Translate this page into:
Purification and characterization of carboxymethylcellulase from Bacillus pumilus EWBCM1 isolated from earthworm gut (Eudrilus eugeniae)
⁎Corresponding authors. biosankaralingam@yahoo.co.in (Subbiah Sankaralingam), suribaskar@hotmail.com (Kathirvelu Baskar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present work describes purification and characterization of an extracellular cellulase from a newly isolated cellulolytic bacterial strain Bacillus pumilus EWBCM1. The candidate strain was tested for its abilities to hydrolyze the structural polysaccharides through the depolymerising activities of carboxymethylcellulase. The purification of cellulase was carried out by ammonium sulphate precipitation, DEAE cellulose and sephadex G-100. Purified cellulase was found to be 47.56 fold along with 1.46 U/mg of protein and the molecular weight of cellulase was 47 kDa through SDS-PAGE electrophoresis. The optimum activity of cellulase was registered at pH 10 at 50 °C. In the present study, metal ions inferred the addition of 5 mM CaCl2 (128%) was recorded good activity than the other tested metal ions. Maximum activity of purified cellulase was recorded in 3% of NaCl and 5% of surfactants medium. The bioactive of purified cellulase was carried out with various commercial detergent and antimicrobial activity.
Keywords
Purification
Characterization
SDS-PAGE
CMCase
Bacillus pumilus
1 Introduction
Cellulose is an important structural material of living cells and it is profusely found on the earth because they are the chief component of plant biomass in terrestrial as well as aquatic ecosystem. These are the renewable polymeric substances which are hydrolyzed it into soluble sugars by the synergistic action of cellulase enzymes. Cellulase is a multifaceted biological molecule to breakdown the cellulose into simplest glucose residues (Darabzadeh et al., 2018). The functional mechanism of cellulase enzymes are combined action of three vital constituents such as endo-β-glucanase (EC 3.2.1.4), exo-β-glucanase (EC 3.2.1.91) and β-glucosidase (EC 3.2.1.21) in order to make simple units (Mostafa et al., 2020).
In general, cellulose is made up of D-glucose units that are associated by β-1,4-glycosidic linkage; mostly, crystalline and amorphous structures. Usually, the numerous microorganisms are found in the environment and they are repeatedly used as potential source to hydrolyze the cellulose by using the cellulase enzyme for the agricultural along with industrial purpose (Nehad et al., 2019). Several earlier literatures have been reported that the breakdown of lignocellulosic waste material through the cellulase is a cost effective method and it is believed to be good bioactive potential for the reduction of cost along with improvement of bioprocess technology. At the same time, current estimated and decreasing of processing charge up to 13% by the way of bioprocess technology for the reduction of cellulase cost (Willis et al., 2010).
Most probably, the aerobic bacterial strains have the capacity to secrete cellulase using lignocellulosic biomass because cellulose is the significant carbohydrates as energy sources for the microorganisms. Among these bacterial strains, the species of Bacillus has dominant cellulase producer such as Bacillus subtilis (Soeka and Sulistiani, 2019), Bacillus megaterium (Sankaralingam et al., 2018), Serratia liquefaciens (Mohammed, 2020) and Pseudomonas fluorescens (Goel et al., 2019). More over Clostridium thermocellum, Fibrobacter succinogenes, and Ruminucoccus albus are the aerobic bacterial strains frequently utilized in aqua feed industry (Lopez-Contreras et al., 2004).
Today, various fermentation technologies have been applied for the production of cellulase by using microorganisms. For this, solid as well as submerged fermentation methods are frequently utilized and both methods are completely differ from one another especially these methods are completely depending up on the water level in the production medium along with environmental conditions (Mostafa et al., 2020). Most often, the cellulase are commercially utilized in various industries like textile and detergent. Among these, the potential of bio-shining’ of fabric materials in household laundry detergents are found in order to improve the softness of fabric along with brightness (Hebeish et al., 2009).
Earlier literatures have quoted the cellulase enzyme is consumed as animal feed for the improvement of nutritional value to prevent the digestion problems due to the presence of probiotic efficiency. At the same time, they have utilized for the fruit juice clarification, bakery industry and also the de-inking of paper is another recent trend. Apart from these various applications, such as paper, medicine and food industry are the major areas of cellulase applications (Maravi and Kumar, 2020).
Most of the earlier literatures have documented the cellulase producing bacterial strains from various sites for the industrial request whereas the cellulase from earthworm gut isolate is available only very limited references. Hence, the present study was evaluates the bioactive potential of the earthworm gut bacterium B. pumilus EWBCM1 as a source of CMCase with promising applications in various industries. Keeping the aforesaid information into contemplation, the present work was concentrated on the purification and characterization of CMCase from gut bacterium B. pumilus EWBCM1 and its biotechnological applications.
2 Materials and Methods
2.1 Cultural Conditions of Microorganism
The gut sample was serially diluted in CMC agar media for the screening of cellulase production and the plates were maintained at 35 °C for 72 hrs. The bacterial strain was grown in 100 mL of CMC broth: carboxy methyl cellulose-1.0 g; NaNO3-0.2 g; FeSO4-0.001 g; MgSO4-0.05 g; K2HPO4-0.1 g; KCl-0.05 g; pH-7 and CMC was used as substrate for cellulose hydrolysis incubated at 35 °C for 72 hrs. The growth of bacterial strains was visualizing the zone of hydrolysis in the presence of Congo red at 15 min. The enzyme producing potential bacterial strain was exhibited maximum clear zone around the bacterial growth and the selected strain was kept on sterile CMC agar slants at 4 °C in order to avoid the contamination. Morphological, biochemical and molecular characteristics of the isolates were studied based on the in Bergey's Manual of Determinative Bacteriology (Bergey's 1964).
