Translate this page into:
Quercetin treatment against NaF induced oxidative stress related neuronal and learning changes in developing rats
⁎Corresponding author. pratapkreddyou@osmania.ac.in (Pratap Reddy Karnati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study evaluates the protective role of quercetin against sodium fluoride (NaF) induced neurotoxicity in developing rat brain using biochemical, behavioural and histopathological parameters. Timed pregnant Wistar rats were chosen for the study and they were distributed into three groups (group 1: control, group 2: fluoride and group 3: fluoride- quercetin). The study duration is for 53 days (i.e. Gestational and post gestational period), where in the control group received normal tap water; Fluoride group received 20 ppm fluoride and the third group received both 20 ppm of Fluoride and 20 mg of quercetin through oral gavage. Behavioural studies were done using postnatal pups of 21 and 30 days age. The brains of postnatal pups of 14, 21 and 30 days age were collected and used for biochemical and histopathological analysis. The present study indicates the reversal of NaF induced alterations, such as decreased body and brain weights, increased lipid peroxidation, epinephrine levels as well as decreased superoxide dismutase, catalase, glutathione, acetylcholine and norepinephrine levels. Quercetin administration has reversed learning inability and morphological changes in neuron structure. Thus, quercetin exerts neuroprotective property in developing rat brain by ameliorating the NaF induced oxidative stress, alterations in behavioural, neurotransmitters and histopathological alterations.
Keywords
Sodium fluoride (NaF)
Quercetin
Antioxidants
Oxidative stress
Monoamines
1 Introduction
Fluoride induces fluorosis which is characterized by dental and skeletal damages. It is also associated with early damage of soft tissues including brain. The physiological changes have been alarming the research fraternity and it is important to identify possible preventive, curative and therapeutic possibilities to handle the condition (Wenjing et al., 2011). Fluoride enters into the body through food, drinking water, toothpastes, fluoride rinses and fluoride containing compounds (Mullenix et al., 1995; Nurgul et al., 2014). The concentration of fluoride permitted in drinking water as per the WHO is 0.7–1.2 ppm (Kumar and Takalkar, 2010). The high concentrations of fluoride in groundwater in some parts of India have raised an alarm regarding the possible damage or toxicity due to chronic fluoride exposure on humans and animals. Fluoride also affects non-skeletal organs such as the liver, kidney, heart and brain (Nabavi et al., 2012c,d). Chronic exposure of fluoride could induce the loss of structure and function of skeletal muscles, brain and spinal cord (Olusegum et al., 2013; Vani and Reddy, 2000). Fluoride is capable of crossing blood brain barrier and placental barrier and causing low birth weights (Diouf et al., 2012; Gouri et al., 2011). Fluoride passes through blood brain barrier and leads to neurodegeneration in the brain, causing learning disabilities, memory deficits, and alters the activities of enzymes in the brain (Madhusudhan and Piler, 2009; Shivarajashankara et al., 2002a,b). In the developing stage, fluoride enters weanling pups through mother’s milk and leads to neuron alteration, morphological changes and neurotransmitters abnormalities in the foetal brain (Varner et al., 1998). Accumulation of fluoride in foetal stage leads to alteration of growth, cell differentiation and subcellular organization in the brain (Yaning et al., 2005). In endemic fluoride areas children lose their intelligence quotient (Wang et al., 2004).
Recent studies reported the potential benefits of natural products such as Curcumin, Silymarin, and Resveratrol against sodium fluoride induced oxidative stress in soft tissues of rats (Nabavi et al., 2011, 2012a–d, Yousef et al., 2010). Micronutrients such as vitamin C, D, E and calcium, treatments did not show expected amelioration. Quercetin is a ubiquitous flavonoid taken as a part of diet and it is extensively available in broccoli, apple, onions, tomatoes, red wine, potatoes, soya beans, tea and coffee (Renugadevi and Milton, 2010). Quercetin exists in the form of glycosides i.e. quercetin glycosides (quercetin glucoside, quercetin galactoside, or quercetin arabinoside) in fruits and vegetables get absorbed passively in small intestine in quercetin aglycone form and quercetin aglycone is also available as a dietary supplement, but it is limited to 1 g/day (Costa et al., 2016). Quercetin aglycone undergoes biotransformation by involving phase II enzymes to yield glucuronidated, sulfated, and methylated metabolites. The phase II enzymes are UGT (uridine 5′-diphosphoglucuronosyltransferase), SULT (sulfotransferase), and COMT (catechol-O-methyltransferase). Quercetin has the ability to cross the blood brain barrier but the amount is very less i.e. in the order of picomolar to nanomolar are found in brain tissue (Costa et al., 2016; Sharma et al., 2015).
Quercetin is a powerful antioxidant, due to the presence of two pharmacophores that is, the catechol group in the B ring and the OH group at position 3 within the molecule which scavenges the free radicals (Nabavi et al., 2015; Sharma et al., 2015). Concentration of 5–50 μM quercetin works as an excellent ROS scavenger in In vitro condition (Saw et al., 2014). Zhang et al. (1999) showed quercetin neuroprotection against hydroxydopamine induced damage in both In vitro (PC12 cell lines) and In vivo by decreasing the expression of iNOS, NO levels and also restricted neuron loss in brain and the quercetin pre-treatment has given more neuroprotection. Till date there are no reports on quercetin’s neuroprotective property against sodium fluoride induced toxicity in developing rat brain. The present study reports the beneficial role of quercetin against sodium fluoride induced neurotoxicity in developing rat brain through oxidative, neurotransmitters, histopathological and behavioural analysis.
2 Materials and methods
2.1 Chemicals
Quercetin, epinephrine norepinephrine and sodium fluoride were purchased from Sigma–Aldrich chemicals. All others chemicals used for biochemical analysis and histopathological analysis were of analytical grade from Himedia.
2.2 Animals
Pregnant Wister albino rats were obtained from NCLAS, National Institute of Nutrition, Hyderabad, India. The pregnant rats were maintained under standard laboratory conditions in single rat polypropylene cages, exposed with 12 h light/dark cycle at room temperature 24 ± 2 °C. The animals were provided standard diet and drinking water with ad libitum. The laboratory conditions were maintained as per the guidelines given by the CPCSEA (Committee for the Purpose of Control and Supervision on Experimental Animals) at Osmania University, Hyderabad, India. Timed pregnant rats aged (160–180 days) were randomly divided into three groups (n = 6/gp). The control group received normal tap water; the experimental group received sodium fluoride 20 ppm/kg bw (Banala and Karnati, 2015) through drinking water and protective group received quercetin 20 mg/kg bw (Nabavi et al., 2012c,d) by gavage and 20 ppm Sodium fluoride through drinking water (Table 1). The experimental animals were maintained for 53 days. Postnatal pups of 14, 21 and 30 days of age were selected for experimental studies. The brains were dissected from of 14, 21 and 30 days old pups and stored at 20 °C. The brains were used for biochemical and histopathological studies.
Groups
Dose
Control
Tap water
Experimental
Fluoride water (20 ppm)
Protective
Fluoride–quercetin (20 mg/kg bw)
2.3 Methods
Body and brain weight: The body and brain weights of postnatal pups of 1, 7, 14, 21 and 30 days age old from each respective groups were noted and analysed.
2.4 Oxidative stress markers
2.4.1 Lipid peroxidation (LPO)
Lipid peroxidation in cerebral cortex of brain tissue was measured by modified method of Garcia et al. (2005). 1 mL of 10% homogenate was added to 1 mL of 20% TCA and heated at 70 °C for 10 min, and cooled at room temperature and centrifuged at 3000 rpm for 10 min. 400 μL of supernatant was mixed with 200 μL of 0.5% TBA reagent in a test tube covered with a glass marble and heated in a boiling water bath for 10 mins and the tubes cooled to room temperature. The absorbance of the pink coloured trimethine condensation product was measured at 533 nm using a spectrophotometer. The results were expressed as nano mole MDA/gm weight of tissue (Fabio et al., 2012; Reham et al., 2015).
2.4.2 Superoxide dismutase (SOD)
Superoxide dismutase activity in the cerebral cortex of brain tissue was estimated by modified protocol of Marklund and Marklund (1974). The assay system in a final volume of 1.0 mL consisting 600 μL of 83.3 mM Tris–Hcl buffer, (pH 8.2), 100 μL of 0.5 mM DETPA, 50 μL of enzyme, 50 μl of Tris-EDTA, 50 μL of 0.01 N HCl, 100 μL of H2O2 was mixed well and the reaction was initiated by adding the 50 μL of 3.97 mM Pyrogallol. Increased absorbance was read at 420 nm using a spectrophotometer. The enzyme activity was expressed as Units/mg protein (Fabio et al., 2012; Reham et al., 2015).
2.4.3 Catalase
The catalase activity in the cerebral cortex of brain tissue was estimated as described by the method of Pari and Latha (2004). The results were based on the ability of enzyme (catalase) units which quenches 1 mole of hydrogen peroxide in 1 min.
2.4.4 Glutathione
The glutathione content was analysed using the modified method of Ellman (1959). Absorbance of the reaction mixture was recorded at 417 nm and results were expressed as μg/mg protein.
2.4.5 Monoamines estimation
2.4.5.1 Preparation of sample
The brain tissue was homogenated in 0.25 M sucrose solution and centrifuged at 4 °C at 1000 rpm for 10mins. 1 mL acid butanol was added to supernatant, mixed well and centrifuged at 1000 rpm for 10 mins. Butanol layer was separated and 2.5 mL of heptane and 1.5 mL of ddw were added, vortexed and centrifuged at 1000 rpm for 5 mins. To the aqueous layer, 200 mg of acid alumina and 1 mL of 2 M sodium acetate were added, vortexed for 5 mins then pH adjusted to 8 with 1 N NaOH and centrifuged at 1000 rpm for 5 mins. The precipitate (acid alumina) was used for the catecholamine assay (Chirumari and Reddy, 2007).
