Translate this page into:
Regulation and molecular mechanism of histone acetylation modification in sevoflurane-induced POCD in mice
⁎Corresponding author at: Department of Anesthesiology, The First Affiliated Hospital of Bengbu Medical College, 287 Changhuai Road, Bengbu City 233004, Anhui Province, China. benglulingyunzhi@126.com (Yunzhi Ling)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
The objective is to explore the effect of sevoflurane anesthesia on histone acetylation and the mechanism of learning and memory ability in mice, so as to provide reference for the development of drugs with cognitive protection and brain injury reduction. Method: In this study, 40 healthy male BALB/c mice are selected as the study subjects and randomly divided into four groups: control group (group A), sevoflurane group (group B), sevoflurane + SAHA group (group C) and SAHA group (group D). A postoperative cognitive dysfunction (POCD) model induced by sevoflurane is established. After extracting the hippocampus, Morris water maze test is used to detect the spatial learning and memory ability of mice, and conditioned fear test is used to detect conditioned fear memory ability. The expression of brain derived neural factor (BDNF) and synaptotagmin-1 (SYT-I) mRNA in hippocampus is detected by real-time fluorescence quantitative PCR. Western blotting is used to detect the expression of Acetylated histone-3 (Ac-H3), BDNF and SYT-I in mouse hippocampus. Results: After sevoflurane anesthesia, the escape latency of mice in Morris water maze experiment is significantly prolonged, and the target quadrant time ratio is significantly reduced. Compared with group A, the time ratio and times of stiffness in group B are significantly lower (P < 0.05). There is no significant difference between group C and group A (P > 0.05). The expression level of Ac-H3, BDNF and SYT-I protein in group B is significantly lower than that in group A (P < 0.05). Compared with group B, the expression level of Ac-H3, BDNF and SYT-I protein in group D is significantly higher, and the difference is statistically significant (P < 0.05), while the effect of sevoflurane anesthesia might be weakened by using the histone deacetylase inhibitor SAHA. Conclusion: Sevoflurane anesthesia can inhibit the expression of BDNF and SYT-I by inhibiting the acetylation of histone-3 in the hippocampus of mice, thus leading to POCD.
Keywords
Histone deacetylase inhibitor
Sevoflurane
POCD
BDNF
Syt-I
1 Introduction
In recent years, with the wide application of general anesthetics, the influence of general anesthetics on patients' learning and memory has attracted more and more attention, which has become the focus of clinical anesthesiologists (Lu et al., 2017). General anesthetics can reversibly inhibit the function of central nervous system, cause the loss of sensation and consciousness, the weakening of visceral reflex activity, muscle relaxation and other reactions. Sevoflurane has been widely used in the induction and maintenance of anesthesia because of its advantages of less respiratory tract stimulation and lower airway spasm caused by tracheal intubation. Clinical research shows that sevoflurane may damage the learning and memory function of patients, cause neurobehavioral dysfunction and neurodegenerative diseases, and lead to postoperative cognitive dysfunction (POCD) (Sabourdin et al., 2019). POCD is a common complication of central nervous system after general anesthesia. Generally speaking, the clinical manifestations are cognitive impairment, including impairment of language expression, memory, and social adaptability, which has a serious impact on the life of patients. The pathogenesis of POCD is complex, and most of the existing studies focus on the physiological status of patients and the type of surgery and other factors (Chen et al., 2018).
It has been reported that sevoflurane can inhibit the formation and maintenance of emotional memory (Liu et al., 2017). The formation and maintenance of long-term memory depend on stable gene expression. However, changes in gene function can be inherited on the premise that DNA sequence remains unchanged. Such changes usually depend on histone modification, noncoding RNA regulation and DNA methylation to affect gene activation and silencing. Histone is a kind of basic protein which combines with DNA in the nucleus of eukaryote, including 5 subtypes of H1, H2A, H2B, H3 and H4. Studies have shown that the acetylation of H3 and H4 is closely related to learning and memory (Zhang et al., 2017). Histone acetylation modification refers to the acetyl group modification of ε lysine residues in the N-terminal basic amino acid concentration region of histone under the regulation of histone acetyltransferase and histone deacetylase, which reduces the positive charge of histone and weakens its ability to combine with negatively charged DNA, resulting in loose chromatin structure and promoting its integration with transcription genes and other transcription elements. Eventually, it results in the change of gene transcription (Alam et al., 2017). In recent years, studies have shown that SAHA can penetrate the blood-brain barrier by detecting the acetylation degree of histone and the content of SAHA (N-hydroxy-N′-phenyloctanediamide). It has been reported that SAHA injection can improve the expression level of cognitive related genes in neurodegenerative diseases such as Alzheimer's disease, promote acetylation modification of histone, repair neuronal damage and improve cognitive function (Ocasio et al., 2017). Other studies have shown that SAHA can improve the learning and memory dysfunction induced by sevoflurane in newborn mice (Borisova et al., 2018; Tang et al., 2017; Tao et al., 2017). It is suggested that sevoflurane may affect postoperative cognitive function by regulating histone acetylation.