2.2 CMCase Assay
The bacterial isolate was subjected for centrifugation at 5000 rpm for 20 min at 4 °C to get the supernatant. The enzyme was carried out by using culture supernatant as per the method of Ghose (1987) with CMC. 0.5 mL of enzyme source with 0.5 mL of 1% CMC solution was prepared in 0.2 M citrate phosphate buffer at pH 7 and maintained at 45 °C for 30 min. The addition of 2 mL of DNS reagent reaction was done to terminate the reaction and solutions were carried out in boiling water for 5 min. Finally, 7 mL of dis·H2O in each tube was made to dilute the reaction mixture and the absorbance was carried out at 540 nm (Systronics, 119) against blank solution made in the same manner without enzyme.
2.3 Acetone Precipitation
The complete enzyme purification process was carried out at 4 °C. After the completion of centrifugation, the collected supernatant was blended with 2 volumes of acetone for the protein precipitation. The precipitate was collected and dried in lab temperature for the dialysis of enzyme.
2.4 Purification of CMCase
The crude enzyme was blended with 100 mL of 0.2 M citrate phosphate buffer at pH 7.0 to concentrate the protein sample. After the dialysis with dis·H2O, 50 mL of enzyme sample was loaded onto a column chromatography of Sephadex G100 (18 × 2 cm). The same buffer was used to elute the sample at 20 ml h−1 for the collection of 5 mL fractions and the each fraction was carried out to test the protein content along with enzyme activity. Then the positive fractions were pooled in order to concentrate the sample by freeze-drying method and it was dialyzed as above said procedure to be loaded separately (25 mL) on to DEAE-cellulose column. The column was eluted with step wise gradient of 0–0.8 M NaCl at a flow rate of 10 ml h−1 and 5 mL fraction was collected. Once again, the collected fraction was dialyzed for the elimination of Na+ and Cl− and the lyophilized enzyme source was stored at 0 °C for further experimental analysis (Mawadza et al., 2000).
2.5 Analysis of SDS-PAGE and Molecular Mass
The molecular weight of the CMCase enzyme was done through SDS-PAGE following the procedure of Laemmli (1970). The protein content of the enzyme was estimated method of Lowry et al. (1951). The molecular mass of electrophoresis protein was studied following the procedure of Weber et al. (1972). For this, standard molecular marker (Medox Cat. No. MX-0211-01) was loaded on to the gel at the time of loading of samples and molecular mass of marker proteins were plotted in log scale against the electrophoretic mobilities of the marker proteins.
2.6 Zymogram
Zymogram was performed by following the method of Li et al. (2009) with minor modification.
2.7 Determination of Kinetic Parameters
The kinetic parameters (Michaelis–Menton constant) Km and maximal velocity Vmax of CMCase activity were studied from Lineweaver-Burk plot with the optimal assay conditions as 50 °C, pH 9 for B. pumilus EWBCM1 at 30 min for CMC concentrations ranging from 0.5 mg to 5 mg/mL. The evaluation of these graphs yielded the kinetic parameters for the CMC hydrolyzing activity of the enzyme was done (Graphpad Prism 5.04 software).
2.8 Characterization of CMCase
2.8.1 Effect of pH and Temperature on Activity of CMCase
The impact of pH on purified CMCase was carried out in 1% (w/v) appropriate buffers such as citrate buffer at pH 4.0–6.0, sodium phosphate buffer at pH 7.0–8.0, tris-HCl at pH 9.0 and glycine-NaOH at pH 10.0–12.0 for 30 min. The evaluation of temperature and pH effects on cellulase activity were carried out at various incubation temperatures ranging between 20 and 80 °C and also at pH values ranges from 4 to 12 for 30 min.
2.8.2 Effect of Various Metals Ions on CMCase Activity
The effect of metal ions on the CMCase activity (endoglucanase) was studied in presence of 5 mM LiCl2, (NH4)2SO4, MgCl2, CaCl2, CoCl2, KCl2, MnCl2, HgCl2, CuSo4 and NiSo4 for 30 min at 50 °C before the supplementation of substrate. More over the relative CMCase activity was also checked (Egas et al., 1998).
2.8.3 Effect of Various Surfactants on CMCase Activity
The impact of surfactants on CMCase activity was studied by the enzyme with various surfactants. Before adding the substrate, the various concentrations of surfactants such as triton X-100 (1% to 5%), tween-20 (1–5%), β-mercaptoethanol (1–5%), SDS (0.1–5%) for 15, 30, 45 and 60 min at 50 °C were carried out. Afterwards, the relative CMCase activity was tested at the optimum temperature (Lo et al., 2001).
2.8.4 Compatibility and stability of CMCase with Commercial Detergents
The CMCase was pre-incubated in various commercial detergents like Surf excel®, Ujala Techno bright®, Jil®, Ponvandu®, Rin®, Ariel® and Power active®. These chemicals were purchased in local market at Sivakasi, Tamil Nadu, India and the solution was prepared in double dis·H2O (7 mg/ml). The enzymes in detergent were deactivated by heating at 100 °C for 10 min. After that, CMCase was pre-incubated in detergent solutions at 50 °C for 1 hr. The residual activity of CMCase was done at 50 °C (Lima et al., 2005).
