Translate this page into:
Protective effects of rutin and Moringa oleifera extract against monosodium glutamate-induced hepatotoxicity in rats
*Corresponding author: E-mail address: sherifrabea@science.helwan.edu.eg (S. Rabea Mohamed).
-
Received: ,
Accepted: ,
Abstract
Monosodium glutamate (MSG) is a common seasoning and flavor booster found in nearly every processed product. MSG induces oxidative stress along with degenerative changes in the hepatic and renal cells. This study aims to evaluate the protective effects of rutin(RT) and Moringa oleifera(MOE) extract against MSG-induced hepatotoxicity in rats. Forty adult male albino rats were randomly divided into four groups. The control group (CNT) received no treatment. The second group received MSG orally (60 mg/kg/day) for 30 days. The third and fourth groups were pretreated with RT (150 mg/kg/day) and MOE (500 mg/kg/day), respectively, for 30 days, followed by oral administration of MSG (60 mg/kg/day) for an additional 30 days, two hours after the RT or MOE dosage. The study assesses whether RT and MOE can reduce liver damage by leveraging their antioxidant, anti-inflammatory, and anti-apoptotic properties. This is evaluated through various biochemicals, histological, and molecular markers associated with oxidative stress, inflammation, and apoptosis in liver tissues. The results indicated that MSG induces organ dysfunction (AST and ALT levels), oxidative stress (GSH, SOD, CAT, GPx, GR, and NO), inflammation (IL-1β and TNF-α), histological alterations (H&E, PAS, Sirius red COX-2, and iNOS stains), and ultra-structural abnormalities of hepatocytes. The authors found that RT and MOE significantly mitigate MSG-induced liver damage in rats. Treatment with RT and MOE reduced oxidative stress, inflammatory responses, and apoptosis markers while enhancing antioxidant defenses, suggesting that both RT and MOE have therapeutic potential in preventing MSG-related liver toxicity due to their strong antioxidant and anti-inflammatory effects.
Keywords
Immunohistochemical studies
Inflammation
Liver
Monosodium glutamate
Moringa olifera
Oxidative stress
Rutin
1. Introduction
The liver is an important organ that serves various functions, including supplying glucose, plasma proteins, and bile (Risi et al., 2023). Several hazardous compounds may have an effect on the liver, and alternative treatments that target the pathophysiological processes causing liver damage must be researched. Free radical damage to oxidative components in cells, such as lipids, proteins, and nucleic acids, causes a discrepancy between the generation of reactive oxygen species (ROS) and the synthesis of antioxidants (Halliwell, 1994). Natural antioxidants enhance the body’s function amid stressful situations. Natural medicines have been utilized for centuries to treat a variety of ailments (Alok et al., 2014). Industrial toxins and food additives are both hazardous synthetic pollutants, with some food additives intended to maintain or improve the flavor of food. Monosodium glutamate (MSG) is the most common flavoring component and food additive (Chakraborty, 2019). When consumed in excess, MSG can lead to a variety of liver problems (Garattini, 2000). Glutamate is an excitatory neurotransmitter in the brain that promotes rapid synaptic transmission (Zhou and Danbolt, 2014). The liver processes glutamate, which is ultimately excreted by the kidneys (Sharma, 2015). In the GI tract, glutamic acid is turned into alanine, which is then transformed into lactate by the liver. It is absorbed from the intestine via active transport mechanisms associated with amino acids (Garattini, 2000). MSG enhances food flavor, stimulates the hunger center, and causes weight gain (Zhou and Danbolt, 2014). When consumed in excess, MSG harms humans and other animals while also improving flavor and appetite (Halliwell, 1994). MSG-treated rats have demonstrated gonadal issues, brain damage, stomach cancer, and hypothalamic depletion of specific neurotransmitters (Alok et al., 2014). Excess MSG consumption has previously been related to harmful effects on the liver and kidneys (Chakraborty, 2019). This is because MSG causes oxidative stress and degenerative changes in hepatic and renal cells (Garattini, 2000). Certain antioxidants and anti-inflammatory medicinal plants, or their components, can mitigate the effects of MSG (Hajihasani et al., 2020).
RT (3-rhamnosyl-glucosyl quercetin) is a flavonoid glycoside that can be widely found in some vegetables and fruits (Kim et al., 2013). It has been established that rutin’s anti-inflammatory and antioxidant properties protect vital organs from the detrimental effects of ROS (Liang et al., 2018). Rutin, in particular, binds to iron ions and prevents the Fenton reaction caused by hydrogen peroxide, thus inhibiting one of the important mechanisms that generate reactive free radicals (Zargar et al., 2017). Rutin prevents isoniazid-induced liver damage by reducing oxidative stress (Abdel-Ghaffar et al., 2019). Buckwheat seeds, tomatoes, onions, apples, tea, passion flowers, wine, and leaves are high in rutin (Aksu et al., 2017). Rutin has a variety of pharmacological properties, including the capacity to scavenge superoxide radicals as well as anti-allergic, antibacterial, anti-ulcer, anti-inflammatory, anticarcinogenic, anti-diabetic, and antimutagenic properties (Nafees et al., 2015). Rutin was shown to reduce methotrexate-induced hepatotoxicity in a rat model to (Erdogan et al., 2015).
Moringa oleifera, a monogeneric Moringaceae plant, appears to be indigenous to India’s tropical woods and is cold- and drought-tolerant. It is well-known for its numerous uses as a food additive and nutritional supplement (Anwar et al., 2007). Moringa oleifera is ideal for food applications due to its high nutritional content, which includes essential amino acids, vitamins, minerals, and oleic acids. MOE is renowned for its medicinal properties, which include the treatment of numerous immune system illnesses, as well as antioxidant, anti-diabetic, and tumor-fighting properties (Dhakad et al., 2019). Moringa oleifera’s bioactive compounds have been discovered in practically every portion of the plant (Liang et al., 2019). The specific ingredients recovered from Moringa oleifera include flavonoids, glucosinolate and isothiocyanate, phenolic acid (every component located inside foliage), sterols, alkaloids (found within leaves, seeds, and roots), and terpenes (found distributed between pods) (Baldisserotto et al., 2018). The components found in leaves and seeds are the most usually stated. Alkaloids and phenols are more frequent in the leaves, while flavonoids, saponins, and anthocyanins are more concentrated in the seeds (Gupta et al., 2018). Several Moringa oleifera components have therapeutic properties, including liver-protective effects that inhibit cholesterol synthesis and inflammation, oxidation, cancer, diuretics, and bacterial infection (Abd Eldaim et al., 2017).
This study aims to evaluate the protective effects of rutin and Moringa oleifera extract against MSG-induced hepatotoxicity in rats.