To sum up, there is no consensus on the regulatory effect and molecular mechanism of sevoflurane on POCD, and the intervention mode of sevoflurane induced POCD is not clear. Therefore, the relevant research is particularly urgent. In this study, healthy male BALB / c mice are selected as the research object to explore the regulatory effect and molecular mechanism of histone acetylation modification in mice POCD induced by sevoflurane, so as to provide reference for the development of drugs with cognitive protection and brain injury reduction.
2 Materials and methods
2.1 2.1Laboratory animals and groups
40 healthy male BALB/c mice selected for the study (8–10 weeks old, and the weight is about 20–25 g). All animals are raised in separate cages with national standard rodent feed, 4 animals per cage. There is no significant difference in body weight between groups. The feeding environment is natural light, room temperature is controlled at 20–26 °C, humidity is controlled at 40–50%, and adaptive feeding is carried out for 2 weeks. The animal handling and experimental procedures conform to the national standards for experimental animals and are approved by the ethics committee.
All mice are randomly divided into 4 groups, 10 mice in each group, namely control group (group A), sevoflurane group (group B), sevoflurane + SAHA group (group C) and SAHA group (group D).
2.2 Establishment of sevoflurane-induced POCD model in mice
The mice are placed in a homemade plexiglass anesthesia box. One side of the anesthesia box is connected to the anesthesia machine (Drager, Germany), and then 95% oxygen with a flow rate of 1L/min needs to be continuously injected into the anesthesia box. The other side of the anesthesia box is connected with the Datex Ohmeda S/5 multifunctional monitor (General Electric Company, USA), which is used to determine the concentration of sevoflurane, oxygen and carbon dioxide in the anesthesia box. Mice in group A are exposed to 95% oxygen for 6 h. Mice in group B inhaled 3.0% sevoflurane for 6 h. In group C, SAHA (Beijing Fubo Biotechnology Co., Ltd., China) is injected intraperitoneally at a dose of 25 mg/kg, and 3.0% sevoflurane is inhaled continuously for 6 h after 1 h. Mice in group D are intraperitoneally injected with SAHA at a dose of 25 mg/kg.
2.3 Morris water maze test for spatial learning and memory in mice
The pool in the Morris Water Labyrinth (Shanghai Xinsoft Information Technology Co., Ltd., China) is divided into four quadrants on average, and different positioning markers are set up. The platform is fixed 1 cm below the water surface at the center of the third quadrant. Firstly, the mice are put into the quadrant midpoint of random selection after 4 days of positioning voyage training. The mice are trained to find a platform under the water. Four times a day, each training time is 60 s, training interval of 5 min. When the mice find the platform within 60 s and stayed for more than 10 s, they are recorded as having successfully found the platform. The image acquisition and analysis system (Shanghai Xinsoft Information Technology Co., Ltd., China) is used to collect the swimming trajectory of mice, and record the swimming speed of mice and the time to find the platform (escape latency). If the mice are not found within 60 s, they are helped to find the platform, and the escape latency was recorded as 60 s. At this time, the mice were gently guided to the platform, and the mice are guaranteed to stay on the platform for more than 10 s. After the training of positioning navigation is completed, space exploration test is needed. After removing the platform, the mice are placed in the Morris water maze and recorded the ratio of time spent in the target quadrant to total time spent.