2.8.5 Effect of Sodium Chloride
The various concentrations such as control, 0.5, 1, 1.5, 2, 2.5, 3, 3.5 and 4% NaCl with cellulase enzyme and incubated at 35 °C for 30 min.
2.8.6 Antibacterial Activity of Cellulase
Antibacterial activity of CMCase by gut bacterial strain was checked as described by Geels and Schippers (1983) methods. The following strains namely Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia and Shigella dysenteriae were utilized for this study. The selected isolates were collected and maintained by the Department of Microbiology, Ayya Nadar Janaki Ammal College at Sivakasi
3 Results
3.1 CMCase Purification
Totally, 18 bacterial strains were isolated and the candidate strain was the dominant enzyme producer among these isolates based on the zone formation. Then the selected bacterial strain was subjected for further purification and characterization of enzyme. The quantity, activity, purity and yield of CMCase obtained from B. pumilus EMBCM1, observed after the various stages of purification are shown in Table 1. Initially, the purification of enzyme by acetone precipitation exhibited 67.82% purity of enzyme. After the acetone precipitation, purification of CMCase was done by Sephadex G-100 column and the positive fraction was reached 6.62 folds of purification with the yield of 47.56% (Fig. 1a). After the completion of gel filtration process, it was carried out on DEAE cellulose column and the potent activity of CMCase was located in one peak with 16.86 folds of purification with the yield 38.58% of the original activity (Fig. 1b). The molecular mass of the enzyme was 47 kDa (Fig. 1c) by SDS-PAGE exposed single band in gel and zymogram analysis was also exhibited clear zone against dark background (Fig. 1d).
STEP
Crude enzyme
Acetone precipitate
Sephadex G-100
DEAE-Cellulose
Protein (mg/ml)
2.84
1.90
1.02
0.65
Enzyme activity (IU/ml)
0.622
0.844
1.480
2.400
Specific activity (U/mg)
0.219
0.444
1.450
3.698
Total activity (U/mg)
621.96
421.80
295.80
239.98
Purification fold
1
2.03
6.62
16.86
Yield (%)
100
67.82
47.56
38.58
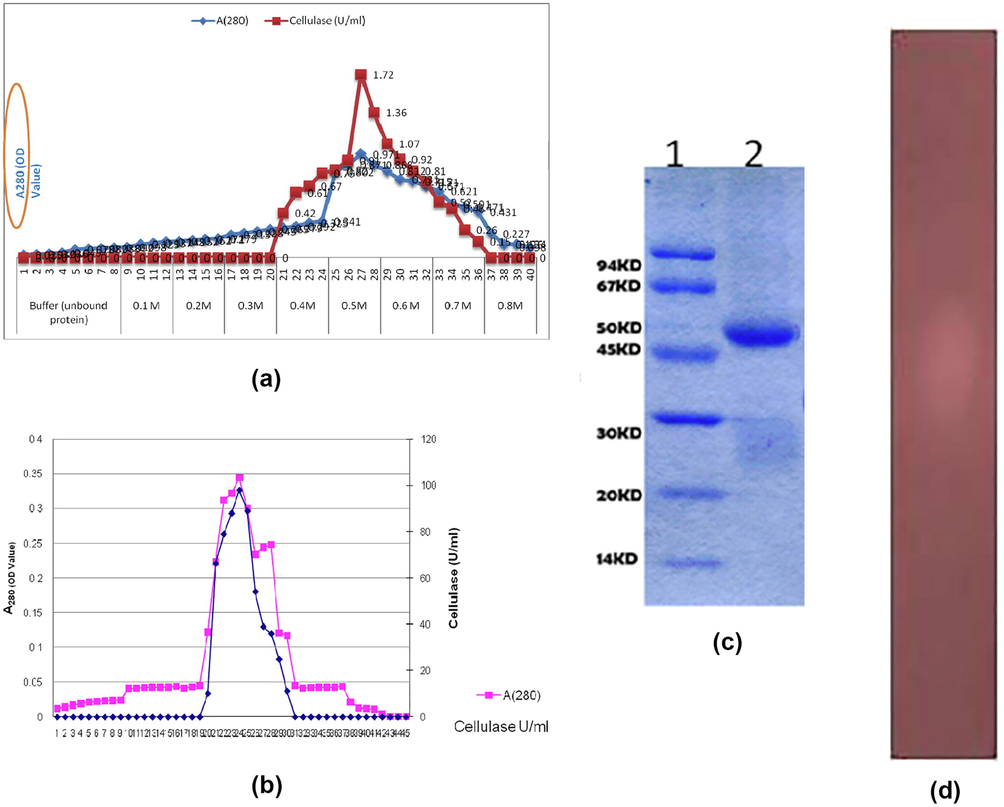
a: Elution profile of CMCase using Sephadox-G100 to check the enzyme activity. b: Elution profile of CMCase using DEAE cellulose for the purification process. c: Molecular weight determination by SDS – PAGE Lane 1 – Molecular weight marker Lane 2 – Sephadax G-100 purified Bacillus pumilus cellulase (47 kDa). d: Zymogram of CMCase from Bacillus pumilus EWBCM1.
