoprotective effect of trans-Chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Canadian J. Phys. Pharmacol.. 2016;94(8):879-887.
- [CrossRef] [Google Scholar]
- Portulaca oleracea L. extract ameliorates the Cisplatin induced toxicity in chick embryonic liver. Indian J. Biochem. Biophys.. 2010;47:185-189.
- [Google Scholar]
- Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev.. 2013;114:4470-4495.
- [Google Scholar]
- Hepatoprotective and antioxidant activity of Amaranthus spinosus against CCl4 induced toxicity. J. Ethnopharmacol.. 2009;125:364-366.
- [Google Scholar]
- Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. Food Funct.. 2014;5:605-611.
- [Google Scholar]
- Propofol protects human umbilical vein endothelial cells from Cisplatin-induced injury. Vasc. Pharmacol.. 2014;61:72-79.
- [Google Scholar]