2.4 Detection of conditioned fear memory in mice by conditioned fear test
The conditioned fear experiment is divided into two days. The first day is conditional fear memory training. The mice are placed in a reaction box with copper bars at the bottom. They are able to move freely for 2 min. Mice are given a single frequency (80 dB, 30 s) of sound stimulation, followed by electrical stimulation (0.35 mA, 2 s). The two stimuli form a cycle, after 10 consecutive cycles, conditional fear training is completed. The stiffness time ratio and stiffness times are recorded during the whole process. If the mice show strong squealing and jumping behavior during electrical stimulation, stiffness and other reactions occur during acoustic stimulation, it indicates that the model is successful. The second day is the conditional fear memory test. Sound stimulation is only given to mice, but no electrical stimulation is given. The interval of two sound stimulations is 2 s and 10 cycles are successively performed. The ratio of stiffness time and the number of stiffness times are recorded.
2.5 Extraction of hippocampal tissue from mice
The mice are killed by cervical spondylolisthesis and their heads are cut off. Brain tissue is exposed and separated and rinsed three times in ice PBS (phosphate buffer saline) (Tianjin Guangcheng Chemical Reagent Co., Ltd., China). After the hippocampus tissue is isolated and removed from the ice, it is frozen at − 80 °C for storage.
2.6 Extraction of total RNA from mouse hippocampus
The frozen hippocampus is removed. 1 ml of β-mercaptoethanol lysate (Shanghai Jingke Chemical Technology Co., Ltd.) is added to every 50 mg of hippocampal tissue and it is ground on ice. 10 μL protein kinase K (Shanghai Youyu Biotechnology Co., Ltd., China) and 590 μL RNase free ddH2O (Beijing baileibo Technology Co., Ltd., China) are added, mixed evenly, and reacted in a 56 °C water bath. After 20 min, centrifugation (12000 rpm) is required for 10 min and supernatant is taken. Anhydrous ethanol (supernatant: anhydrous ethanol = 2:1) (Tianjin Guangcheng Chemical Reagent Co., Ltd., China) is added and mixed evenly. Then, it needs to be moved into the adsorption column of the collection pipe and centrifuged (12,000 rpm) for 1 min. Then, the waste liquid in the collection pipe needs to be discarded. 350 μL deproteinized tissue solution RW1 is added to the adsorption column CR3, rinsed and centrifuged (12,000 rpm) for 1 min. 10 μL DNase I storage solution (Shanghai Kemin Biotechnology Co., Ltd., China) is added into 70 μL RDD solution (Shanghai Beinuo Biotechnology Co., Ltd., China), and mixed evenly to prepare DNase I solution. 80 μL DNase I solution is added to the adsorption column, and placed at room temperature for 15 min, centrifuged (12,000 rpm) for 1 min. 350 μL deproteinized tissue solution RW1 is added to the adsorption column and rinsed. After centrifugation (12,000 rpm) for 1 min, the waste liquid needs to be discarded. After that, repeated centrifugation and the discard of the waste liquid is needed. Then, the RNA in the column needs to be dried at room temperature. After 2 min, it is necessary to take out the new RNase test tube and put the adsorption column into it. Then, 50 μL RNase free double distilled water is added, blown repeatedly and mixed evenly. It is put at room temperature for 2 min to make RNA fully dissolved. After centrifugation (12,000 rpm) for 2 min, RNA solution is obtained.
2.7 Detection of BDNF and Syt-I mRNA expression in hippocampus of mice by real-time fluorescence quantitative PCR
The primers used in the experiment are all obtained by retrieving GenBank gene sequence and designing. After pre experimental screening, they are finally obtained by Shanghai biotechnology. The sequence is as follows:
BDNF: Forward 5′-AGCCTCCTCTGCTCTTTCTGCTGGA-3;
Reverse 5′-CTTTTGTCTATGCCCCTGCAGCCTT-3;
Syt-Ⅰ: Forward 5-TGGCATCAACCTCCTCCTCTAC-3;
Reverse 5′-TGGCATCAACCTCCTCCTCTAC-3;
GAPDH: Forward 5′-AGGCCGGTGCTGAGTATGTC-3;
Reverse 5′-TGCCTGCTTCACCACCTTCT-3′
The total RNA extracted is reverse transcripted to produce a cDNA. Operation is carried out according to the instruction system of reverse transcription kit (Takara Company, Japan). The reaction system is as follows: 1 μg Total RNA, 4 μL L 5 * PrimeScript RT Master Mix, RNase Free ddH2O supplement total volume to 20 μL. It is incubated at 42 °C for 1 h, 99 °C for 5 min, and then quickly placed at 4 °C for 5 min. It is diluted to 0.1ug/ml and then frozen at −20 °C for reserve. Real-time fluorescent quantitative PCR kit (Takara Company, Japan) is used for polymerase chain reaction (PCR), and the amplification is completed. The reaction system is as follows: 0.4 μL PCR Forward Primer, 0.4 μL PCR Reverse Primer, 10 μL SYBR, 2 μL DNA solution, 7.2 μL RNase Free dH2O. Pre-denaturation is carried out for 60 s at 95 °C, denaturation for 5 s at 95 °C, annealing for 30 s at 60 °C, and elongation for 0.09 s at 0.09 °C. After 40 cycles of repeated reaction, the experimental results are calculated by 2−ΔΔCT method.