2. Materials and methods
2.1 Materials
2.1.1 Drugs and chemicals
Pure MSG (99%) was sourced from Egypt’s Morgan Pharmaceutical Company. Rutin, 95% pure was sourced from Sigma Company. 10% formalin, xylazine, and ketamine were from the chemical company El-Gomhoryia in Cairo, Egypt. Kits for oxidative stress indicators were from Biodiagnostics in Cairo, Egypt. Scientific Thermo Fisher, USA, provided enzyme-linked immunosorbent test (ELISA) kits for inflammatory markers. Antibody against Cox-2 were from Santa Cruz Biotechnology, Inc. Anti-iNOS were from Thermo Scientific, Walthman, Massachusetts, USA. Additional common chemicals and regular stains of analytical quality were produced by local companies in Egypt.
2.1.2 Preparation of moringa oleifera leaf extract
Fresh Moringa oleifera leaves gathered from the Helwan governorate in the summer were recognized and validated at Egypt’s National Research Center in Giza. The fallen leaves were pulverized after drying and cleaning. Leaves from Moringa oleifera were extracted in water. Forty grams of dried powder, and 100 milliliters of hot water were combined and stirred frequently for an hour. Whatman paper No. 1 was used to filtering in order to acquire the extract. A rotary evaporator at 55°C was used to concentrate the filtrate to 8% of its original volume (Shah et al., 2015).
2.1.3 Phytochemical analysis of moringa oleifera leaf extract
The phenolic composites were examined by high-performance liquid chromatography (HPLC) manufactured by the Waters Corporation in Massachusetts, USA, utilizing a Waters 2695 Alliance instrument (Waters Inc., Milford, CT, USA) connected with an SPD-40V HPLC/UHPLC UV-Vis detector from Shimadzu (Mizzi et al., 2020).
2.2 Experimental design and treatment schedule
Forty mature male albino rats weighing between 120 and 150 grams were imported from the Medical Ain-Shams Research Institute in Cairo, Egypt. Under veterinary guidance, the experiment complied with the regulations of Ain-Shams Research Institute’s animal housing. The experimental methodology was conducted in line with the accepted ethical research requirements of Ain Shams University, and the Experimental Animal Research Unit’s code number is [RE (189)22].
Rats were housed in standard settings, which included feeding, a 12-hour light-dark cycle, 24±2oC temperature, and 50±10% humidity. Following an acclimatization period of seven days, the rats were divided into four equal groups (n = 6);
Group 1: The Control (CNT) group was not given any medication.
Group 2: The MSG group was given a daily oral dosage of 60 mg/kg of MSG (99% pure, Morgan Chemical Industry, Egypt) for 30 days (Hamza and Al-Harbi, 2014).
Group 3: RT+MSG group was administered 150 mg/kg of rutin orally followed by rutin after two hours for each of the 30 days (Qu et al., 2018).
Group (4): The MOE+MSG group was orally administered 500 mg/kg of Moringa leaf extract (MOE) and MSG after two hours daily for 30 days (Sadek et al., 2017).
2.3 Collection of samples for biochemical analyses
The rats were killed by intraperitoneal injections of 10 mg of xylazine and 90–100 mg of ketamine/kg-1 once the plan was completed. Using microcapillary heparinized tubes, blood samples were taken from each rat’s retro-orbital plexus after a 12-hour fast. The serum was then extracted from the blood samples by centrifuging them for 15 minutes at 3,000 rpm. Serum was stored at -20°C for hepatic function-related biochemical analysis. The liver was carefully taken out of each group and divided in half. The first part was used to create tissue homogenates (10% w/v), which were created by centrifuging at 3000 × g for 10 minutes at 4°C after adding a specific amount of tissue to an ice-cold (pH 7.4) 50 mM Tris-HCl solution. The final supernatant was kept at -20°C for biochemical examination, which comprised specific oxidative stress indicators, antioxidant biomarkers, inflammatory markers, and apoptotic biomarkers. The second half was treated with 10% formalin with a neutral buffer for use in histological, histochemical, and immunohistochemical assessments, while the third part was fixed in glutaraldehyde (5%) for use in transmission electron microscopy (TEM) of hepatocyte ultrastructure.
2.4 Biochemical analysis
2.4.1 Oxidative stress markers
The hepatic glutathione (GSH) was determined by the colometric method utilizing Elaman’s reagent, a non-enzymatic antioxidant marker (Ellman, 1959). The potential of superoxide dismutase (SOD) to block diminution within the nitroblue tetrazolium stain was used to determine SOD activity (Sun et al., 1988). Furthermore, catalase, or CAT, activity was calculated utilizing the rate of H2O2 degradation (Aebi, 1984). The rate of oxidation of NADPH at 340 nanometers and the rate at which NADPH decreases in the presence of glutathione were employed to evaluate GSH peroxidase (GPx) plus GSH reductase (GR) in reference to Paglia and Valentine (1967) and Factor et al. (1998), respectively. Employing a commercial kit, the liver tissue’s nitric oxide (NO) values were measured (Biodiagnostics, Cairo, Egypt). Using a process that turns NO into nitrous acid, the kit creates an azo dye by combining it with N-(1-naphthyl) ethylenediamine plus sulfanilamide. Next, spectrophotometric measurement of the dye’s absorbance was performed at 540 nanometers (Bryan and Grisham, 2007). Malondialdehyde was utilized to measure lipid peroxidation (MDA) according to Ohkawa et al. (1979).
2.4.2 Inflammatory markers
An enzyme-linked immunosorbent assay (ELISA) kit was utilized to quantify tumor necrosis factor-α along with interleukin-1β, coming from Scientific Thermo Fisher, USA, in accordance with the manufacturer’s instructions for IL-1β (Cat. number. BMS6002) and TNF-α (Cat. number. BMS607-3).
2.4.3 Apoptotic markers
Pro-apoptotic marker Bax’s values were measured utilizing a commercially available ELISA kit (rat Bax; Catalogue No. E4513) obtained from BioVision, Inc. Bcl-2, an antiapoptotic marker, was measured utilizing commercial ELISA kits (rat Bcl-2; catalog no. CSB-E08854r) that were acquired from Cusabio. Using ELISA kits from MyBioSource (San Diego, CA, USA), Caspase-3 (rat Casp-3; Catalogue No. MBS261814) was tested in the hepatic homogenate; all estimates were made in compliance with the manufacturer’s procedure.
2.4.4 Liver function markers
Utilizing kits made by the Biodiagnostic Company, Giza, Egypt, an enzymatic colorimetric endpoint approach was used to quantify the serum activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (Reitman and Frankel, 1957). The Biodiagnostic Company, located in Giza, Egypt, created kits for the colorimetric end point method of determining serum total bilirubin levels (Malloy and Evelyn, 1937).
2.5 Histopathological examination
After being fixed in formalin, the samples were dried in increasing ethanol grades after being cleansed in xylene; paraffin wax was used to encase it. A number of histological stains were applied to cut and stain paraffin 5 μm thick sections:
-
1)
For an officer histological investigation, consider hematoxylin and eosin (Slaoui and Fiette, 2011).
-
2)
With regard to PAS-positive (PAS+ve) materials, the periodic acid-Schiff’s (PAS) approach was utilized (Lai and Lü, 2012).
-
3)
Sirius red stain for staining of collagen fibers is utilized to evaluate fibrosis degree (Dapson et al., 2011).