2.4.6 Catecholamine assay
A modified protocol of Kari et al. (1978) was used to estimate the levels of Epinephrine and Norepinephrine using fluorescence meter (Jasco FP750). Epinephrine read at (Excitation: 410 nm; Emission: 500 nm) and Norepinephrine read at (Excitation: 387 nm; Emission: 487 nm). The results were expressed as μg of mono amine/gm weight of tissue.
2.4.7 Acetylcholine
Acetylcholine was estimated using the method of Hestrin (1949). 10 mg of the cerebral cortex was weighed and homogenized by adding 1 mL of 3% perchloric acid and centrifuged at 1200 rpm for 10mins. The supernatant was collected and used for the estimation. The optical density was measured at 540 nm. The results were expressed as μg of mono amines/gm weight of tissue.
2.4.8 Histopathological studies
Brain tissues stored in 10% formalin, were dehydrated through a graded ethanol series. Paraffin embedded tissue blocks were made using the L blocks. 5 micron sections were made on rotary microtome (rotary microtome, model No.: 45). The brain sections were stained with Haematoxylin and eosin stain (Lillie and Fullmer, 1976). The stained sections were analysed using Olympus microscope.
2.4.9 Behavioural studies
2.4.9.1 Maze learning test
A maze is a puzzle in the form of a complex branching passage through which the starved animals try to find the food/goal animal find. Maze learning is the process of learning a route typically by rats through a maze in order to obtain reinforcement. This process is a popular experiment in behavioural laboratory and it is the main method of studying spatial learning. The rats were starved for 12 h before the maze learning experimentation. The postnatal day pups 21 and 30 days of age from all experimental groups were trained two days before the experimentation, the animals were tested and the results were analysed (Winocur and Morris, 1990).