2.8 Detection of Ac-H3, BDNF and Syt-I protein expression in hippocampus of mice by Western blot
Frozen hippocampal tissue is removed and cut into small pieces. First of all, it is necessary to add some radio immunoprecision assay (RIPA) lysate (Shanghai Yuanye Biotechnology Co., Ltd.). According to the proportion of 200 μL lysate added to every 20 mg of hippocampus, it is fully ground on ice. After centrifugation (1400g) for 5 min, the supernatant needs to be taken. The bicinchoninic acid (BCA) protein concentration determination kit (Thermo Fisher Scientific, USA) is used to prepare the color development solution with the ratio of A solution and B solution of 50:1. The absorbance of the sample is measured by ultraviolet spectrophotometry, and the protein concentration is calculated by standard curve method. An appropriate amount of sample is added to the 5 × SDS sample buffer. After mixing, it is reacted at 100 °C for 5 min.
5 μL marker (Shanghai Bogu Biotechnology Co., Ltd., China) and protein sample to be tested (20 μL/well) are added into the sample adding hole. After the power supply is connected, the gel concentrate needs constant voltage 80 V electrophoresis. After 15 min, it needs to be changed to a constant voltage of 100 V. After 40 min, the electrophoresis needs to be finished. According to the method of wet membrane conversion, PVDF (polyvinylidene fluoride, Millipore, USA) is placed in the membrane conversion device with a constant voltage of 100 V for 2 h. TBST (Tris-Buffered Saline and Tween 20, Shanghai Youyu Biotechnology Co., Ltd., China) solution containing 5% skimmed milk (Inner Mongolia Yili Industrial Group Co., Ltd., China) is used as a sealing solution, and PVDF membrane is put into it. It is sealed in a shaker at room temperature for 2 h. First antibody diluent: Ac-H3 (dilution ratio of 1:2000, Abcam, UK), Rabbit anti BDNF polyclonal antibody (dilution ratio of 1:5000, Abcam, UK), rat anti SYT-I polyclonal antibody (dilution ratio of 1:200, Abcam, UK), Rabbit anti β - actin polyclonal antibody (dilution ratio of 1:5000, Abcam, UK), is added and sealed in the hybrid bag and incubated overnight on the shaking table at 4 °C. After taking out the hybrid bag, it needs to be balanced on the shaking table at room temperature for 30 min and rinsed with TBST for 3 × 15 min. After that, it is necessary to add the second anti diluent and rinse with TBST for 3 × 15 min. High sensitivity ECL (electrochemiluminescence) chemiluminescence Kit (Shanghai Biyuntian Biotechnology Co., Ltd., China) is used to prepare the color solution. After exposure, the gray value of the strip is analyzed by ImageJ software, and the relative expression of the target protein is calculated.
2.9 Statistical methods
Spss20.0 software is used for statistical analysis. The measurement data obeying normal distribution are expressed as mean ± standard deviation (x ± s), and two independent samples t test is used for group comparison. The counting data are expressed by number, and the comparison between groups is performed by x2 test. The difference is statistically significant (P < 0.05).