-
4)
COX-2 and iNOS immunostains to check liver tissue for COX-2 and iNOS positivity.
COX-2 was detected in paraffin-embedded liver slices using specific antibodies, including an avidin-biotin complex immunoperoxidase method (Santa-Cruz Biotechnology) (Mohamed et al., 2022). The sections were treated with 3% hydrogen peroxide to suppress the activity of the endogenous peroxidase enzyme. The primary goat antiserum was added and incubated at room temperature for two hours to detect COX-2 (1:150 dilution) after blocking with 10% non-immunized goat serum. Goat serum that had not been vaccinated was used as a negative control, and primary antibodies were excluded. The avidin-biotin complex and horseradish peroxidase (Dako A/S, Glostrup, Denmark) were used after washing the biotinylated secondary antibodies. In order to detect peroxidase activity, the sections were counterstained with hematoxylin after adding diaminobenzidine.
Immunohistochemical staining enables identification of iNOS antigen utilizing the avidine-biotin peroxidase complex (ABC) approach (Abd El Fattah and Ismail, 2015): employing a rabbit polyclonal antibody called anti-iNOS (Thermo Scientific, Walthman, Massachusetts, USA). The following is how the ABC approach was applied: After using the primary antibody for 60 minutes, sections were treated with the secondary antibody, which is a biotinylated antiserum to rabbit/mouse IgGs. The reaction was seen using a chromogen (3,3-diaminobenzidine tetrahydrochloride). After staining, the paraffin slides were investigated under the light microscope, and a Zeiss camera (LEICA DM4 P, Wetzlar, Germany) was used to take pictures.
Using an image analyzer and image analysis software (Imagej version 1.46, Rasband, Bethesda, Maryland, USA), the color area percent of Sirius red stained collagen fibers, PAS positive substances, COX-2 positive immune reaction, and iNOS positive immune reaction were determined in five different fields from five distinct sections of differing rats in each group, at × 400 magnifications. The data was also statistically analyzed.
2.6 Transmission electron microscopy
All groups provided small (1 mm3) liver tissue samples for both ultrathin and semithin sectioning. The specimens underwent a 24-hour immersion in 2.5% phosphate-buffered gluteraldehyde (pH 7.3), followed by a 2-hour postfixation in 1% OSO4, and a dehydration process using varying amounts of alcohol. The specimens were submerged in propylene oxide and then placed in epoxy resin. 1 micron thick sections were stained with 0.5% toluidine blue and then examined under a light microscope. Ultrathin scale sections (Thickness of 50 nm) were cut, put on nickel grids, and colored with both lead citrate and uranyl acetate (Helal et al., 2013). The Regional Mycology and Biotechnology Center at Al-Azhar University in Cairo, Egypt, used TEM (JEOL1010 EX II, Japan) to examine and take pictures of specimens.
2.7 Statistical analysis
The mean ± SD of the data was reported. GraphPad Prism (version 8, GraphPad Software, Inc., USA) was used for statistical analysis of the obtained results. To assess and identify differences between the groups, a one-way ANOVA test was also performed, which was followed by Tukey’s post hoc analysis. P<0.05 was chosen as the threshold for statistically significant differences.
3. Result
3.1 Phytochemical analysis of MOE:
Fig. 1 displays components of MOE from an HPLC study. The retention duration and percentage areas are displayed. Furthermore, the chromatogram shows large peaks such as glycerine, gallic acid, hexadecanoic acid, and oleic acid, but no minor peaks are seen.
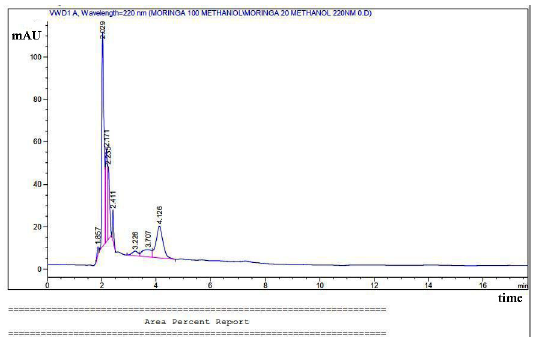
- HPLC components of MOE as glycerine, gallic acid, hexadecanoic acid, and oleic acid. HPLC: High-performance liquid chromatography, MOE: Moringa oleifera extract.
Overall results showed MSG administration led to significant liver dysfunction, oxidative stress, inflammation, histological changes, and ultrastructural abnormalities. Both RT and MOE treatments significantly reduced these MSG-induced alterations. Biochemical analysis showed decreased levels of liver enzymes (ALT, AST), reduced oxidative stress markers (MDA), and increased antioxidant activity (SOD, CAT). Histological and immunohistochemical analyses revealed reduced inflammation and improved liver structure, while electron microscopy confirmed the protective effects at the cellular level.
3.2 Oxidative stress and antioxidant enzyme activity markers:
When compared to the CNT group, the MSG group’s level of lipid peroxidation products, as determined by MDA levels, was considerably higher (P<0.05). The MDA levels in the RT+MSG and MOE+MSG groups were significantly lower (P<0.05) than those in the MSG group (Fig. 2a). Furthermore, there was a noteworthy (P<0.05) increase in NO levels in the MSG group relative to the CNT group. In contrast to the MSG group, the RT+MSG and MOE+MSG groups showed a significant (P<0.05) drop in NO levels (Fig. 2b). Additionally, GSH levels in the RT+MSG and MOE+MSG groups were considerably greater (P<0.05) than in the MSG group, although lower (P<0.05) GSH levels were found in the MSG group compared to the CNT group (Fig. 2c). The significant suppression of GPx, GR, CAT, and SOD activities (P<0.05) in the MSG group relative to the CNT group showed the cells’ antioxidant capacity. The co-administration of Rutin and MOE rose (P<0.05) the liver’s levels of the enzymes GPx, GR, CAT, and SOD in comparison to the MSG-only treatment (Fig. 2d-g) respectively.