3.2 Kinetic parameters of CMCase
The kinetic parameters (Km and Vmax) for the purified CMCase were determined at 50 °C and pH 9 for B. pumilus EWBCM1for concentrations ranging from 0.5 to 5 mg/mL of CMC as substrate. The Km and Vmax values for the purified CMCase of B. pumilus EWBCM1 were 1.688 mg/mL and 108.2 µm/min/mg, respectively (Table 2).
Michaelis-Menten
Best-fit values
Vmax
108.2
Km
1.688
Std. Error
Vmax
42.62
Km
0.4412
95% Confidence Intervals
Vmax
102.0 to 177.4
Km
0.0 to 1.151
Goodness of Fit
Degrees of Freedom
5
R square
0.5715
Absolute Sum of Squares
220.9
Sy.x
6.647
Constraints
Km
Km > 0.0
Number of points analyzed
7
Outliers (excluded, Q = 1.0%)
0
3.3 Characteristics (properties) of the CMCase enzyme
3.3.1 Effect of Temperature
The CMCase was stable at various temperature ranges from 20 to 80 °C and the maximum activity of 100% was registered at 50 °C. The activity of enzyme was 88.10 ± 1.01, 91.67 ± 0.92 and 92.71 ± 1.20% respectively at 20, 30 and 40 °C. However, only 68.35 ± 1.05% of activity was retained at 80 °C. The CMCase activity was 86.2 ± 0.85 and 81.32 ± 1.05 at 60 and 70 °C respectively (Fig. 2).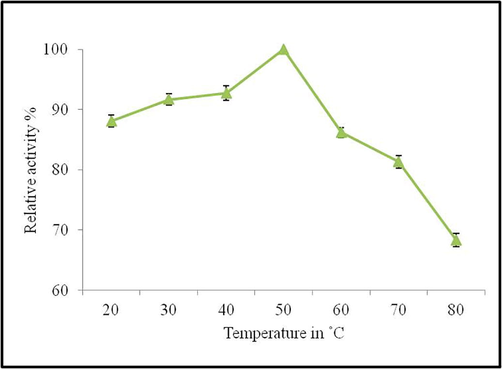
Effect of temperature on the relative activity of CMCase from Bacillus pumilus EWBCM1.
3.3.2 Effect of pH
The result of pH on enzyme activity was studied by incubating the mixture in various pH and the obtained CMCase activity ranges from pH 6 to 11, being maximum at pH 11 with the relative activity of 100% after which at pH 12, the relative activity was declined to 52.36 ± 0.97%. At pH 4, 5 and 6 relative activity observed was 9.9 ± 0.16, 49.68 ± 1.8 and 83.66 ± 1.52% respectively (Fig. 3a).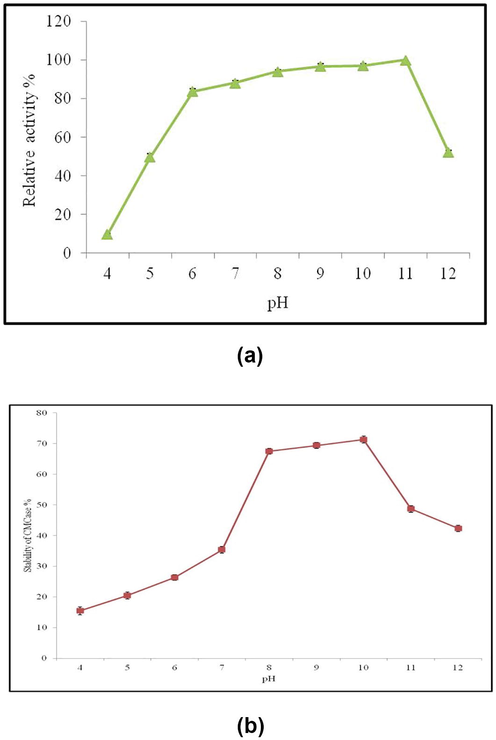
a: Effect of pH on relative activity of CMCase from Bacillus pumilus EWBCM1 b: Effect of pH on stability of CMCase from Bacillus pumilus EWBCM1.
The stability of CMCase gradually increased from pH 4 to 8 it reached a plateau and after pH 8 to 10 the enzyme activity was decreased. At pH 4, 5, 6 and 7 the activity of CMCase was 15.47 ± 1.30%, 20.44 ± 1.15%, 26.35 ± 0.90% and 35.35 ± 1.05% respectively. At pH 8, 9 and 10 the activity of CMCase was 67.49 ± 0.96%, 69.41 ± 0.96% and 71.32 ± 1.15% respectively. Whereas, the activity of CMCase was registered at pH 12 (42.34 ± 1.03%) (Fig. 3b).