3 Results and discussion
3.1 3.1Morris water maze experiment results
Morris water maze test results are shown in Fig. 1. It can be seen that compared with group A, the swimming track of group B mice is disordered, not concentrated near the target quadrant, and it takes longer to find the platform. Compared with group B, the situation of group D is improved. It can be seen from figure A that in the first four days of directional navigation training, the escape latency of mice in each group has no significant difference (P > 0.05). It can be seen from figure B that in the spatial memory test on the fifth day, compared with group A, the escape latency of group B is significantly longer, and the time ratio of target quadrant is significantly lower, and the difference is statistically significant (P < 0.05). There is no significant difference in escape latency and target quadrant time ratio between group C and group A (P > 0.05). Compared with group B, the escape latency of group D is shortened, the time ratio of target quadrant is increased, and the spatial memory ability is improved to some extent, but the difference is not statistically significant (P > 0.05).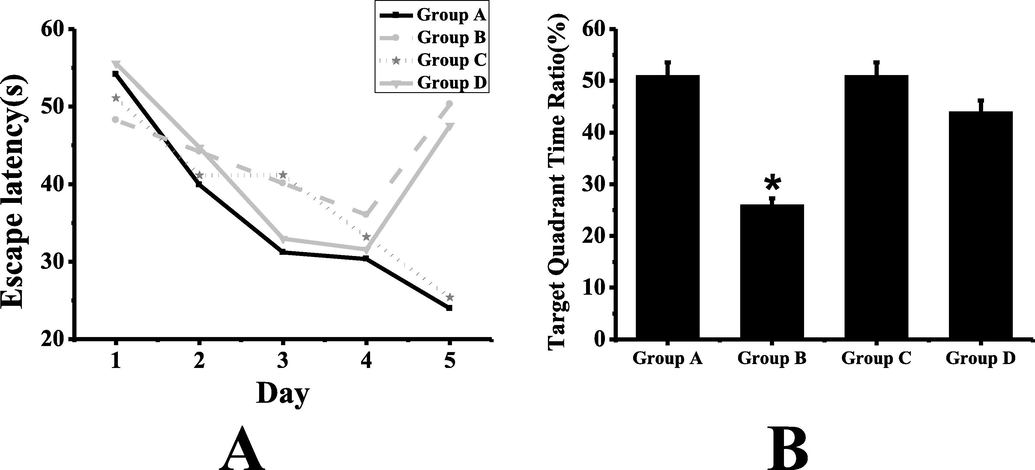
Morris water maze test results (A: comparison of escape latency of mice in each group; B: comparison of target quadrant time ratio of mice in each group; *: compared with group A, P < 0.05).
3.2 Conditional fear experiment results
The results of conditioned fear experiment are shown in Fig. 2. It can be seen from a chart that group C has the longest time of rigidity and group B has the shortest time. As can be seen from the figure B, the number of stiffness in group A and group C is almost the same, and that in group B and group D is almost the same. It can be seen that the time ratio and times of stiffness in group B are significantly lower than those in group A (P < 0.05). There is no significant difference between group C and group A (P > 0.05). Compared with group B, the time ratio and times of rigidity in group D are increased to some extent, and the ability of fear memory is improved to some extent, but the difference is not statistically significant (P > 0.05).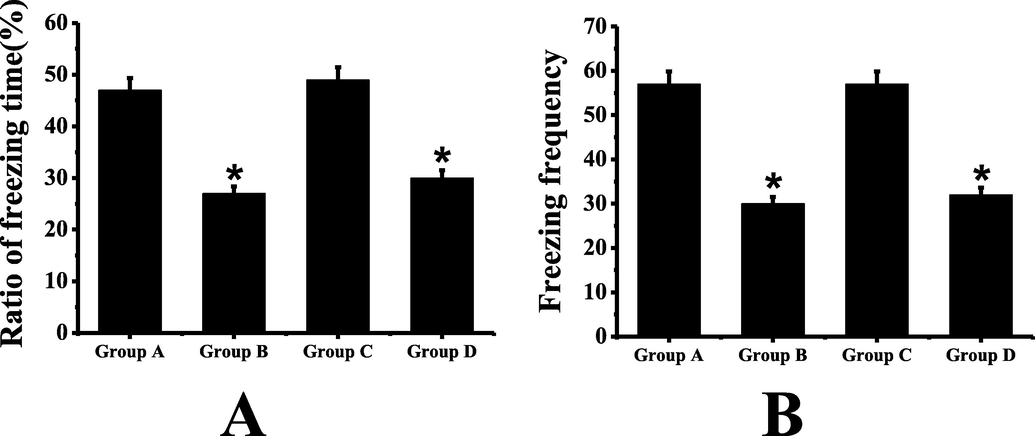
Conditional fear test results (A: comparison of stiffness time of mice in each group; B: comparison of stiffness times of mice in each group; *: compared with group A, P < 0.05).