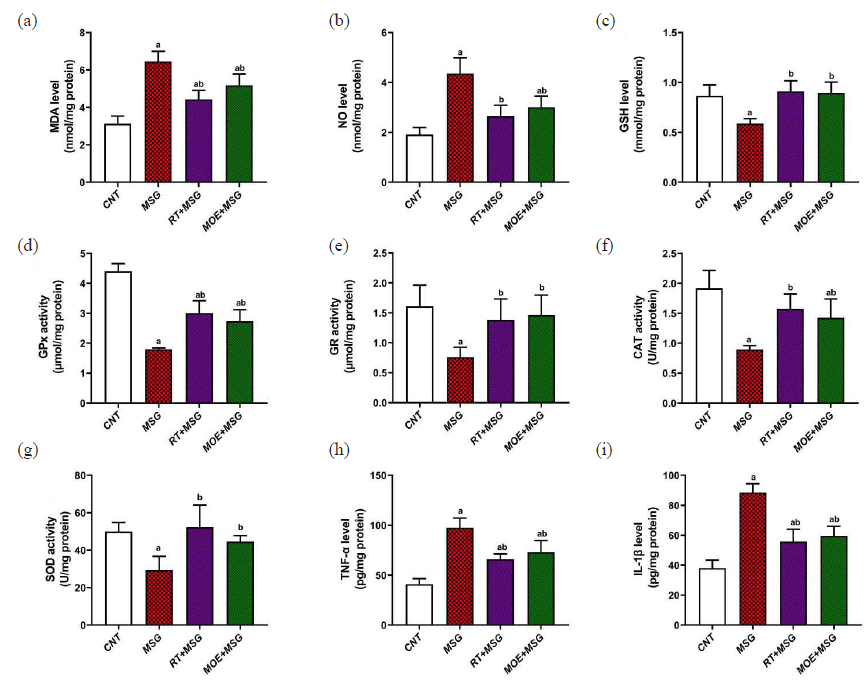
- The impact of RT and MOE on oxidative stress (a-g) and inflammatory markers (h-i) in male rats’ hepatic tissues caused by MSG. (a) MDA level, (b) NO level, (c) GSH level, (d) GPx activity, (e) GR activity, (f) CAT activity, (g) SOD activity, (h) TNF-α level, (i) IL-1β level. Mean ± SD (n = 5) is represented by each value. a P < 0.05 against the CNT rats and b P < 0.05 against the MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, MDA: Malondialdehyde, NO: Nitric oxide, GSH: Glutathione, GP: Glutathione peroxidase, GR: Glutathione reductase, CAT: Catalase, SOD: Superoxide dismutase, SD: Standard deviation. CNT: Control, TNF: Tumor necrosis factor, IL: Interleukin
3.3 Inflammatory markers:
MSG treatment brings about significant increase (P<0.05) in inflammatory reactions of TNF-α plus IL-1β in hepatic tissues relative to the CNT group. Otherwise, the co-administration of Rutin and MOE showed significantly reduced (P<0.05) TNF-α plus IL-1β values tested in relation to the MSG group (Fig. 2h and i).
3.4 Apoptosis markers:
MSG significantly (P<0.05) reduced the anti-apoptotic marker Bcl-2 in liver tissues as compared to the CNT group. However, Bcl-2 levels in the RT+MSG and MOE+MSG groups were significantly higher (P<0.05) than in the MSG group (Fig. 3a). In comparison to the CNT group, the MSG group’s hepatic tissues showed significantly higher levels (P<0.05) of the pro-apoptotic marker Bax (Fig. 3b). In addition, the Bax levels in the RT+MSG and MOE+MSG groups were significantly lower (P<0.05) than in the MSG group. Additionally, compared to the CNT group, MSG demonstrated a significant increase (P<0.05) in caspase-3 levels. However, compared to the MSG group, the RT+MSG and MOE+MSG groups showed a substantial perceptible drop (P<0.05) in caspase-3 levels (Fig. 3c). These results demonstrated that rutin and MOE had an anti-apoptotic effect in rats given MSG.
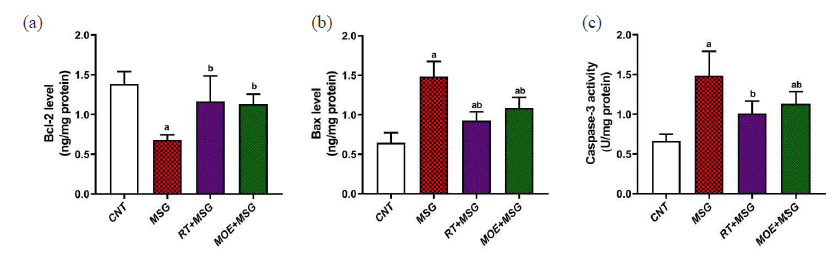
- The impact of RT and MOE on MSG-induced apoptosis in male rats’ hepatic tissues. (a) Bcl-2 level, (b) Bax level, and (c) Caspase-3 activity. Mean ± SD (n = 5) is represented by each value. a P < 0.05 against the CNT rats and b P < 0.05 against the MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, CNT: Control group, SD: Standard deviation.
3.5 Liver function markers:
There was a marked increase (P<0.05) in readings of hepatic functions including ALT, AST, and total bilirubin levels among the MSG group in contrast to the CNT group. However, the RT+MSG and MOE+MSG groups showed a significant decrease (P<0.05) in the hepatic function readings in contrast to the MSG group (Fig. 4).
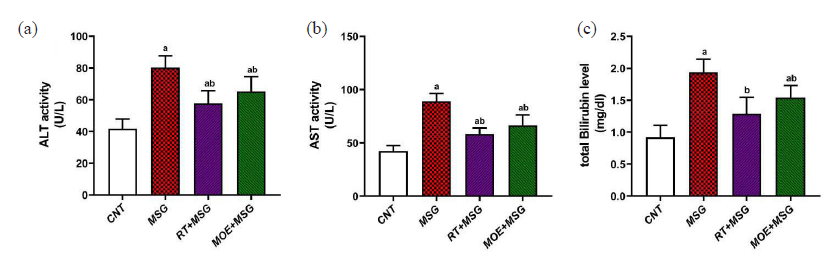
- The impact of RT and MOE on MSG-induced abnormalities in liver function in rat serum. (a) ALT activity, (b) AST activity, and (c) total bilirubin level. The values represent the mean ± SD (n = 5). a P < 0.05 against the CNT rats and b P < 0.05 against the MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, CNT: Control group, SD: Standard deviation.
Table 1 outlines the role of RT and MOE on biochemical analyses including oxidative stress, inflammation, apoptosis, and liver function in MSG-intoxicated rats.
| Parameters | CNT | MSG | RT+MSG | MOE+MSG |
|---|---|---|---|---|
| MDA level (nmol/mg protein) | 3.124±0.407 | 6.444±0.550a | 4.435±0.474ab | 5.184±0.595ab |
| NO level (nmol/mg protein) | 1.916±0.277 | 4.356±0.631a | 2.647±0.434b | 3.009±0.441ab |
| GSH level (mmol/mg protein) | 0.866±0.108 | 0.588±0.050a | 0.910±0.105b | 0.894±0.109b |
| GPx activity (µmol/mg protein) | 4.398±0.261 | 1.797±0.044a | 3.003±0.412ab | 2.738±0.377ab |
| GR activity (µmol/mg protein) | 1.611±0.352 | 0.758±0.165a | 1.382±0.349b | 1.460±0.335b |
| CAT activity (U/mg protein) | 1.918±0.295 | 0.895±0.066a | 1.574±0.245b | 1.424±0.314ab |
| SOD activity (U/mg protein) | 49.941±4.839 | 29.384±7.358a | 52.283±11.831b | 44.573±3.285b |
| TNF-α level (pg/mg protein) | 40.901±5.618 | 97.636±9.573a | 65.963±5.194ab | 72.946±11.750ab |
| IL-1β level (pg/mg protein) | 38.119±5.250 | 88.501±5.864a | 55.745±8.266ab | 59.555±6.398ab |
| Bcl-2 level (ng/mg protein) | 1.385±0.154 | 0.680±0.064a | 1.165±0.319b | 1.130±0.126b |
| Bax level (ng/mg protein) | 0.646±0.127 | 1.481±0.194a | 0.924±0.115ab | 1.084±0.136ab |
| Caspase-3 activity (U/mg protein) | 0.662±0.088 | 1.485±0.306a | 1.007±0.159b | 1.131±0.153ab |
| ALT activity (U/L) | 41.792±6.094 | 80.360±7.226a | 57.720±8.043ab | 65.268±9.234ab |
| AST activity (U/L) | 42.322±5.242 | 89.244±7.047a | 58.392±5.587ab | 66.364±9.796ab |
| Total Bilirubin level (mg/dl) | 0.921±0.186 | 1.936±0.208a | 1.289±0.258b | 1.542±0.190ab |
The values represent the mean ± SD (n = 7). a P < 0.05 vs. CNT rats, b P < 0.05 vs. MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, CNT: Control group, MDA: Malondialdehyde, NO: Nitric oxide, GSH: Glutathione, GPx: Glutathione peroxidase, GR: Glutathione reductase, CAT: Catalase, SOD: Superoxide dismutase ALT: Alanine aminotransferase, AST: aspartate aminotransferase, SD: Standard deviation. TNF: Tumor Necrosis Factor.