3.4 3 Effect of Metals Ions
The result of metal ions on CMCase was observed by pre-incubating at 50 °C for 30 min at various concentrations. The results on the activity of CMCase enzyme with the addition of metal ions tested are given in Fig. 4a. The stimulatory effect was high only with the addition of 5 mM CaCl2 up to 127.60 ± 1.09%. At the same time, the inhibition of CMCase activity was noticed with the addition of other metal ions tested and they were MgCl2 > CuSo4 > (NH4)2SO4 > KCl2 > HgCl2 > LiCl2 > NiSo4 > CoCl2 > MnCl2.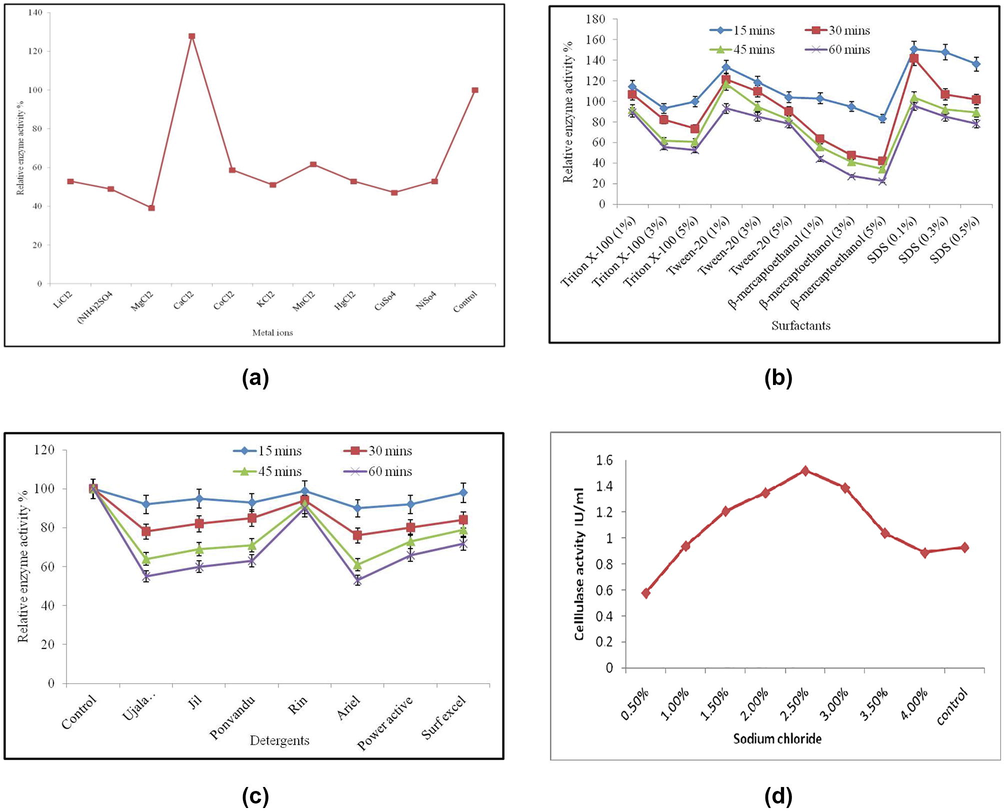
a: Effect of metal ions on activity of CMCase from Bacillus pumilus EWBCM1 b: Effect of surfactants on activity of CMCase from Bacillus pumilus EWBCM1 c: Effect of detergents on activity of CMCase from Bacillus pumilus EWBCM1 d: Effect of sodium chloride on enzyme activity.
3.5 4 Effect of Surfactants
In this study, the result of surfactant on enzyme activity was studied by incubation of reaction mixture at various concentrations. The relative activity was high at 1% surfactant concentration and it gradually reduced but not completely inhibited with the increase in the concentration of the surfactant up to 5% concentration. Among the four commonly tested surfactants, SDS at 0.1% concentration resulted maximum activity (Fig. 4b).
3.5.1 Effect of Commercial Detergents
The stability of CMCase in the presence of commercial detergents exhibited the best result when tested with the detergent Rin®. Whereas, only partial stability was observed when tested with Surf excel®, Power active® and Ponvandu®. The formulations, Ujala Techno bright®, Jil® and Ariel® almost prevented the activity; hence were not compatible for the CMCase (Fig. 4c). The CMCase activity was gradually diminished by increase in the time of exposure for all detergents. For example, at 15 min of incubation all the detergents tested showed a relative activity of more than 90%. At the same time, 60 min exposure, the relative activity of all the detergents tested except Rin® were less than 90%. The reduction of CMCase activity was in the order of Ariel® > Ujala Techno bright® > Jil® > Ponvandu® > Power active® > Surf excel®.
3.5.2 Effect of Various Concentrations of Sodium Chloride
Various concentrations of sodium chloride influence the enzyme activity, among them 2.5% concentration supplemented medium at 30 min incubations was optimal concentration for enzyme production (Fig. 4d).
3.6 Antibacterial activity of CMCase
The purified enzyme was tested against five clinical trials namely Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia and Shigella dysenteriae diameter of the inhibition zones for these bacterial isolates were 9, 13, 13, 15, 11 mm at 100 (µg/mL) for respective bacteria (Table 3). P.C-Positive control. N.C-Negative control.
Antibacterial activity
Microorganisms
MTCC & ATCC No.
P.C
N.C
50 (µg/ml)
100 (µg/ml)
Escherichia coli
MTCC25922
25
–
5
9
Staphylococcus aureus
MTCC25923
27
–
9
13
Pseudomonas aeruginosa
MTCC27853
28
–
10
13
Klebsiella pneumonia
MTCC432
18
–
12
15
Shigella dysenteriae
ATCC13313
21
–
7
11
4 Discussion
4.1 Purification of CMCase Enzyme
In the production of enzymes, fermentation technology is one of the significant strategies that could be carried out in order to increase the economic value of agro industrial waste matter because the generation of lignocellulosic feed stocks is very high in around the world (Soeka and Sulistiani, 2019)
Purification of enzyme is generally done for the improvement of functional mechanism and also to concentrate the protein content. Initially, it is achieved either through precipitation of crude enzyme from the broth through pre-chilled acetone or ammonium sulphate precipitation. The crude precipitate is then passed through chromatographic column for further purification by methods like Sephadox G-100 and DEAE- cellulose (Ali and EI-Dein, 2008). Molecular mass of CMCase could be calculated by running the protein in SDS-PAGE (Mawadza et al., 2000). The kinetic parameters (Km and Vmax) for the CMCase can also be studied (Nguyen and Quyen, 2010). Further, the purified enzyme protein can be characterized for its stability in various temperature, pH, metal ions, surfactants and commercial detergents (Oyekola et al., 2007).