2.4.9.2 Open field test
The open field test was used to measure behavioural responses such as emotional reactivity/anxiety and locomotory activity. Open field test was performed using 21 and 30 days old pups from all groups and the results were analysed (Seibenhener and Wooten, 2015).
2.5 Statistical analysis
Statistical analyses were performed using SPSS version 20 software. mean ± SEM (Standard Error), One way ANOVA post hoc multiple comparison tests were tested at p < 0.5∗ and <0.05∗∗
3 Results
NaF treated rat pups failed to gain normal body weight, as compared to the controls and the protective group showed reversal in the body weight when significantly (p < 0.05) compared to the experimental group (see Fig. 1).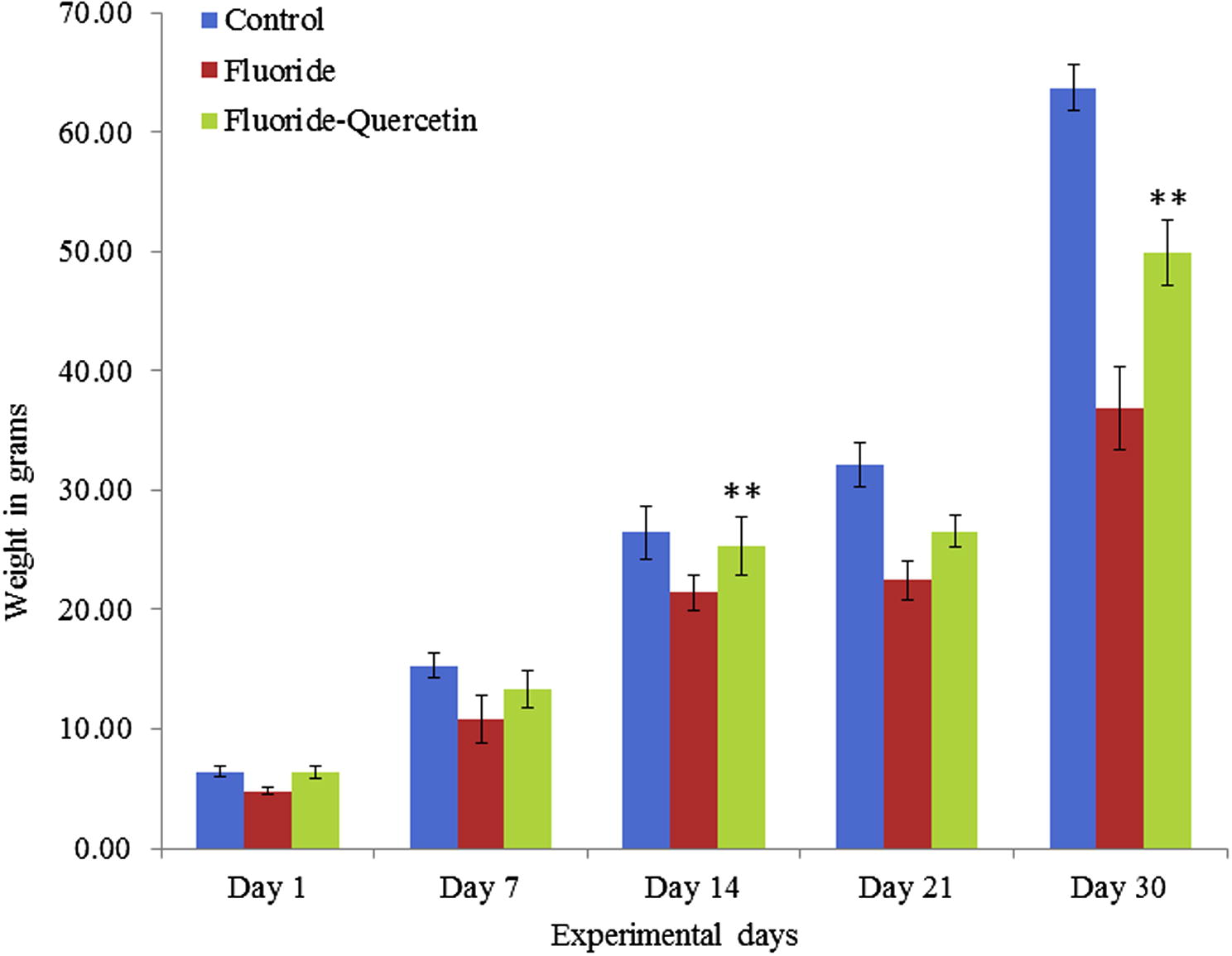
Effect of quercetin and NaF on body weights of developing rat pups. The values are mean ± SEM; n = 6, (∗∗p < 0.05).
Prenatal exposure of NaF has restricted the postnatal rats from gaining brain weight, the difference is significant (p < 0.05) when compared to that of controls and the protective group, whereas the postnatal pups which received quercetin–NaF have shown significant (p < 0.05) recovery in brain weights in all age groups (Fig. 2).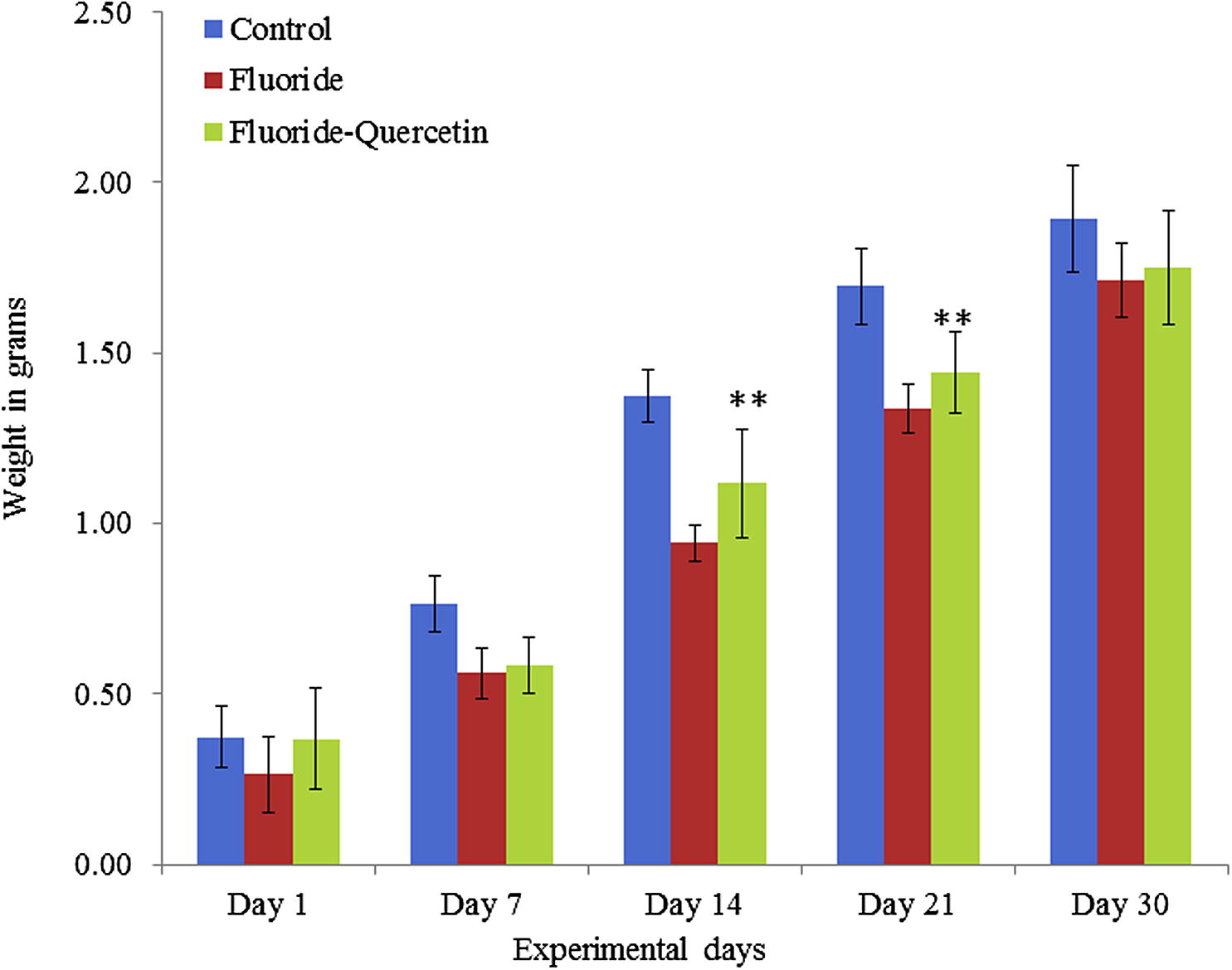
Impedance of NaF on brain weight of developing rats in presence and absence of quercetin. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
Lipid peroxidation was significantly (p < 0.05) increased in the NaF group as compared to the control group. Whereas the lipid peroxidation was significantly (p < 0.05) reduced in the quercetin–NaF induced group as compared to the NaF group (see Fig. 3).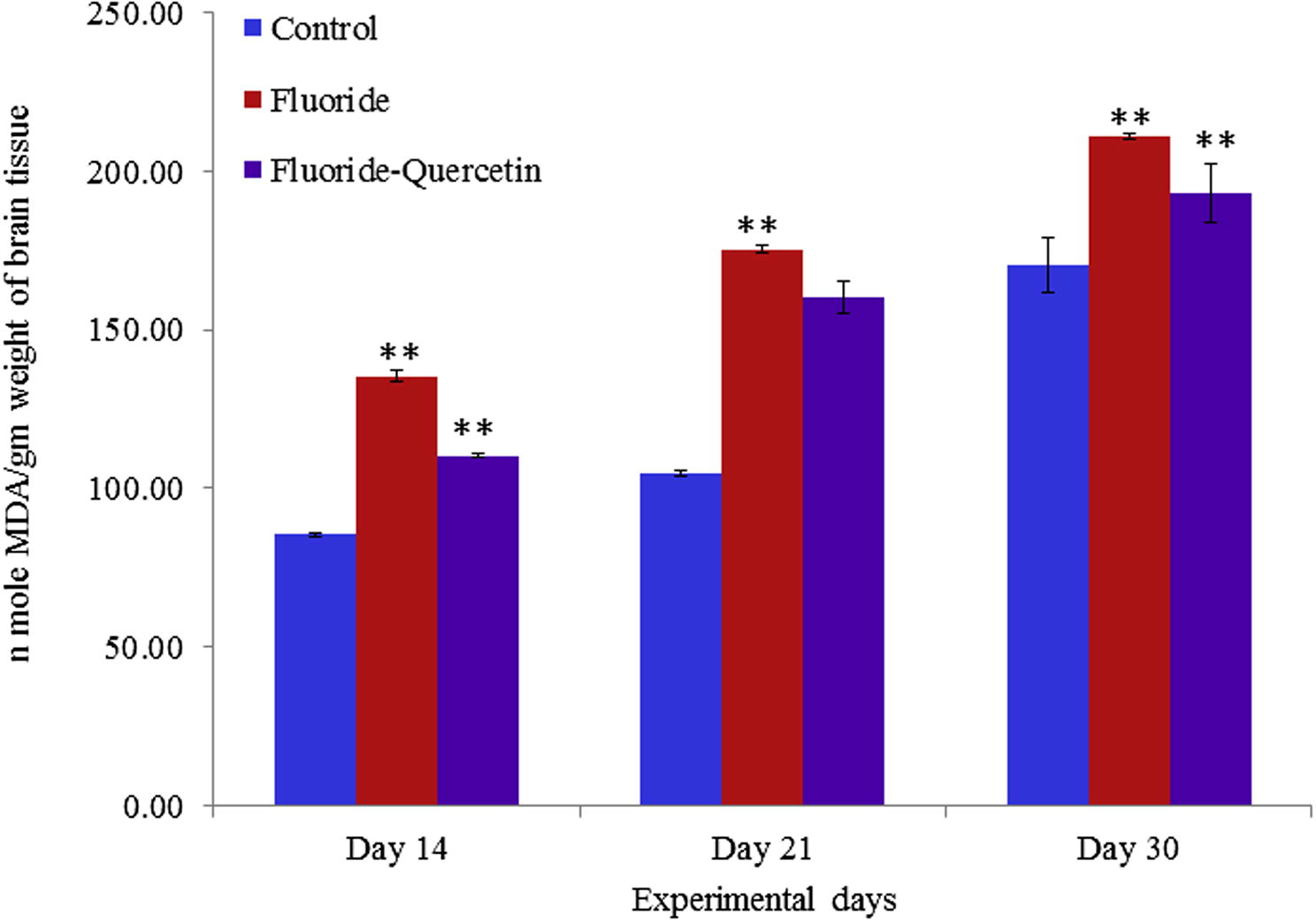