3.6 Liver histopathology:
The CNT group’s liver sections displayed the typical histoarchitecture of the liver, with flat Kupffer cell-lined sinusoidal gaps between plates of hepatocytes extending from the central vein (Fig. 5a). Severe histopathological alterations were observed in the MSG group, including confined inflammation, dilated congested portal veins, marked dilatation of the hepatic sinusoids, growth in the bile duct walls, thick portal arteries, and Kupffer cell proliferation. In addition, a large number of tiny, broken-up pyknotic nuclei and apoptotic cells were seen (Fig. 5b). The aforementioned modifications in the MSG group were lessened in the RT+MSG and MOE+MSG groups (Fig. 5c and d).
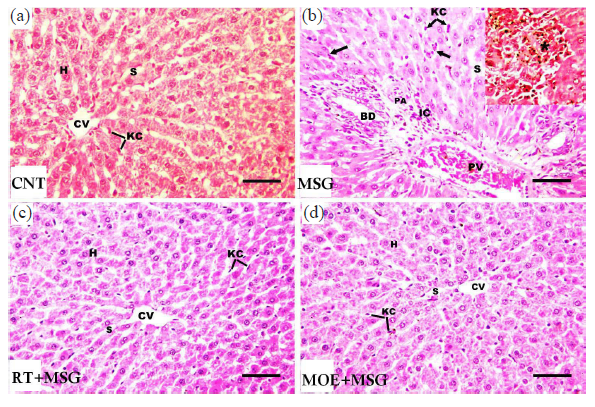
- Light photomicrographs of hepatic tissues from tissues from rats of the study groups. (a) The CNT group revealed the typical structure of the liver, with hepatocyte (H) plates extending from the central vein (CV), separated with sinusoidal spaces (S) that lined with flat Kupffer cells (KC). (b) MSG group showed marked dilatation of hepatic sinusoids (S), Kupffer cell (KC) proliferation, focal inflammation (star), dilated congested portal veins (PV), infiltration of inflammatory cells (IC) in the vicinity of the portal areas, large portal arteries (PA), and proliferation in the bile duct (BD) walls. In addition, apoptotic hepatocytes and several tiny, broken pyknotic nuclei were seen (black arrows). (c) The RT+MSG-treated group ameliorated the above changes. (H), (CV), (S), and (KC) are seen as normal. (d) The MOE+MSG-treated group showed no visible lesions. (H), (CV), (S), (KC) are clearly seen. RT: Resveratrol treatment, MSG: Monosodium glutamate, MOE: Morin oil extract, H&E: Hematoxylin and eosin. (H&E stain, scale bar = 50 µm) (40x magnification).
3.7 Collagen deposition and glycoprotein content in liver:
Masson trichrome staining of CNT hepatic tissue revealed normal collagen fiber content and distribution surrounding the central vein and hepatic parenchyma (Fig. 6). In addition, the MSG group demonstrated a considerable increase (P<0.05) of dense collagen, especially around the portal vein and in the portal area (Figs. 6 and 7). RT+MSG and MOE+MSG groups demonstrated a considerable decrease (P<0.05) of collagen amount and distribution in contrast to the MSG group (Figs. 6 and 7).
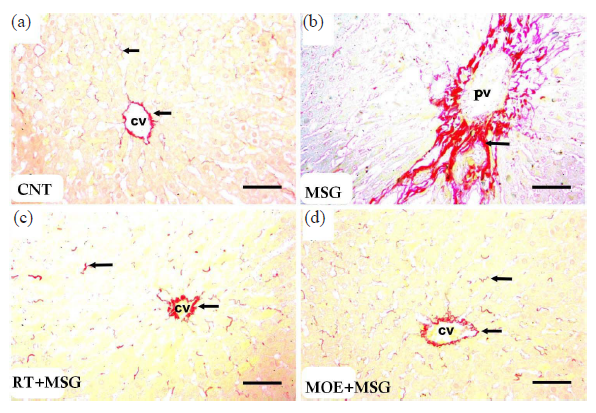
- Light photomicrographs of collagen fibers in hepatic tissues from tissues from rats of the study groups. (a) The CNT group showed normal collagen amount and distribution around the central vein (CV) and in liver parenchyma (arrows). (b) MSG group showed increased content of dense collagen, especially around the portal vein (PV) alongside the portal area (arrow). (c) RT+MSG-treated group showed normal collagen amount and distribution around the central vein (CV) and in hepatic parenchyma (arrows). (d) MOE+MSG-treated group showed normal collagen amount and distribution surrounding the central vein (CV) as well as its liver parenchyma (arrows). RT: Resveratrol treatment, MSG: Monosodium glutamate, MOE: Morin oil extract. (Sirius red stain, scale bar = 50 µm) (40x magnification).
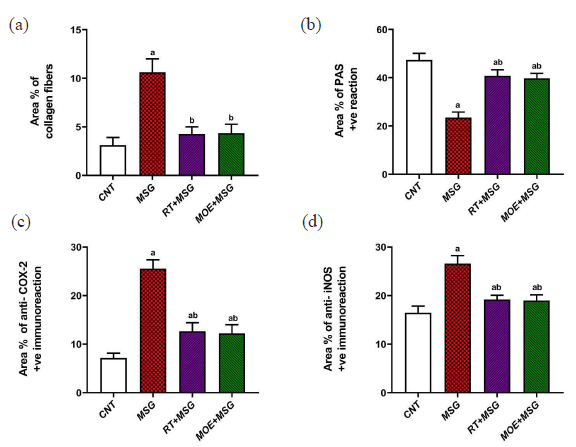
- Area percentage of some parameters in hepatic tissue among the study groups: (a) collagen fiber content, (b) PAS +ve reaction, (c) anti-COX-2 +ve expression, and (d) anti-iNOS +ve expression. Each bar value represents mean ± SD (n = 5). a P < 0.05 versus the CNT rats, b P < 0.05 versus the MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, CNT: Control group, PAS: Periodic acid schiff, iNOS: Inducible nitric oxide synthase, COX-2:Cyclooxygenase-2 and SD: SD: Standard deviation.