Doi (2008) cited the isolation of cellulase producing bacterial strain from various natural resources is a crucial role for the industrial applications. However, the cellulase producing bacterial strains from extremophiles are the hot topic because these bacterial strains have the capacity to synthesize more quantity as well as thermostable than the terrestrial area. In the present work, the candidate strain Bacillus pumilis isolated from the earthworm gut among the 18 isolates; it was identified by biochemical tests along with 16 s rRNA gene sequencing. This is in accordance with the earlier report of Rastogi et al. (2010) who have reported the terrestrial hot spring is the chief zone for the isolation of cellulase producing bacterial strain due to the production of high amount of cellulase yield. Most of the previous literatures have documented the purification and characterization of CMCase from various bacterial isolates. Conversely, cellulase bacterial consortium is more complex and it is done by the synergistic action of enzymes (cellobiohydrolases, endo-glucanases and βglucosidases) for the complete breakdown.
In the present study, CMCase purification was carried out in gel filtration chromatography for increasing the enzyme activity. The fractions showed maximum CMCase activity of 1.480 IU/mL with 47.56% yield and specific activity as 1.450 U/mg proteins accounting for 6.62 fold increase in purification. Further purification of CMCase by ion exchange chromatography through DEAE-Cellulose column was carried out. The fractions showed maximum CMCase activity of 2.40 IU/mL with 38.58% yield and specific activity as 3.698 U/mg proteins accounting for 16.86 fold increase in purification. The present report is identical to the earlier report of Zhou et al. (2015) who postulated the purification of cellulase from the Bacillus sp. by using ammonium sulphate, DEAE- Sephadex A-25 column including gel filtration column. The CMCase from the candidate strain was found to be 7.9-fold as well as 65.41% final yield and the obtained specific activity was 3.77 U/mg. Sadhu et al. (2013) also supported that the 8.0 fold of purified cellulase from Bacillus sp. and the recorded specific activity was 68.1 U/mg protein.
In the present study, molecular weight determination of CMCase using SDS-PAGE analysis was 47 kDa. Several reports on the molecular weight of CMCase are available. The molecular mass of the CMCase isolated from the bacterial strains, CH43 and HR68 estimated through SDS-PAGE was 40 kDa (Mawadza et al., 2000). In another study, purified CMCase from B. pumilus was reported to have a molecular weight range of 30–65 kDa (Ariffin et al., 2006). Sadhu et al. (2013) reported that the molecular mass of endoglucanase is varied from bacterial strains and the obtained molecular weight as 97 kDa in Bacillus sp. isolated from cow dung.
Km is a measure of the apparent affinity of enzyme for its substrate. In the present study, the kinetic parameter was carried out in B. pumilus EWBCM1 for the concentrations ranges from 0.5 to 5 mg/mL of CMC as substrate. Kumar et al. (2018) reported that the registered Km and Vmax value of cellulase from Schizophyllum commune were 0.0909 mg/mL and 45.45 μmol/min mg respectively. The Km and Vmax of purified CMCase towards B. pumilus EWBCM1 were 1.688 mg/mL and 108.2 µm/min/mg. whereas, the Km and Vmax values registered from the endogluconase (EG) from Aspergillus awamori VTCC-F099 were 5.83 mg/mL and 333.33 U/mg protein, respectively (Nguyen and Quyen, 2010).
4.2 Effect of Temperature
In the present study, the CMCase from B. pumilus showed activity based on temperature range from 20 to 80 °C and the maximum activity of 100% was observed at 50 °C. The present study is similar to the earlier study of Endo et al. (2001) who studied the optimal activity of CMCase from an alkaline Bacillus sp. was registered at pH 10 and the finest temperature as 55 °C. Some of the earlier studies have reported that the CMCase from Bacillus sp. reveal an optimum activity from 40 to 60 °C (Christakopoulos et al., 1999). Earlier researchers have postulated the thermophilic activity of endoglucanase (Cel9P) is based on the presence of substrate from the newly isolated Paenibacillus sp. (Fu et al., 2010; Islam and Roy, 2019).
4.3 Effect of pH
Goel et al. (2019) reported that the characterization of cellulases from bacterial strain was optimum at pH 10. In the present study, the B. pumilus CMCase possessed a noteworthy relative activity ranges from pH 6 to 11, being maximum at pH 11 with the relative activity of 100% after which, at pH 12 the relative activity declined to 52.36 ± 0.97%. The present report is endorsed by the earlier report of Endo et al. (2001) who studied the optimal activity of pH was found to be at 11. Kim et al. (2005) also reported that the maximum activity of CMCase was exhibited at pH 12 in Baclillus sp. Johnvesly and Naik (2001) studied an alkaline pH ranges from 8 to 10 was more suitable for the growth as well as it enzyme activity in Bacillus sp.