Lipid peroxidation in cerebral cortex of developing brain on chronic NaF exposure with and without quercetin treatment. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
Superoxide dismutase activity was significantly (p < 0.05) reduced in the NaF treated group in 14, 21 and 30 days aged post-natal pups as compared to the control group, whereas the superoxide dismutase activity was significantly (p < 0.05) reversed in quercetin–NaF received group as compared to the NaF treated group (see Fig. 4).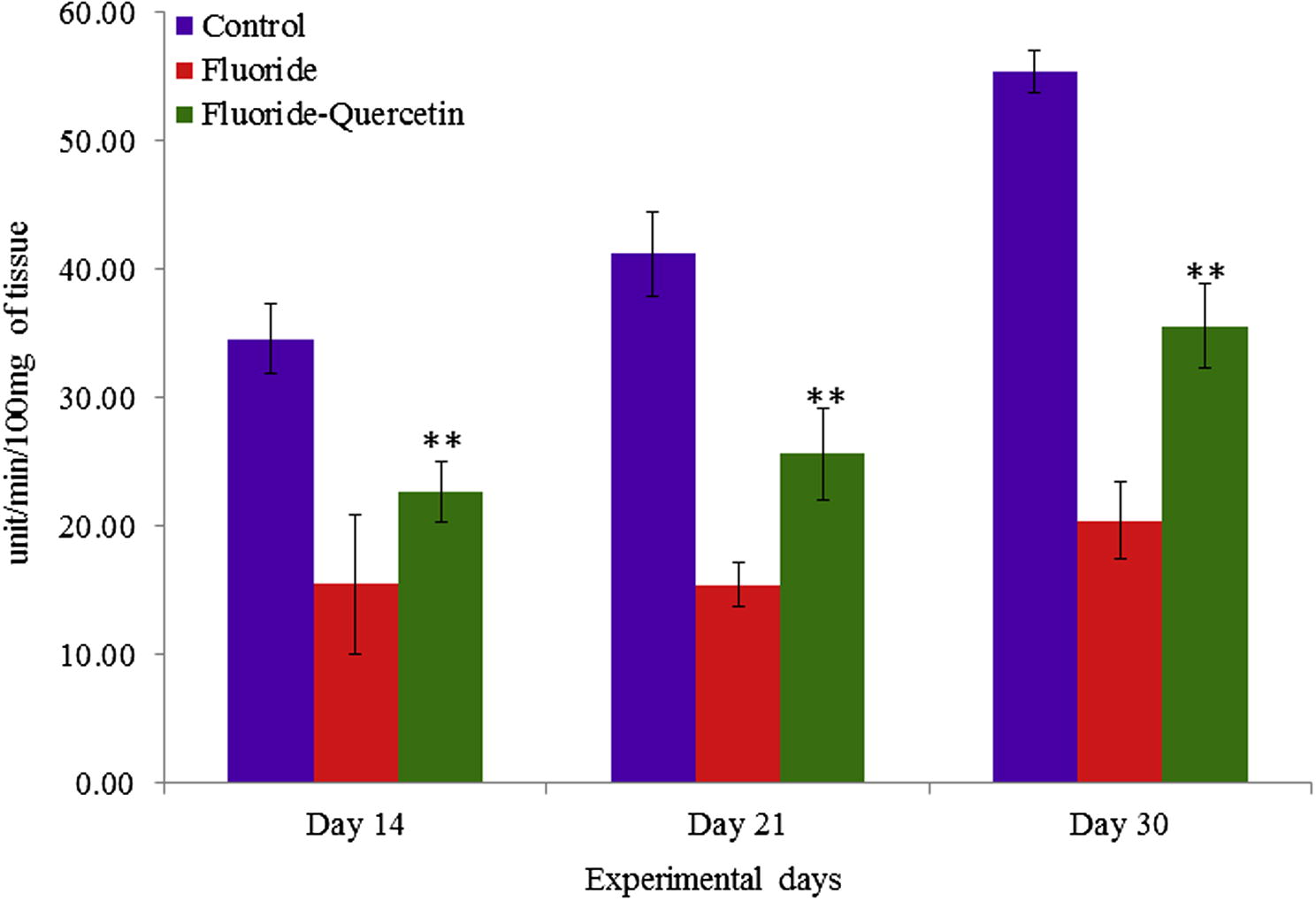
Effect of NaF on superoxide dismutase activity in cerebral cortex of developing rat brain with and without quercetin administration. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
The NaF treatment led to a significant (p < 0.05) decrease in catalase activity when compared to the controls whereas the groups that received quercetin along with NaF have shown significant (p < 0.05) recovery of catalase activity in developing rat brains (p < 0.05) (see Fig. 5).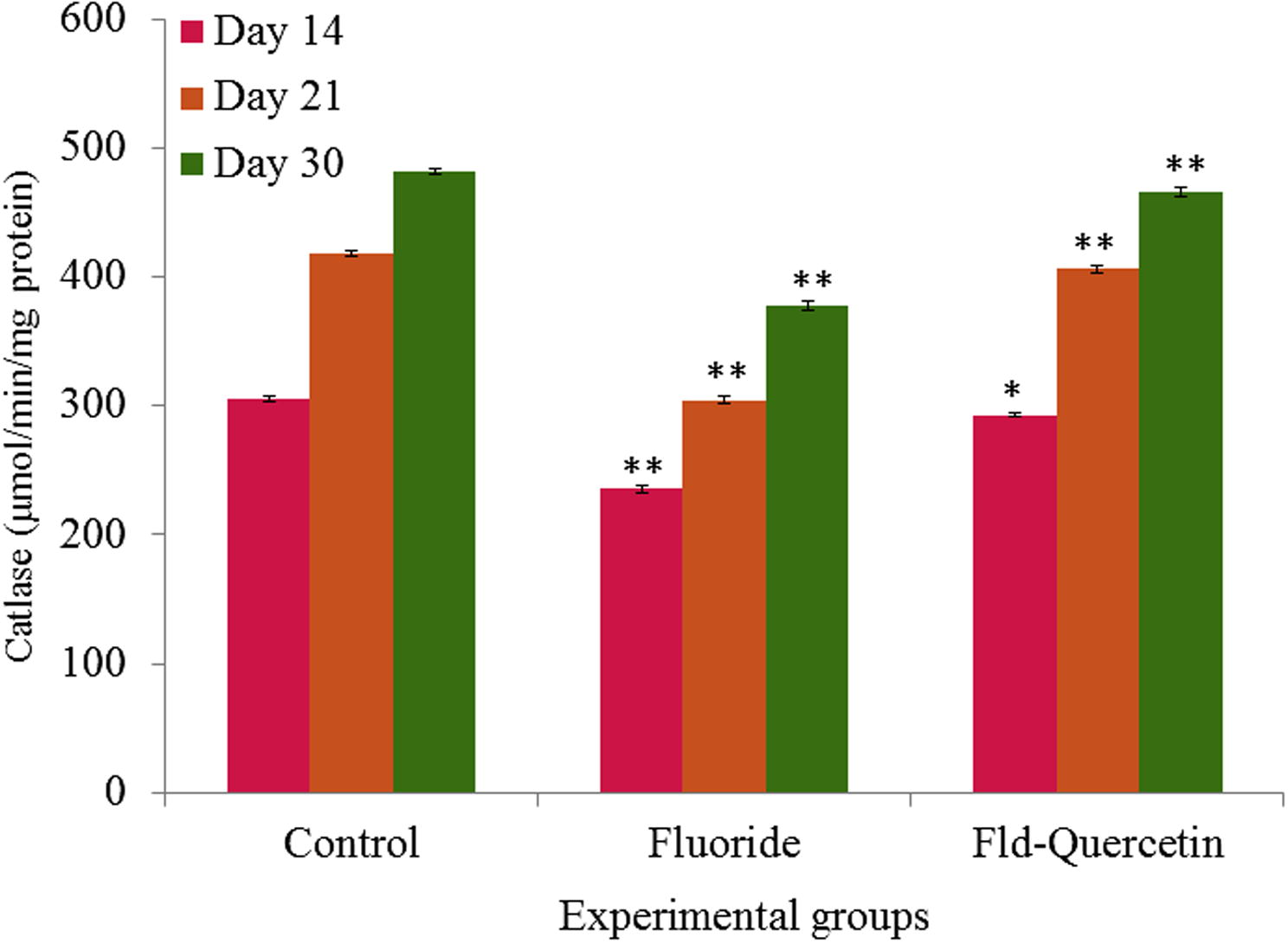
Effect of NaF on catalase activity in cerebral cortex of developing rat brain with and without quercetin treatment. The values are mean ± SEM, n = 6, (∗p < 0.5; ∗∗p < 0.05).
NaF induced oxidative stress led to significant (p < 0.05) depletion of glutathione levels in the brains of NaF treated developing rats compared to controls. The co-treatment of quercetin along with NaF has shown significant (p < 0.05) recovery in restoring the levels of glutathione in the brains of developing rats when compared to the NaF treated group (see Fig. 6).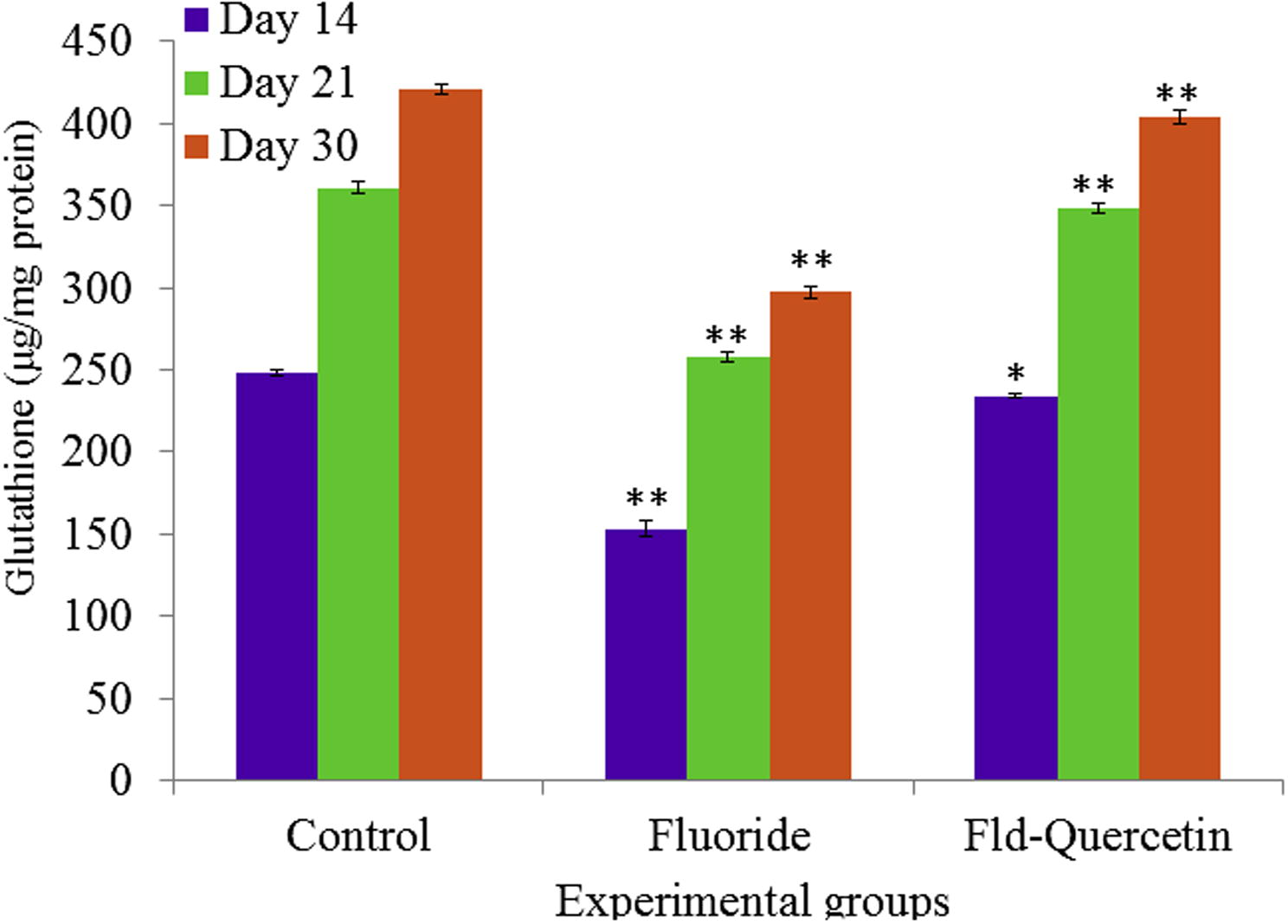
Effect of NaF on glutathione levels in cerebral cortex of developing rat brain with and without quercetin administration. The values are mean ± SEM, n = 6, (∗p < 0.5; ∗∗p < 0.05).