PAS-stained liver from the CNT group showed normal glycoprotein content in hepatic tissues (Fig. 8), and while in the MSG group, there was a considerable drop (P<0.05) of glycoprotein content in many hepatocytes contrasted to the CNT group (Figs. 7 and 8). RT+MSG and MOE+MSG groups revealed a notable increase (P<0.05) of Pas-positive material contrasted to the MSG group (Figs. 7 and 8).
- Light photomicrographs of PAS-positive material (glycogen) in hepatic tissues from rats of the study groups. (a) The CNT group displayed typical PAS-positive cytoplasmic material. (b) In several hepatocytes, the MSG group displayed reduced PAS+ve materials. (c) The group treated with RT+MSG exhibited enhanced PAS-positive material. (d) MOE+MSG-treated group showed almost restoration of the PAS+ve materials (PAS, scale bar = 50 µm). CNT: Control group, MSG: Monosodium glutamate, RT: Resveratrol treatment, MOE: Morin oil extract, PAS: Periodic acid-schiff staining. (40x magnification).
3.8 COX-2 & iNOS immunohistochemistry:
Immunohistochemical observations of COX-2 and iNOS in the liver of the CNT group showed a mild reaction limited to hepatocytes surrounding the sinusoidal lining plus the central vein (Fig. 9 and 10, respectively). While the MSG group showed extensive COX-2 and iNOS immunostained hepatocytes, particularly close to the central vein and around the sinusoidal lining (Fig. 9 and Fig. 10) respectively. RT+MSG and MOE+MSG groups showed few COX-2 and iNOS immunopositive hepatocytes interspersed with numerous unstained normal cells (Figs. 9 and 10), respectively. Area percent of anti-COX-2 and anti-iNOS was markedly elevated (P<0.05) in the MSG group compared to the CNT group. But after treatment with RT and MOE, the area percent of COX-2 and iNOS immuno-positive hepatocytes decreased significantly (P<0.05) (Fig. 7), respectively. Table 2 demonstrated the impact of RT and MOE on the area% of collagen fibers, PAS positive reaction, anti-COX-2 positive immunoreaction, and anti-iNOS positive immunoreaction in MSG-intoxicated rats.
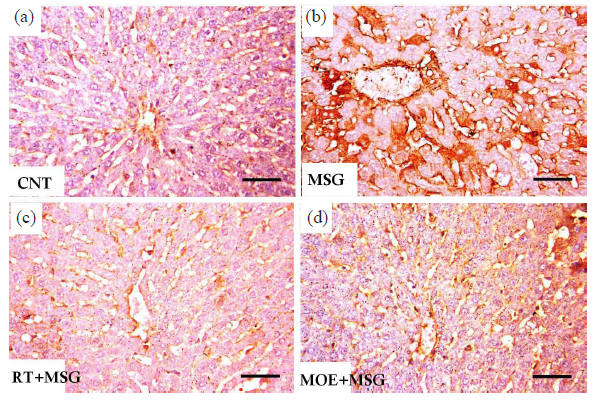
- Light photomicrographs of anti-COX-2 immunostained liver of rats from the study groups. (a) The CNT group showed minimal tissue immunoreactivity limited to hepatocytes in the vicinity of the sinusoidal lining and central vein. (b) The MSG group showed extensive immunostained hepatocytes, especially around the sinusoidal border and the central vein. (c) The RT+MSG-treated group revealed several unstained normal cells mixed with a small number of immunopositive hepatocytes. (d) The MOE+MSG-treated group revealed a significant drop in the quantity and density of immunostained cells. CNT: Control group, MSG: Monosodium glutamate, RT: Resveratrol treatment, MOE: Morin oil extract, COX-2: Cyclooxygenase-2, Anti-COX-2 Immunostain: Anti-Cyclooxygenase-2 immunohistochemistry staining. (Anti-COX-2 immunostain, scale bar = 50 µm) (40x magnification).
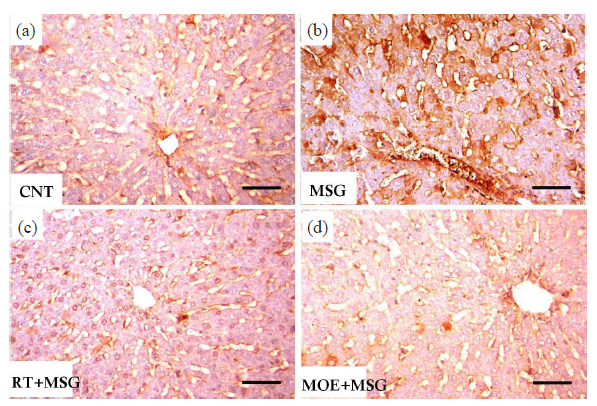
- Light photomicrographs of anti-iNOS immunostained liver of rats from the study groups. (a) The CNT group showed minimal tissue immunoreactivity limited to hepatocytes in the vicinity of the central vein alongside the sinusoidal lining. (b) The MSG group showed extensive immunostained hepatocytes, especially near the central vein and around the sinusoidal lining. (c) The RT+MSG-treated group revealed several unstained normal cells mixed with a small number of immunopositive hepatocytes. (d) The MOE+MSG-treated group revealed a significant drop in the quantity and density of immunostained cells. CNT: Control group, MSG: Monosodium glutamate, RT: Resveratrol treatment, MOE: Morin oil extract, iNOS: Inducible nitric oxide synthase, Hepatocytes: Liver Cells, Anti-iNOS Immunostain: Anti-inducible nitric oxide synthase immunohistochemistry staining. (anti-iNOS immunostain, scale bar = 50µm) (40x magnification).
| Parameters | CNT | MSG | RT+MSG | MOE+MSG |
|---|---|---|---|---|
| Area % of collagen fibers | 3.128±0.771 | 10.612±1.377a | 4.260±0.741b | 4.356±0.912b |
| Area % of PAS + ve reaction | 47.364±2.708 | 23.478±2.324a | 40.778±2.480ab | 39.778±2.021ab |
| Area % of anti- COX-2 +ve immunoreaction | 7.138±0.994 | 25.538±1.828a | 12.652±1.741ab | 12.220±1.772ab |
| Area % of anti- iNOS +ve immunoreaction | 16.486±1.330 | 26.580±1.657a | 19.188±0.887ab | 18.970±1.197ab |
The values represent the mean ± SD (n = 7). a P < 0.05 vs. CNT rats; b P < 0.05 vs. MSG group. RT: Rutin, MOE: Moringa oleifera extract, MSG: Monosodium glutamate, CNT: Control group, PAS: Periodic acid schiff, COX-2: Cyclooxygenase-2, iNOS:Inducible nitric oxide synthase, SD: Standard deviation.