4.4 Effect of Metal ions
Coniglio et al. (2016) reported that the metal ions could influence the enzyme reaction in metabolic pathway and the activity of enzyme was also encouraged in microbes. In this study, the effect of metal ions on CMCase revealed that the optimal activity was registered in 5 mM CaCl2 (127.60 ± 1.09%) supplemented medium than the other ions. This is in accordance to the previous study of Sankaralingam et al. (2018) who studied Ca+ ions has induced relative activity of cellulase than the other divalent ions in marine B. megaterium. Iqbal et al. (2011) also stated the presence of 1 mM concentration of Co2+ and Mn2+ were influenced the activity of cellulase by Bacillus sp.
4.5 Effect of Surfactants
The present study, the result of surfactants on CMCase activity inferred that the optimal activity was registered in % surfactants added medium. In contrary to the earlier report of Iqbal et al. (2011) who studied the cellulase activity was reduced with increasing concentration of SDS as well as by the time of exposure. Some of the earlier references have revealed the optimal activity of cellulase registered in the media supplemented with SDS, Tween 20, Triton X 100 in Bacillus sp. The increased activity of cellulase was recorded with increasing the concentrations of surfactants in microorganism because the surfactants demonstrated the capacity to change the surface property and also to reduce the irreversible inactivation of cellulase (Seki et al., 2015).
4.6 Effect of Commercial Detergents
Application of cellulase from microbial origin exhibited stable at wide range of pH along with temperature and these characteristic features are widely employed in various aoolication (Islam and Roy, 2019). Iqubal et al. (2011) stated that the prevention of pollution issues by using bacterial cellulase because their potential as well-matched with local detergents. Lima et al. (2005) also reported that the bacterial cellulase showed good stability with commercial detergents namely Ariel® and Minerva at 28 °C in pH 10.0. In the present study, the activity along with stability of bacterial cellulase possessed good results while testing with local detergents such as Rin®, Surf excel®, Power active®, Ponvandu®, Ujala Techno bright®, Jil® and Ariel®. Among them, best result was obtained when tested with the detergent Rin®. Data is in accordance to the earlier report of George et al. (2001) who studied the bacterial cellulase was incubated with local detergents showed residual activity of 85, 72 and 66% with the detergents Ariel®, Surf excel® and Henko® after 1 hr incubation.
4.7 Antibacterial Activity of Purified Enzyme
The purified enzyme was tested for the presence of antibacterial activity against five clinical isolates. The strains namely Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia and Shigella dysenteriae diameter of the inhibition zones for these bacterial isolates were 9, 13, 13, 15, 11 mm at 100 (µg/mL) for respective bacteria. The present study is similar to the earlier report of Sankaralinga et al. (2018) who stated the antimicrobial activity of purified enzyme from newly isolated marine strain Bacillus megaterium.
5 Conclusion
The purpose of the present work is to accentuate the values of bacterial cellulase from earthworm gut origin for increasing enzyme yields. The candidate organism has been able to produce thermostable cellulase for the development of bio-detergent in order to avoid the usage of unwanted chemicals. The CMCase was stable over a wide range of pH and temperature which are the important traits for the preparation of animal feeds.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2020/257) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ali, U.F., EI-Dein, H.S.S., 2008. Production and partial purification of cellulase complex by Aspergillus niger and A. nidulans grown on water hyacinth blend. J. Appl. Sci. Res. 4 (7), 875-891.
- Production and characterisation of cellulase by Bacillus pumilus EB3. Inter. J. Engine. Technol.. 2006;3(1):47-53.
- [Google Scholar]
- Purification and Mode of Action of an Alkali-Resistant Endo-1,4-β-glucanase from Bacillus pumilus. Arch. Biochem. Biophys.. 1999;364(1):61-66.
- [CrossRef] [Google Scholar]
- Screening of new secretory cellulases from different supernatants of white rot fungi from Misiones, Argentina. Mycol.. 2016;8(1):1-10.
- [CrossRef] [Google Scholar]
- Darabzadeh, N., Hamidi‐Esfahani, Z., Hejazi, P., 2018. Improvement of cellulase production and its characteristics by inducing mutation on Trichoderma reesei 2414 under solid state fermentation on rice by‐products. Appl. Food Biotechnol. 5 (1), 11–18. doi.org/10.22037/afb.v5i1.18651
- Cellulases of mesophilic microorganisms. Ann. New York Acad. Sci.. 2008;1125(1):267-279.
- [CrossRef] [Google Scholar]
- Extracellular α-amylase from Thermus filiformis Ork A2: purification and biochemical characterization. Extremophiles. 1998;2:23-32.
- [CrossRef] [Google Scholar]
- Endo, K., Hakamada, Y., Takizawa, S., Kubota, H., Sumitomo, N., Kobayashi, T., Ito, S., 2001. A novel alkaline endoglucanase from an alkaliphilic Bacillus isolate: enzymatic properties and nucleotide and deduced amino acid sequences. Appl. Microbiol. Biotechnol. 57, 109–16 doi: 10.1007/s002530100744
- Fu, X., Liu, P., Lin, L., Hong, Y., Huang, X., Meng, X., Liu, Z., 2010. A Novel endoglucanase (Cel9P) From a Marine Bacterium Paenibacillus sp. BME-14. Appl. Biochem. Biotechnol. 160, 1627–1636. doi.org/10.1007/s12010-009-8648-2
- Geels, F.P., Schippers, B., 1983. Reduction in yield depressions in high frequency potato cropping soil after seed tuber treatments with antagonistic fluorescent Pseudomonas spp. J. Phytopathol. 108, 207–214. doi.org/10.1111/j.1439-0434.1983.tb00580.x
- Studies on carboxymethyl cellulase produced by an alkalothermophilic actinomycete. Bioresour. Technol.. 2001;77(2):171-175.