The epinephrine levels were significantly (p < 0.05) increased in rats on NaF exposure when compared to controls. Whereas the epinephrine levels were significantly (p < 0.05) restored in the quercetin–NaF received group as compared to the NaF treated group (see Fig. 7).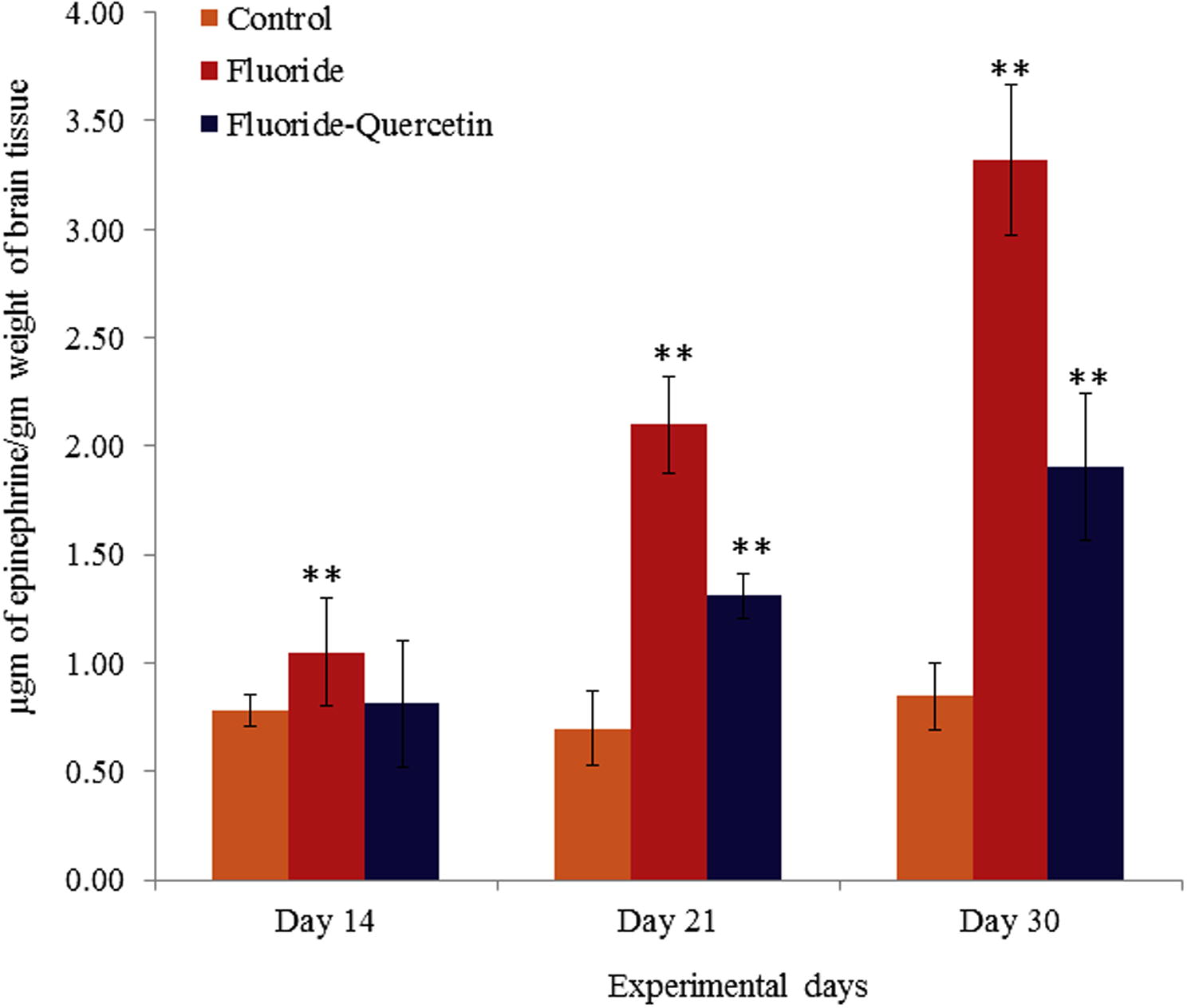
Epinephrine levels in cerebral cortex of developing rat brains on chronic exposure to NaF and quercetin concomitant treatment. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
The nor-epinephrine levels were significantly (p < 0.05) decreased in rats on NaF exposure when compared to controls. Whereas the nor-epinephrine levels were significantly (p < 0.0) restored in the quercetin–NaF received group as compared to the NaF group (see Fig. 8).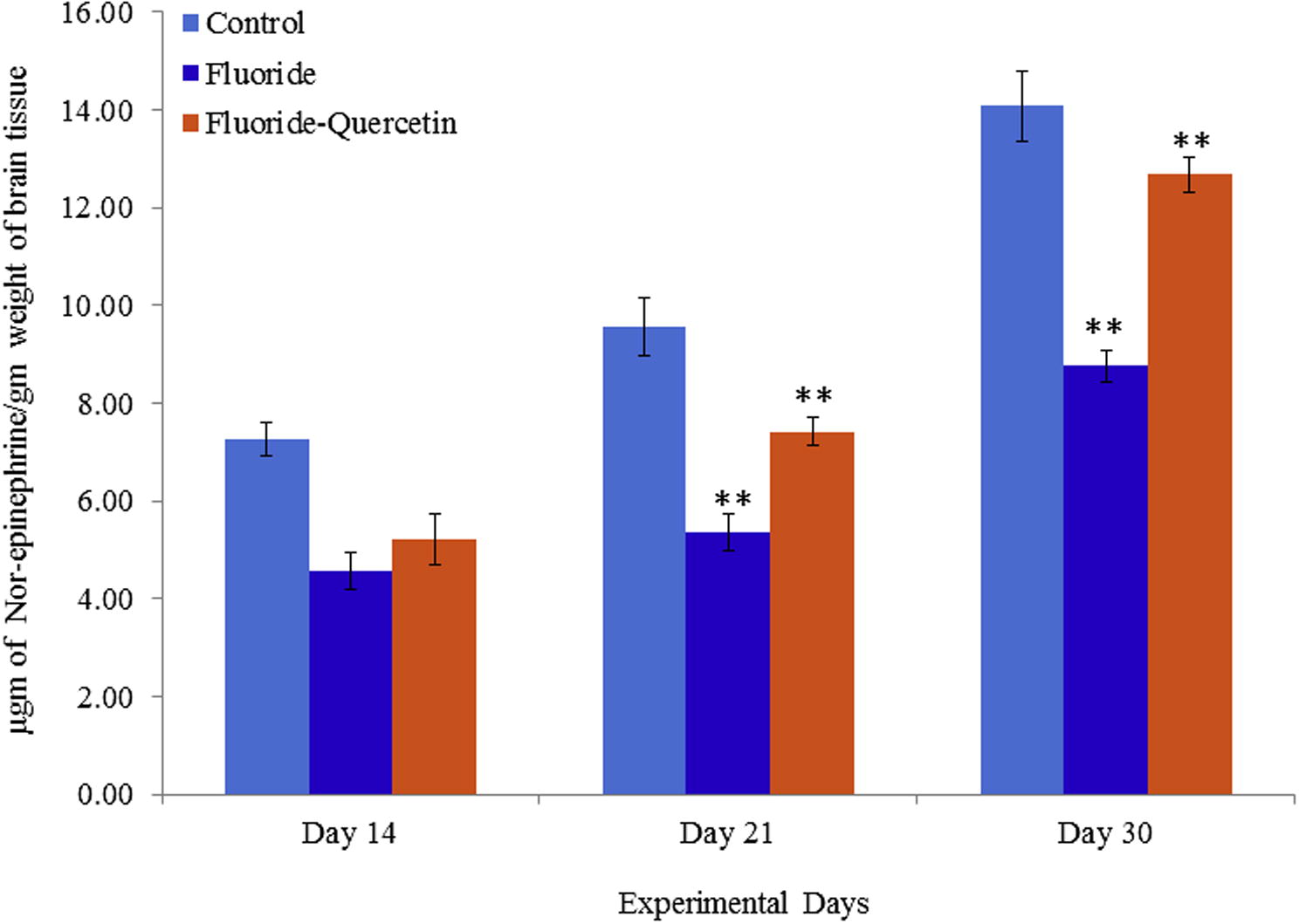
Levels of Nor-epinephrine in cerebral cortex of developing rat brain on NaF induction with and without quercetin administration. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
The acetylcholine levels were significantly (p < 0.05) decreased in rats on NaF exposure when compared to controls, whereas acetylcholine levels were significantly (p < 0.05) restored in the quercetin–NaF received group as compared to the NaF group (see Fig. 9).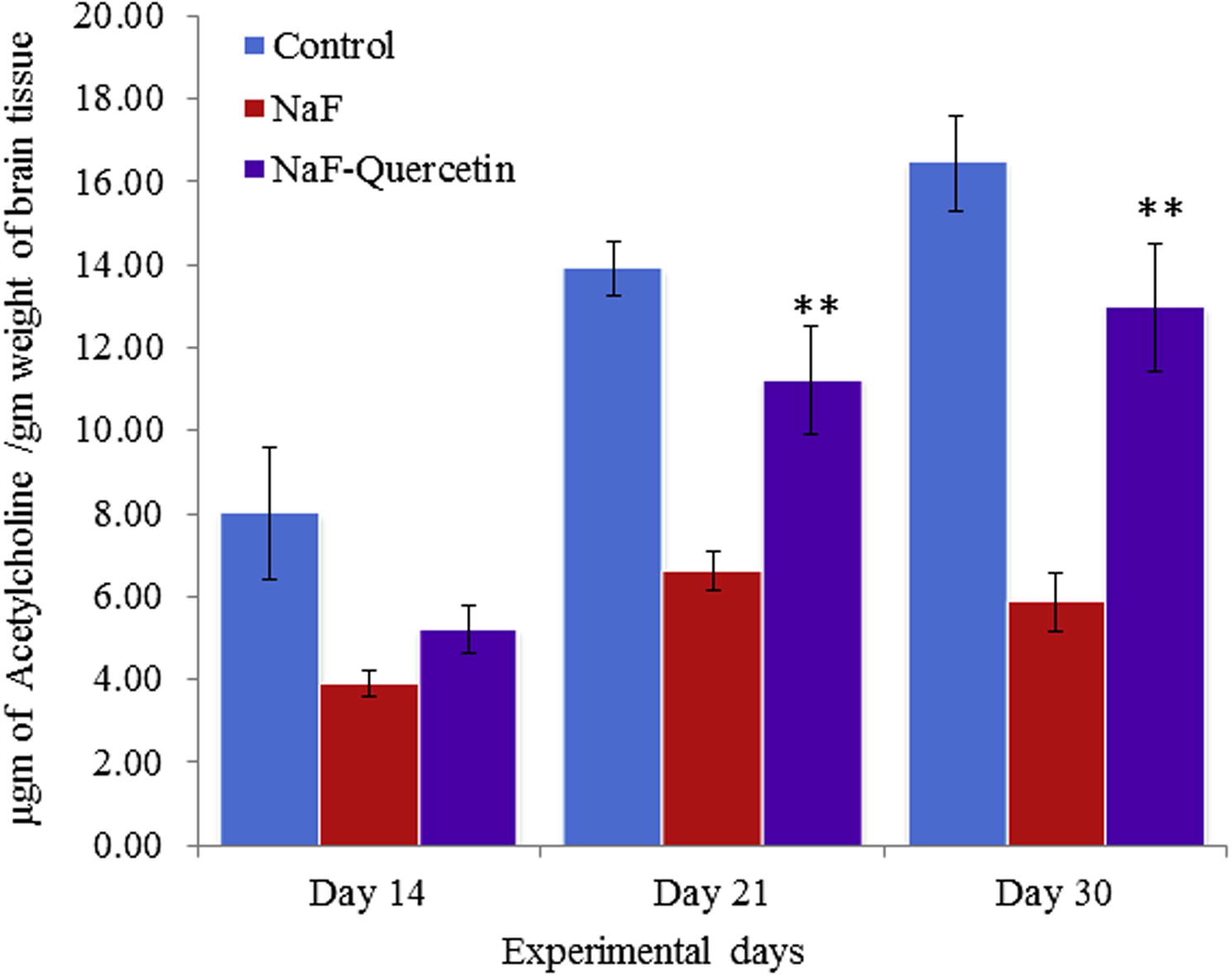