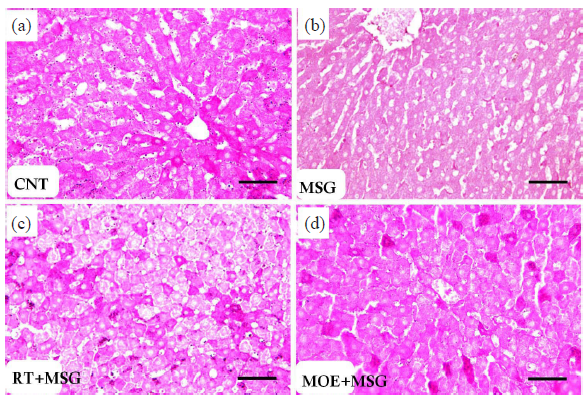
3.9 Transmission electron microscopy:
Normal hepatic structures were clearly visible in sections investigated with TEM in the CNT group; in the MSG group, hepatocytes with pyknotic nuclei, many vacuoles, and numerous enlarged mitochondria were seen (Fig. 11). The ultra-structural alterations of the hepatocytes were improved in rats treated with RT and MOE; rounded mitochondria with barely noticeable cristae were restored, and the nuclei and cytoplasm returned to nearly normal levels (Fig. 11).
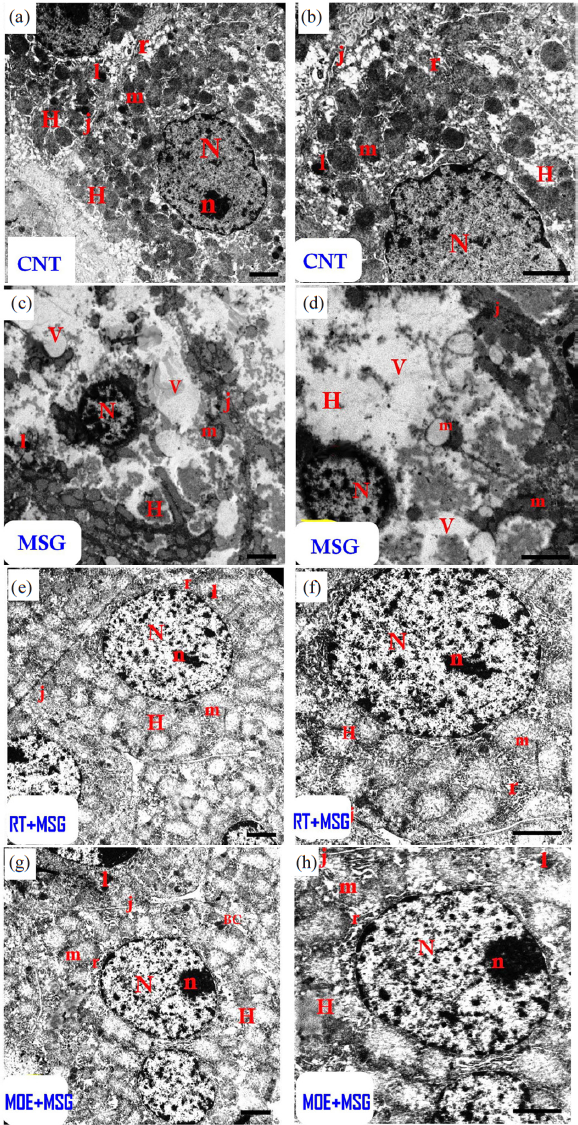
- Electron micrographs of hepatic tissues from CNT rats (a and b) and MSG rats (c and d). (a and b): The CNT group displayed an ordinary hepatocyte (H) with rounded-shaped euchraomatic nuclei (N), large-sized nucleoli (n), rounded mitochondria (m), rough endoplasmic reticulum cisternae (r), small dark lysosomes (l), and a tight junction (j) with the adjacent cell. (c and d): The MSG group demonstrated distorted hepatocytes (H) with pyknotic nuclei (N) with condensed chromatin, vacuolized swelling mitochondria (m) with damaged cristae, tiny lysosomes (l), tight junctions (j), and many cytoplasmic vacuoles. (e-f): The rutin-treated group had more or less normal hepatocytes (H) with normal nuclei (N) with big nucleoli (n), rounded mitochondria (m) containing indistinct cristae, cisternae of rough endoplasmic reticulum (r), small dark lysosomes (l), and a tight junction (j) with the surrounding cell. (g-h): The MOE-treated group seemed almost normal (H), with a normal nucleus (N) with large nucleoli (n), rounded mitochondria (m) containing indistinct cristae, cisternae of rough endoplasmic reticulum (r), small dark (l), bile canaliculi (BC), and a tight junction (j) between the cells. (Scale bar = 2 µm). CNT: Control group, MSG: Monosodium glutamate, H: Hepatocytes (liver cells), BC: Bile canaliculi MOE: Morin oil extract.
The current investigation showed that consumption of RT as well as MOE caused the liver’s histology and ultrastructure to significantly improve in the MSG group, as well as a considerable reduction in liver damage. The biochemical and microscopic action of rutin and MOE on MSG-induced liver damage was examined in this research. The MSG group had pyknotic hepatocytes with leukocyte infiltration, sinusoidal dilatation, kupffer cell proliferation, an inflammatory portal area, and elevated collagen fibers within the liver. PAS positivity in hepatocyte cytoplasm diminished in the presence of the MSG group. That group had a higher proportion of COX-2 and iNOS-positive cells. The treatment with RT and MOE resulted in considerable improvements of histopathological injury and biochemical markers.