3.3 Detection results of real-time fluorescence quantitative PCR
The results of real-time fluorescence quantitative PCR are shown in Fig. 3. It can be seen that the expression levels of BDNF and Syt-I in group B mice are significantly lower than those in group A (P < 0.05). There is no significant difference in BDNF and Syt-I expression between group C and group A (P > 0.05). Compared with group B, the expression levels of BDNF and Syt-I in group D mice are significantly higher, and the difference is statistically significant (P < 0.05).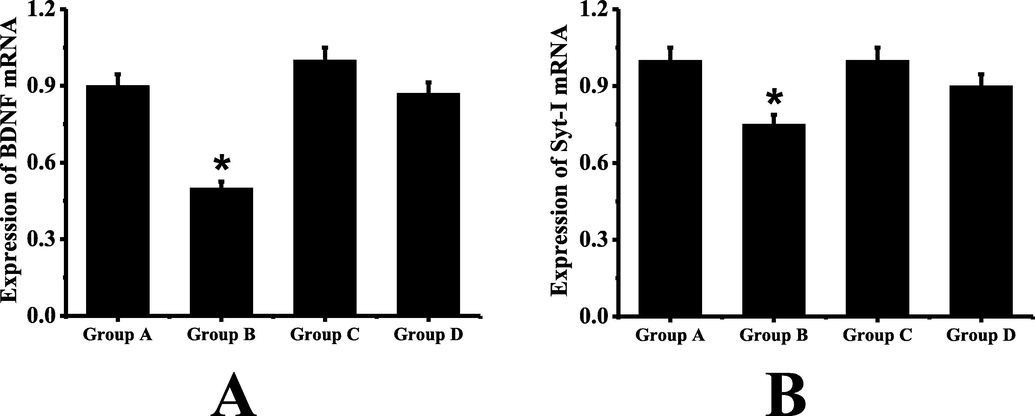
Real-time fluorescence quantitative polymerase chain reaction (A: the expression level of BDNF in mice of each group; B: the expression level of Syt-I in mice of each group; *: Compared with group A, P < 0.05).
3.4 Western blot test results
The results of Western blot are shown in Fig. 4. It can be seen that the expression levels of AC-H3, BDNF and Syt-I in group B mice are significantly lower than those in group A (P < 0.05). Compared with group B, the expression levels of AC-H3, BDNF and Syt-I protein in group D mice are significantly higher, and the difference is statistically significant (P < 0.05). There is no significant difference in the expression levels of A C-H3, BDNF and Syt-I between group C and group D (P > 0.05).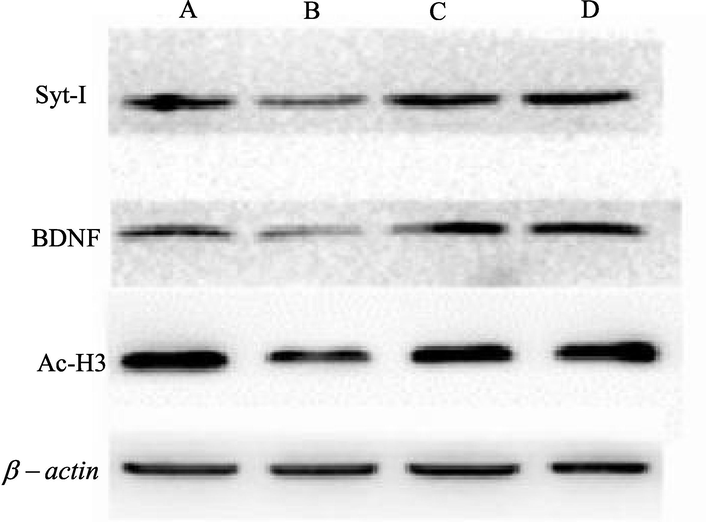
Protein electrophoretogram.