Effect of quercetin and NaF on acetylcholine levels in cerebral cortex of developing rat brain. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
The behavioural studies conducted on the postnatal pups 21 and 30 days of age. Sodium fluoride treated pups showed significant (p < 0.05) decrease in learning ability, cognition, and increase in the latency period of goal achieving compared to the control rat pups. The quercetin received pups showed significant (p < 0.05) recovery in learning ability, cognition and decreased in the latency period of goal achieved when compared to experimental pups treated with sodium fluoride (see Fig. 10).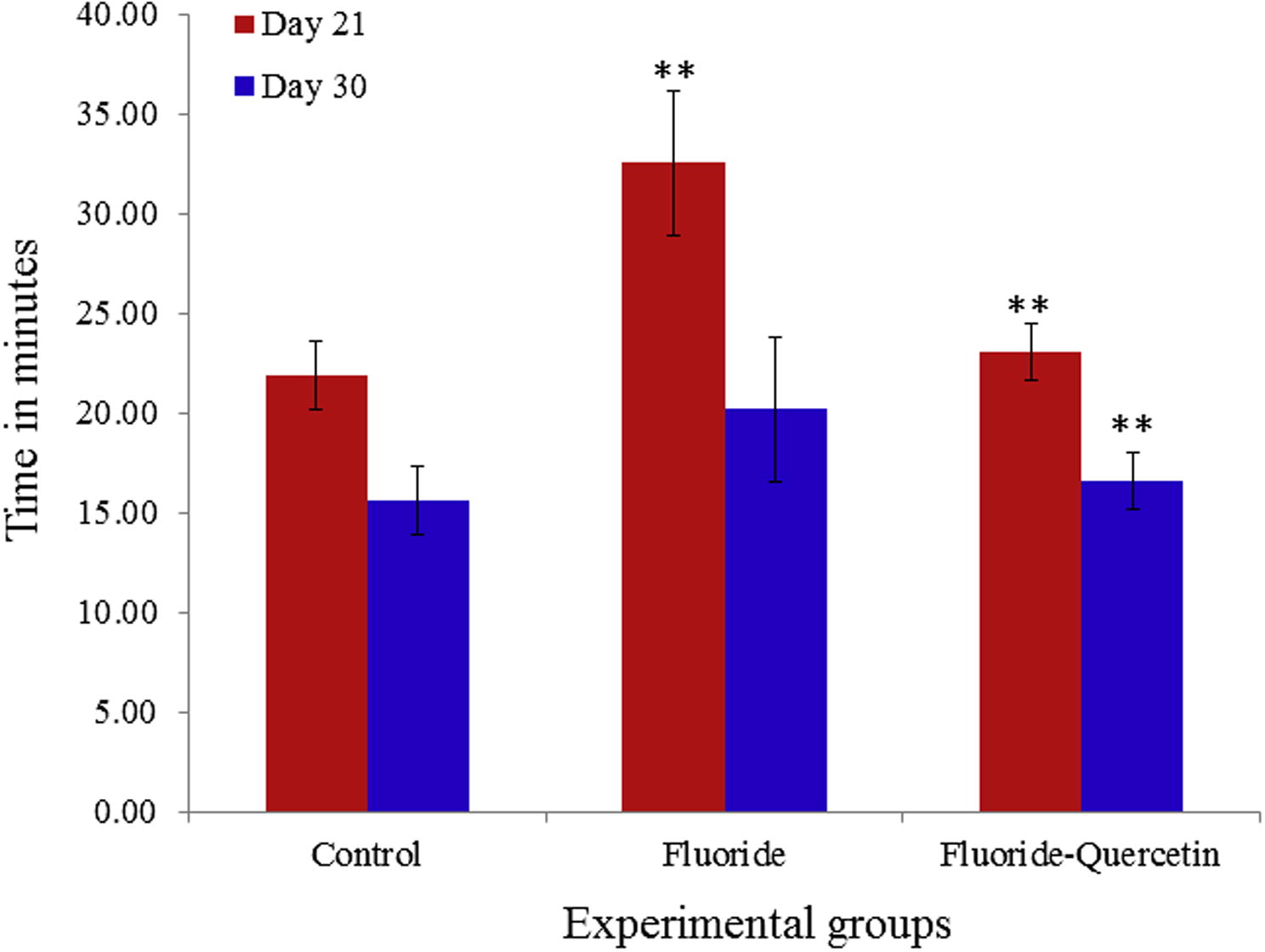
Quercetin effect on learning ability in postnatal rats exposed to NaF. The values are mean ± SEM, n = 6, (∗∗p < 0.05).
Behavioural activities such as head elevation, hind limb elevation, sniffing, grooming, auditory startle, pivoting scores were significantly reduced when compared to the control group. Whereas the behavioural activities were significantly restored in the quercetin–NaF group as compared to the NaF treated group (see Fig. 11).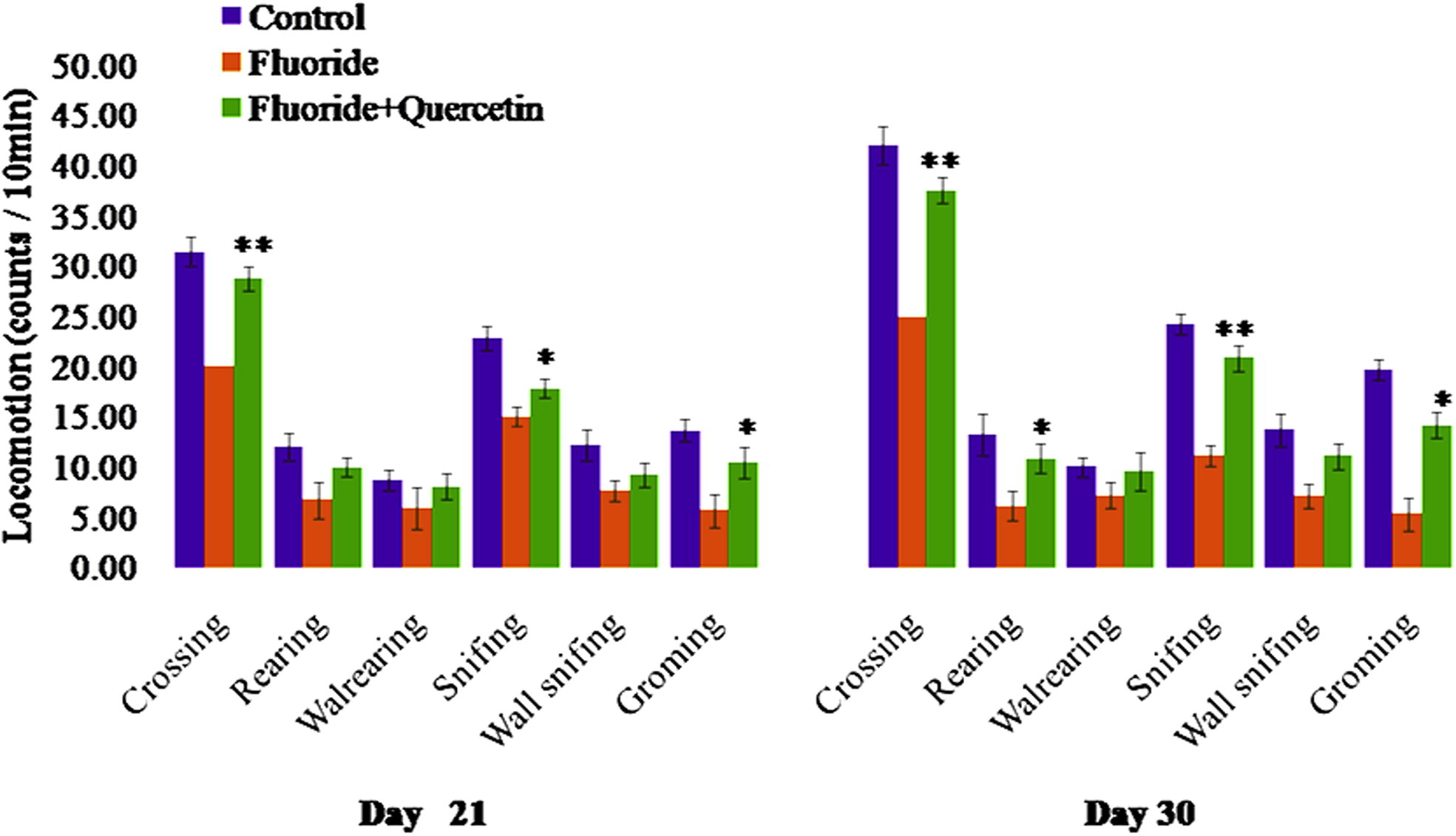
Effect of quercetin administration on behavioural dysfunction induced by NaF in postnatal rats. The values are mean ± SEM, n = 6, (∗p < 0.5, ∗∗p < 0.05).