- [CrossRef] [Google Scholar]
- Purification and characterization of cellulase from Pseudomonas sp. isolated from waste dumping site soil. J. Appl. Biotechnol. Bioeng.. 2019;6(3):118-124.
- [CrossRef] [Google Scholar]
- Effect of post- and pre-crosslinking of cotton fabrics on the efficiency of biofinishing with cellulase enzyme. Carbohydr. Polym.. 2009;78(4):953-960.
- [CrossRef] [Google Scholar]
- Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Adv. Biosci. Biotechnol.. 2011;2(3):149-156.
- [CrossRef] [Google Scholar]
- Isolation and characterization of cellulase-producing bacteria from sugar industry waste. Ame. J. BioSci.. 2019;7(1):16-24.
- [CrossRef] [Google Scholar]
- Studies on production of thermostable alkaline protease from thermophilic and alkaliphillic Bacillus sp JB-99 in a chemically defined medium. Process. Biochem.. 2001;37(2):139-144.
- [CrossRef] [Google Scholar]
- Purification and characterization of an alkaline cellulase from a newly isolated alkalophilic Bacillus sp. HSH-810. Biotechnol. Lett.. 2005;27:313-316.
- [CrossRef] [Google Scholar]
- Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express. 2018;8(1):173.
- [CrossRef] [Google Scholar]
- Cleavage of Structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680-685.
- [CrossRef] [Google Scholar]
- Li, X.-L., Yang, H.-J., Roy, B., Wang, D., Yue, W.-F., Jiang, L.-J., Park, E.-Y., Miao, J.-G., 2009. The most stirring technology in future: cellulose enzyme and biomass utilization. Afr. J. Biotechnol. 8 (11), 2418–2422. DOI: 10.5897/AJB09.201
- Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme Microbial. Technol.. 2005;37(2):272-277.
- [CrossRef] [Google Scholar]
- Lo, H.F., Lin, L.L., Chen, H.L., Hsu, W.H., Chang, C.T., 2001. Enzymatic properties of a SDS-resistant Bacillus sp. TS-23 a-amylase produced by recombinant E. coli. Process Biochem. 36 (8–9), 743–50. doi: 10.1016/S0032-9592(00)00273-9
- Substrate-induced production and secretion of cellulases by Clostridium acetobutylicum. Appl. Environ. Microbiol.. 2004;70(9):5238-5243.
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(265):1951.
- [Google Scholar]
- Isolation, screening and identification of cellulolytic bacteria from soil. Biotechnol. J. Inter.. 2020;24(1):1-8.
- [CrossRef] [Google Scholar]
- Purification and characterization of cellulases produced by two Bacillus strains. J. Biotechnol.. 2000;83(3):177-187.
- [CrossRef] [Google Scholar]
- Optimization of cellulase and chitinase enzymes production by plant growth promoting rhizobacteria. Novel Res. Microbiol. J.. 2020;4(1):641-652.
- [CrossRef] [Google Scholar]
- Mostafa, Y.S., Alamri, S.A., Hashem, M., Nafady, N.A., Abo-Elyousr, K.A.M., Mohamed, Z.A., 2020. Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation. Open Life Sci. 15, 185–197. doi.org/10.1515/biol-2020-0019
- Optimization and purification of cellulase produced by Penicillium decumbens and its application. Egypt. Pharmaceut. J.. 2019;18:391-402.
- [CrossRef] [Google Scholar]
- Optimizing culture conditions for the production of endo-β-1,4-glucanase by Aspergillus awamori strain Vietnam Type Culture Collection (VTCC)-F099. Afr. J. Biotechnol.. 2010;9(38):6337-6344.
- [Google Scholar]
- Isolation, purification and characterisation of an endoglucanase and β-glucosidase from an anaerobic sulphidogenic bioreactor. Enz. Microbial Technol.. 2007;40(4):637-644.
- [CrossRef] [Google Scholar]
- Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol.. 2010;101(22):8798-8806.
- [CrossRef] [Google Scholar]
- Sadhu, S., Saha, P., Sen, S.K., Mayilraj, S., Maiti, T.K., 2013. Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from Bacillus strain isolated from cow dung. Springer Plus 2, 10. doi.org/10.1186/2193-1801-2-10
- Screening and characterization of cellulase by Bacillus megaterium isolated from marine sediments and its antimicrobial activity. World Appl. Sci. J.. 2018;36(5):646-653.
- [CrossRef] [Google Scholar]
- Enhancement of cellulose degradation by cattle csaliva. PLoS One. 2015;10(9):e0138902
- [CrossRef] [Google Scholar]
- Soeka, Y.S., Sulistiani, 2019. Production and characterization of cellulase from the newly isolated Bacillus subtilis A8 on rice bran and corncob. IOP Conference Series Earth and Environmental Science 308, 012033. doi: 10.1088/1755-1315/308/1/012033
- [1] Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol.. 1972;26:3-27.
- [CrossRef] [Google Scholar]
- Methods for discovery and characterization of cellulolytic enzymes from insects. Insect Sci.. 2010;17:184-198.
- [CrossRef] [Google Scholar]
- Construction and expression of two-copy engineered yeast of feruloyl esterase. Electron. J. Biotechnol.. 2015;18(5):338-342.
- [CrossRef] [Google Scholar]