4 Discussion
It is not clear which mechanism sevoflurane may play a role in cognitive impairment. Memory consolidation and retrieval require new protein synthesis, which breaks the balance of protein in the brain, and the phenomenon of long-term potentiation of synapse (LTP) is also related to memory coding, which is manifested in the increase of memory related protein expression (Zhong et al., 2015; Han et al., 2015). Previous studies have shown that histone acetylation plays a key role in synaptic plasticity and memory formation. Among them, the acetylation of H3K14 site can regulate the transcription of BDNF gene and directly participate in the change of synaptic plasticity (Sun et al., 2016). At present, Syt-I is widely considered as the main neurotransmitter released Ca2 + sensor in animal brain, which plays an important role in learning and memory (Fig. 5).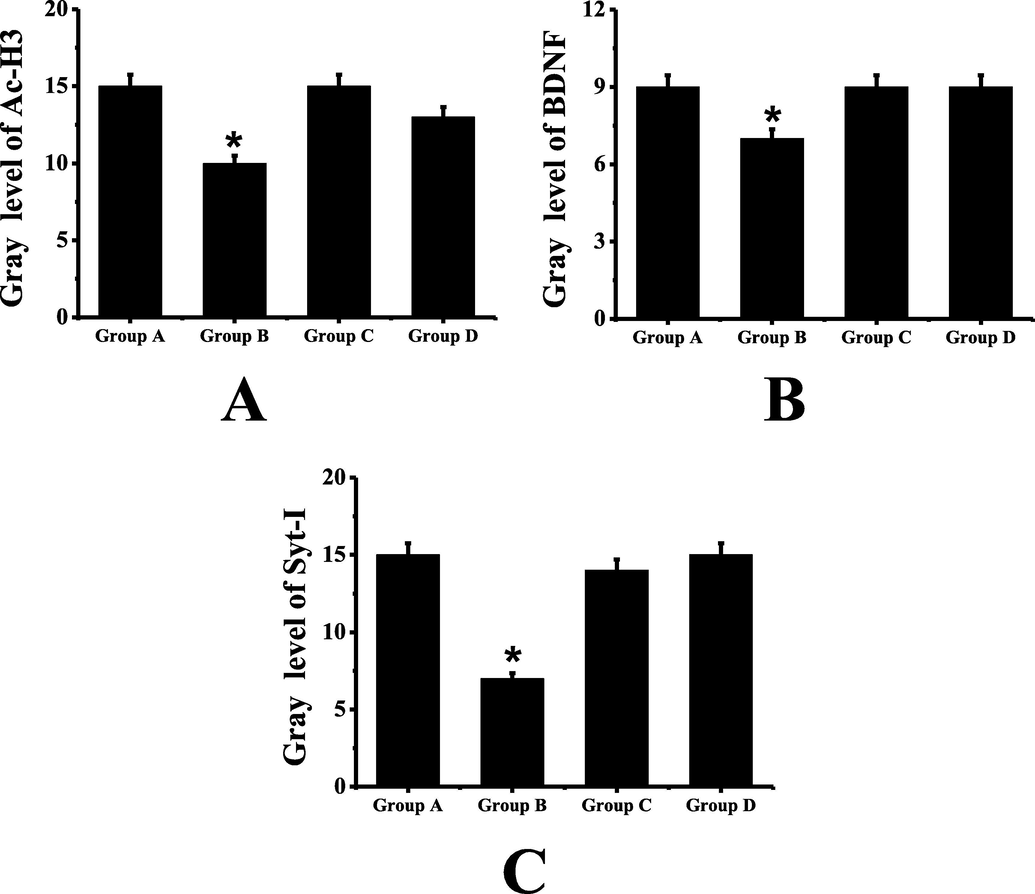
Western blot test results (A: comparison of the expression level of A C-H3 protein in mice of each group; B: comparison of the expression level of BDNF protein in mice of each group; C: comparison of the expression level of Syt-I protein in mice of each group; *: compared with group A, P < 0.05).