The histopathological alterations in the cerebral cortex of brain of postnatal rat pups (day 14, 21 and 30) were observed using haematoxylin and eosin stain. The control group showed normal morphology of neurons. NaF treated pups showed degenerating neurons and darkly stained shrinked nuclei. The quercetin along with NaF treated sections showed less neurodegeneration in the cerebral cortex region when compared to NaF treated rats indicating the neuroprotective capacity of quercetin and the ameliorative effect increased with age and duration of exposure (see Fig. 12).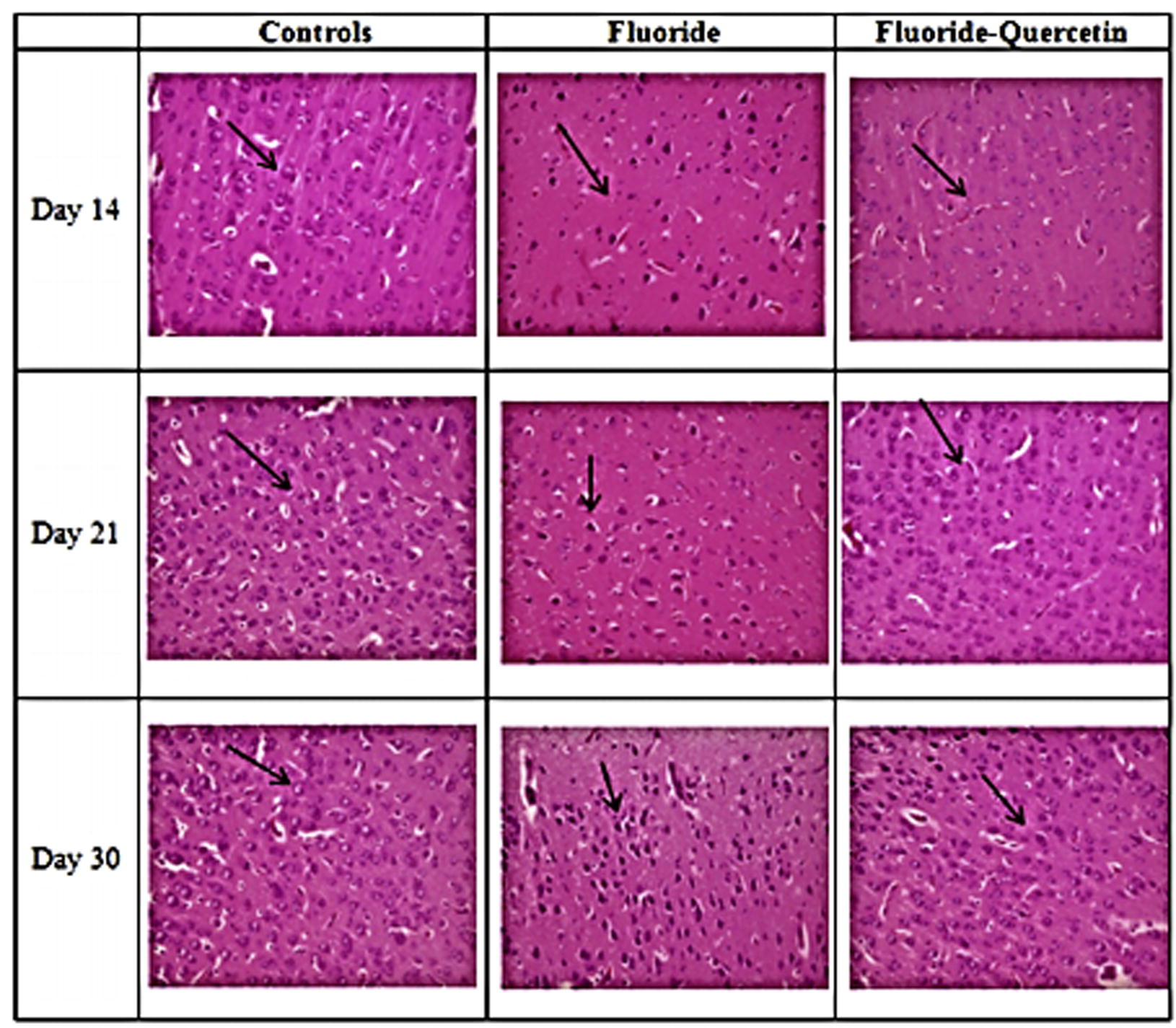
Effect of quercetin on the histological alterations induced on chronic exposure of NaF in the cerebral cortex of 14, 21 and 30 days old rat pups (40X).
4 Discussion
The experimental data demonstrated sodium fluoride induced oxidative damage, leading to significant changes in neurochemical, behavioural and histopathological alterations in developing rat brain whereas the administration of quercetin helped in restoring the levels of antioxidant enzymes, neurochemicals and also replenished the behavioural, and histopathological alterations induced by NaF. The present study provided the evidence about the ameliorative capacity of quercetin against 20 ppm NaF induced oxidative stress, morphological, biochemical and histological alterations in developing rat brain. Earlier reports on sodium fluoride induced histopathological alterations in the hippocampus, cerebral cortex regions of brains of rats and rabbits, showed decreased neuronal generation, neuronal and nucleus shrinkage, chromatolysis neurons making the neurons look dark (Bhatnagar et al., 2002; Shashi, 2003; Shivarajashankara et al., 2002a,b). According to the literature, numerous protective agents such as Curcumin (Kirankumar et al., 2015), extract of Spirulina platensis (Banji et al., 2013), Thymoquinone (Wessam and Wahab, 2013), Resveratrol (Nurgul et al., 2014) Oleanolic Acid (Sarkar et al., 2014), Pentacyclic triterpenes (Majdak, 2014) etc. were exercised to prevent the toxic effects of sodium fluoride in animal models and all which showed promising effects but there are no protective agents which could completely reverse the toxic effects of sodium fluoride or stop its accumulation in various tissues. Hence, the search for better protective agents is still on going.
Earlier studies on quercetin showed it as a protectant against various toxins induced oxidative stress in rats (Costa et al., 2016) and also indicates that it has multiple properties such as anti-oxidative, hematoprotective, hepatoprotective, anti-cancer and anti-allergic (Chouhan et al., 2011; Federico, 2012; Nabavi et al., 2012c,d; Yousef et al., 2010). Quercetin is a good antioxidant and anti-inflammatory agent as it exerts low side effects on liver and gastric systems, and has inhibitory action on prostaglandins production, COX2 expression and nuclear factor κB (NFκB) activation (Pany et al., 2014). As per the study done by Fabio et al. 2012, quercetin tempered serum lipid and glucose status and minified the MSG induced toxic effects by exerting its antioxidant properties in postnatal rats. Quercetin’s role as a neuroprotectant has been reported in old male rats (Nabavi et al., 2012c,d; Reham et al., 2015) but there are no reports published on quercetin against chronic exposure of NaF in developing rat brain. The results achieved in our study coincide with the published reports on NaF induced neurotoxicity and oxidative damage.
Previous studies reported the decreased body and brain weight of the rats, increased lipid peroxidation (Malondialdehyde levels) on sodium fluoride intoxication. The polyunsaturated lipids in the brain are highly vulnerable to oxidative damage leading to lipid peroxidation and a decrease in antioxidant enzymes such as superoxide dismutase in hepatic tissue (Nabavi et al., 2012c,d) and neural tissue (Chirumari and Reddy, 2007; Madhusudhan and Piler, 2009). The present results showed alterations in the levels of antioxidants (SOD, catalase and glutathione) in developing rat pups intoxicated with 20 ppm sodium fluoride, resulting in an increase in lipid peroxidation in treated rat pups. The lipid peroxidation is less in the control and quercetin received group when compared to the fluoride induced postnatal pups. The toxic effects of NaF were ameliorated in the presence of quercetin.
The physiological function of neurotransmitter is accomplished by means of specialized receptors. Norepinephrine is released from nerve ending and its function is primarily to activate epinephrine receptors. Previous studies have reported on the alterations in monoamine neurotransmitter levels in different regions of the rat brain intoxicated with NaF (Yu et al., 2008). In the present study, the levels of epinephrine were elevated and nor-epinephrine levels were depleted in the cerebral cortex of the brain intoxicated by sodium fluoride when compared to the controls. Administration of quercetin controls the alteration of monoamine levels in the cerebral cortex region of the brain in postnatal day pups induced by NaF. The Ach levels were significantly depleted in NaF treated rat pups as compared to the control. The simultaneous treatment of quercetin to the rats receiving NaF has shown significant reversal of acetylcholine levels in comparison to that of NaF treated rats.
Previous behavioural studies show that excessive exposure of fluoride caused diminished intelligent quotient in children compared to the normal children (Wang et al., 2004) and sodium fluoride intoxicated rat exerted loss of memory and learning disability (Yaning et al., 2005). In the present study postnatal rats aged day 21 and day 30 demonstrated loss of memory and learning inability and there was a considerable increase in the goal achieve latency period in the NaF treated group when compared to the control pups. The concomitant administration of NaF and quercetin received pups showed better memory and learning ability and there was a significant decrease in the goal achieve latency period compared to the sodium fluoride treated rat pups.
The present study indicates the evidence on mitigation of NaF induced oxidative damage, behavioural and learning impairment, and alternations in levels of pro and antioxidants neurotransmitters and histological changes on concomitant administration of quercetin in developing rat brain. Quercetin restored the levels of antioxidants enzymes (SOD, catalase and glutathione), monoamines (epinephrine and norepinephrine) levels, as well as ameliorated behavioural and histopathological alterations when compared to the sodium fluoride treated rat pups. The administration of quercetin ameliorated the neuron morphology, nucleus shrinkage and neuronal degeneration in the cerebral cortex of developing rat brain.
5 Conclusion
The present results corroborate that the F induces the oxidative stress by increasing pro-oxidant LPO and decreasing the antioxidants SOD, catalase, glutathione which concomitantly may alter neurotransmitters and neurobehaviour of rats which were further evidenced with neurohistological alterations in the cerebral cortex of developing brain. The co-administration of quercetin to sodium fluoride intoxicated rats was found to be effective in reversing the oxidative stress, altered neurotransmitters, and behaviour along with neurohistological changes in developing rat brain. This study conclusively proves quercetin ability of neuroprotection against F toxicity and indicates that the consumption of quercetin rich food can give protection against fluoride induced neurotoxicity. However, further studies are needed to evaluate the ameliorative effect of quercetin at molecular level particularly as gene and protein expression studies of transcription factors (Nfr2), pro and anti-oxidants on co-administration of quercetin.
Acknowledgement
Authors acknowledge the partial funding under UGC-DSA-I and UGC-BSR award (19-118/2014).
References
- Vitamin A deficiency: an oxidative stress marker in sodium fluoride (NaF) induced oxidative damage in developing rat brain. Int. J. Dev. Neurosci.. 2015;47(Pt B):298-303.
- [CrossRef] [Google Scholar]
- Investigation on the role of Spirulina platensis in ameliorating behavioural changes, thyroid dysfunction and oxidative stress in offspring of pregnant rats exposed to fluoride. Food Chem.. 2013;140:321-331.
- [Google Scholar]
- Neurotoxicity of fluoride: evidence of neurodegeneration in hippocampus of female mice. Indian J. Exp. Biol.. 2002;40:546-554.
- [Google Scholar]
- Dose dependent effects of fluoride on neurochemical milieu in hippocampus and neocortex of rat brain. Fluoride. 2007;40(2):101-110.
- [Google Scholar]
- Silymarin and quercetin abrogates fluoride induced oxidative stress and toxic effects in rats. Mol. Cell Toxicol.. 2011;7:25-32.
- [Google Scholar]
- Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Hindawi Publishing Corporation; 2016.
- [CrossRef]
- Pregnant women living in areas of endemic fluorosis in Senegal and low birth weight new borns: case control study. Rev. Epidemiol. Sante Publique. 2012;60(2):103-108.
- [Google Scholar]
- Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem. Toxicol.. 2012;50:3556-3561.
- [Google Scholar]
- Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol.. 2012;143:383-396.
- [Google Scholar]
- Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods. 2005;144(1):127-135.
- [Google Scholar]
- The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem.. 1949;180(1):249-261.
- [Google Scholar]
- Effects of ketamine on brain monoamine levels in rats. Res. Commun. Chem. Pathol. Pharmacol.. 1978;20:475-488.
- [Google Scholar]
- Curcumin protection against oxidative stress induced neural damage in developing brain of rat with fluoride exposure. Int. J. Bioassays. 2015;4(7):4077-4081.
- [Google Scholar]
- Study of the effects of drinking water naturally contaminated with fluorides on the health of children. Biomed. Res.. 2010;21(4):423-427.
- [Google Scholar]
- Histopathologic Technique and Practical Histochemistry (4th ed.). New York: McGraw-Hill; 1976. p. :205-208.
- Effect of maternal exposure of fluoride on biometals and oxidative stress parameters in developing CNS of rat. Biol. Trace Elem. Res.. 2009;133:71-82.
- [Google Scholar]
- Neuroprotective properties of compounds of vegetable origin: pentacyclic triterpenes. Psychiatr. Psychol. Klin.. 2014;14(4):284-289.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- Protective effects of curcumin and quercetin on thyroid function in sodium fluoride intoxicated rats. Fluoride. 2011;44(3):147-152.
- [Google Scholar]
- Mitigating role of quercetin against sodium fluoride-induced oxidative stress in the rat brain. Pharm. Biol.. 2012;50(11):1380-1383.
- [Google Scholar]
- Role of quercetin as an alternative for obesity treatment: you are what you eat! Food Chem.. 2015;179:305-310.
- [Google Scholar]
- Neuroprotective effects of Silymarin on Sodium fluoride-induced oxidative stress. J. Fluorine Chem.. 2012;142:79-82.
- [Google Scholar]
- In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem.. 2012;132:931-935.
- [Google Scholar]
- Protective effect of quercetin against sodium fluoride induced oxidative stress in rat’s heart. Food Funct.. 2012;3(4):437-441.
- [Google Scholar]
- Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food Chem. Toxicol.. 2014;70:191-197.
- [Google Scholar]
- Comparative study on the influence of fluoride on lipid peroxidation and antioxidant levels in the different brain regions of well fed and protein undernourished rats. J. Trace Elem. Med Biol.. 2013;27:370-374.
- [Google Scholar]
- Neuroprotective role of quercetin in neurotoxicity induced rats: role of neuroinflammation in neurodegeneration. Asian J. Pharm. Clin. Res.. 2014;40:178-183.
- [Google Scholar]
- Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipid peroxidation in STZ diabetic male Wistar rats. BMC Complement. Altern. Med.. 2004;4:4-16.
- [Google Scholar]
- Protective effects of blackberry and quercetin on sodium fluoride-induced oxidative stress and histological changes in the hepatic, renal, testis and brain tissue of male rat. J. Basic Clin. Physiol. Pharmacol.. 2015;26(3):237-251.
- [Google Scholar]
- Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp. Toxicol. Pathol.. 2010;62:471-481.
- [Google Scholar]
- Ameliorative effects of oleanolic acid on fluoride induced metabolic and oxidative dysfunctions in rat brain: experimental and biochemical studies. Food Chem. Toxicol.. 2014;66:224-236.
- [Google Scholar]
- Quercetin protects against aluminium induced oxidative stress and promotes mitochondrial biogenesis via activation of the PGC-1α signalling pathway. Neurotoxicology. 2015;51:116-137.
- [CrossRef] [Google Scholar]
- Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Visualized Exp.. 2015;96:e52434.
- [CrossRef] [Google Scholar]
- Histopathological investigation of the fluoride-induced neurotoxicity in rabbits. Fluoride. 2003;36:95-105.
- [Google Scholar]
- Histological changes in rat brain of young fluoride intoxicated rats. Fluoride. 2002;35:12-21.
- [Google Scholar]
- Brain lipid peroxidation and antioxidant systems of young rats in chronic fluoride intoxication. Fluoride. 2002;35:197-203.
- [Google Scholar]
- The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signalling pathway. Food Chem. Toxicol.. 2014;72:303-311.
- [Google Scholar]
- Effect of fluoride accumulation on some enzymes of brain and gastrocnemius muscles of mice. Fluoride. 2000;33:17-26.
- [Google Scholar]
- Chronic administration of aluminium fluoride or sodium fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res.. 1998;784:284-298.
- [Google Scholar]
- Effects of high fluoride and low iodine on biochemical indexes of the brain and learning-memory of offspring rats. Fluoride. 2004;37:201-208.
- [Google Scholar]
- Effects of fluoride on synaptic membrane fluidity and PSD-95 expression level in rat hippocampus. Biol. Trace Elem. Res.. 2011;139:197-203.
- [Google Scholar]
- Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J. Basic Appl. Zool.. 2013;66:263-270.
- [Google Scholar]
- Hippocampal and prefrontal cortex contributions to learning and memory: analysis of lesion and aging effects on maze learning in rats. Behav. Neurosci.. 1990;104(4):544-551.
- [Google Scholar]
- Effects of high fluoride and low fluoride on brain histopathology in offspring rats. Fluoride. 2005;38(2):127-132.
- [Google Scholar]
- Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and hepatotoxicity in rat. Food Chem. Toxicol.. 2010;48:3246-3261.
- [Google Scholar]
- Neurotransmitter and receptor changes in the brains offetuses from areas of endemic fluorosis. Fluoride. 2008;41(2):134-138.
- [Google Scholar]
- Effects of fluoride exposure on synaptic structure of brain areas related to learning-memory in mice. Fluoride. 1999;41(2):139-143.
- [Google Scholar]