The results show that SAHA, an inhibitor of histone deacetylase, can attenuate sevoflurane induced POCD. At the same time, sevoflurane anesthesia can inhibit the expression of BDNF and Syt-I by inhibiting the acetylation of histone-3 in the hippocampus of mice, thus leading to POCD. This study reveals the regulatory effect and molecular mechanism of histone acetylation modification in mice POCD induced by sevoflurane. It is found that AC-H3/BDNF/Syt-I can mediate sevoflurane anesthesia to affect learning and memory cognition of mice, inhibit the acetylation modification level of histone-3, significantly reduce the mRNA expression level of BDNF and Syt-I, and the protein expression result is consistent with that of mRNA detection. This study is helpful to the rational use of drugs, improve the application effect and reduce the occurrence of adverse reactions. At the same time, solutions are explored to provide reference for the development of drugs with cognitive protection and brain injury reduction. Compared with the previous studies, the innovation of this study is to introduce molecular biological mechanism to explore the effect of sevoflurane anesthesia on learning and memory function of mice and epigenetic mechanism. However, there are some shortcomings in the process of this study, such as the small amount of data collected from the samples leads to a certain degree of deviation of the results. Although Morris water maze and conditioned fear experiment in behavioral science are classic, they have their own limitations, and the design idea is relatively simple. Recently, experts creatively use Morris water maze, pattern separation memory and contextual discrimination test to verify the performance of learning and memory, which provides a new idea for the design of later experimental scheme.
Acknowledgements
This work was supported by Anhui Province Nature Science Foundation (No. 1808085MH305), National Innovation and Entrepreneurship Training Program for College Students (No. 201810367029) and Natural Science Research Projects of Colleges and Universities in Anhui Province (No. KJ2015B004by).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem.. 2017;292(18):7519-7530.
- [Google Scholar]
- Screening of the Structure of Americium Extractants Based on a 2,2’-Bipyridyl Scaffold: a Simple Way to a N2, O2-Tetradentate Ligands Library for Rational Design of An/Ln Extractants. ChemistrySelect. 2018;3(7):1983-1989.
- [Google Scholar]
- An investigation of the mechanism of dexmedetomidine in improving postoperative cognitive dysfunction from the perspectives of alleviating neuronal mitochondrial membrane oxidative stress and electrophysiological dysfunction. Exp. Therap. Med.. 2018;15(2):2037-2043.
- [Google Scholar]
- Single sevoflurane exposure increases methyl-CpG island binding protein 2 phosphorylation in the hippocampus of developing mice. Mol. Med. Rep.. 2015;11(1):226-230.
- [Google Scholar]
- Sevoflurane pretreatment inhibits the myocardial apoptosis caused by hypoxia reoxygenation through AMPK pathway: An experimental study. Asian Pac. J. Trop. Med.. 2017;10(2):148.
- [Google Scholar]
- Neuroprotective effect of miR-665 against sevoflurane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway by targeting insulin-like growth factor 2. Am. J. Trans. Res.. 2017;9(3):1344.
- [Google Scholar]
- Pojamide: an HDAC3-selective ferrocene analogue with remarkably enhanced redox-triggeredferrocenium activity in cells. Organometallics. 2017;36(17):3276-3283.
- [Google Scholar]
- Pupillary Pain Index Changes After a Standardized Bolus of Alfentanil Under Sevoflurane Anesthesia: First Evaluation of a New Pupillometric Index to Assess the Level of Analgesia During General Anesthesia. Anesth. Analg.. 2019;128(3):467-474.
- [Google Scholar]
- Inhibiting NADPH oxidase protects against long-term memory impairment induced by neonatal sevoflurane exposure in mice. Br. J. Anaesth.. 2016;117(1):80-86.
- [Google Scholar]
- The protective effect and mechanism of sevoflurane on LPS-induced acute lung injury in mice. Am J Transl Res. 2017;9(4):1732-1742.
- [Google Scholar]
- Regulatory effects of the AMPKα-SIRT1 molecular pathway on insulin resistance in PCOS mice: An in vitro and in vivo study. Biochem Biophys Res Commun. 2017;494(3–4) S0006291X17319356
- [Google Scholar]
- EIN2 mediates direct regulation of histone acetylation in the ethylene response. In: Proceedings of the National Academy of Sciences of the United States of America. 2017. p. :10274.
- [Google Scholar]
- Neonatal Isoflurane Exposure Induces Neurocognitive Impairment and Abnormal Hippocampal Histone Acetylation in Mice. PLoS ONE. 2015;10(4):e0125815
- [Google Scholar]