4. Discussion
In this study, MDA levels rose, although antioxidant enzymes (SOD, catalase, and GSH) were notably lower in the MSG group. Other toxicants accumulating in the liver as well as testicles have been related to similar outcomes (Ijaz et al., 2021). As stated by Singh and Ahluwalia, changes in cellular redox status, H2O2 generation, SOD depletion, and damage to cell membranes are all caused by an elevation in MDA concentrations as a reaction to MSG-triggered ROS creation (Singh and Ahluwalia, 2012). As mentioned by Khayal et al., 2018, MSG may trigger hepatotoxicity; this data suggests that MSG generates oxidative stress, which can be damaging to a variety of tissues (Khayal et al., 2018). Elbassuoni et al., 2018 gave rats an oral dose of 35 mg/kg of MSG for a duration of two weeks. The researchers discovered an upsurge in ALT, AST, and MDA concentrations in the liver (Elbassuoni et al., 2018). Farombi and Onyema, 2006 treated albino wister rats with 4 mg/gram MSG for 10 days. In his investigation, the liver tissues exhibited a drop in GSH levels and an increase in SOD levels (Farombi and Onyema, 2006). The study uncovered that MSG supplementation increased TNF-α, IL-1, and COX2 levels. As stated by Jiang et al. (2018), COX2 serves as an inducible enzyme that contributes to inflammation of the tissues. NF-κB regulates genes of inflammatory mediators such as TNF-α along with COX2, which play an essential part in tissue injury (Yi et al., 2007). The current research noticed that the MSG group had higher levels of apoptotic indicators such as BCl-2, Bax, and Cas-3. Over-activation of apoptosis in cases with liver illness can harm hepatocytes, while inhibiting apoptosis can cause hepatocyte growth and transformation (Wu et al., 2014). Pavlovic et al. (2007) found that the percent of the number of apoptotic cells in the MSG group increased significantly in a dose-dependent way as compared to the normal CNT group (Pavlovic et al., 2007). The present research discovered that rats given MSG had considerably greater levels of liver function indicators (ALP, ALT, and AST) than the CNT group. These outcomes confirm the research by Onyema et al. (2006), who discovered MSG-triggered hepatocyte destruction as well as elevated AST and ALT levels (Onyema et al., 2006). Toxic chemicals, such as MSG, enter hepatic tissue through cellular membrane interactions plus membranous transporters that are identified in the cell membranes of hepatic endothelial cells at sinuosoids (DelRaso et al., 2003). These toxins impair the function of liver antioxidant enzymes, thereby leading to localized oxidative stress along with ROS production (Noor et al., 2022). Production of ROS raises lipid peroxidation, damages proteins, DNA, and lipids, and ultimately leads to oxidative stress in general (Jomova and Valko, 2011). This affects the activity of numerous enzymes, bringing the liver’s metabolism into balance (Begic et al., 2017). MSG’s cytotoxic action causes significant increases in AST and ALT, along with GGT levels, owing to liver cell death (Shukry et al., 2020). This causes the formation of ROS and hepatic stress; this causes the peroxidation of lipids and the breakdown of hepatic cells, followed by an increase in hepatic enzymes (Tawfik and Al-Badr, 2012).
The present investigation determined that tissue sections from the CNT group had normal hepatic structure, including polygonal hepatocytes with conspicuous round nuclei, appropriate Kupffer cell architecture, eosinophilic cytoplasm, and the positioning of the hepatic sinusoids in regions where the hepatic cords diverge. The MSG experimental group showed some apoptotic hepatocytes with pyknotic nuclei, dilatation of hepatic sinusoids, kupffer cell proliferation, and inflammatory infiltrates in the portal region, dilated congested portal vein, thick portal artery, and overgrowth within the bile duct wall. Such findings supported those of Eweka et al. (2011), which discovered that MSG provokes the central hepatic vein to widen, in contrast to the CNT group. The effect of the toxic chemical within the liver might reflect the atrophic change along with deterioration shown in that research. The hepatic tissue of the untreated MSG group contains a large number of necrotic and inflammatory cells (Eweka et al., 2011). In the present investigation, there was a decline in PAS positive reactions in the MSG group associated with a decreased glycogen level, as well as an increase in collagen fibers in the periportal area. The investigators discovered that MSG damaged the hepatocyte cell membrane with tiny plus pyknotic nuclei, together with a notable rise in vacuole formation of hepatocytes and a decrease in PAS-positive responses caused by glycogen concentration in hepatocytes (Bhattacharya et al., 2011). In the instance of the MSG group, tissue from the liver showed hemorrhagic necrosis, broken collagen fibers, and inflammation (Waer and Edress, 2006). Cytoplasmic damage with many vacuoles was seen in liver cells following MSG i.p. injection in rats (Ortiz et al., 2006). MSG-induced tissue damage in many organs has been linked to oxidative stress (Hajihasani et al., 2020). ROS may impact several liver-specific cells, resulting in fibrosis. High ROS production in cells of the liver triggers cell death. As a result, numerous intermediaries: in Kupffer and Ito cells, the production of transforming growth factor beta and tumor necrosis factor alpha results in an inflammatory and fibrotic response. The current investigation found that the MSG group had a statistically significant rise in iNOS immune expression compared to the CNT group. Induction of iNOS has been associated with elevated levels of reactive nitrogen molecules. Blood sinusoids seemed dilated, with endothelial cells being far apart from surrounding hepatocytes. This might be described as lipid peroxidation; this changes the plasma membrane and disrupts it in a variety of type of cells, particularly endothelial cells. Likewise, the dilated blood sinusoids may be caused by the higher iNOS levels seen in our study. Elevated iNOS induce an upsurge in NO production, which produces sinusoidal vasodilation (Vakkala et al., 2000).
This research found that MSG-intoxicated rats had impaired liver ultrastructure, as seen by cytoplasmic vacuolation, enlarged mitochondria, along with pyknotic nuclei. Hepatocyte vacuolation can be defined as a cellular defense mechanism against harmful chemicals (Dorreia a. Zaghlol and Hanaa a. Abdel-Naeim, 2018). These vacuoles collect hazardous substances and prevent them from compromising with the cells’ basic functioning (Cheville, 2009). Al-Mosaibih reported that pyknosis along with karyolysis of hepatocyte nuclei could be attributable to a decrease in functional efficiency (Al-Mosaibih, 2013). Other investigations also found histological changes in the liver of MSG-treated experimental mice (Waer and Edress, 2006; Nakanishi et al., 2008). The hepatocytes in this study have enlarged mitochondria with disintegration or damage to their cristae. Khalaf and Arafat (2015) found similar outcomes in MSG-treated rats’ thyroid follicular cells (Khalaf and Arafat, 2015). A study discovered that hepatocyte vacuolation became more evident after rats received a 75-day MSG injection around the central vein (Bhattacharya et al., 2011). According to a different study, these vacuoles are essential for gathering toxic materials and keeping them from interfering with the normal function of these cells (Cheville, 2009). A study further said that vacuolation could be caused by a disruption in the cell’s ionization, resulting in the absorption of water and salt, and cellular enlargement. The results of this study are in line with those of a prior investigation that discovered many ultrastructural abnormalities in the liver, including vesiculated rough endoplasmic reticulum, degenerated mitochondria with poorly defined cisternae, and cytoplasmic vacuolation (Osman et al., 2012).
The study indicated that administering RT alongside MSG decreased the peroxidation of lipids in the liver sections of treated rats compared to those in the MSG group. Rutin’s mode of action included the inhibition of LPO plus its capacity to remove free radicals. Ma et al., (2018) discovered that rutin may prevent liver damage in mice through regulation of the activities of CYP450 and antioxidant enzymes (Ma et al., 2018). According to research by Magalingam et al., (2013), RT inhibits peroxidation of lipids in vitro and raises antioxidant enzyme levels to decrease oxidative stress (Magalingam et al., 2013). Just recently, Erdogan et al. (2015) found that RT treatment boosted tissue SOD and GPx activity (Erdogan et al., 2015). This action might be attributed to RT’s superoxide anion scavenging activity, which boosts the body’s natural defense system and lowers glutathione levels while raising levels of a number of internal antioxidant enzymes (Nakamura et al., 2000). According to Nafees et al. (2015), RT’s hepatoprotective properties are associated with increased antioxidant enzyme activity (Nafees et al., 2015). RT’s medicinal promise stems from its antioxidant, anti-inflammatory, antiallergenic, and antiangiogenic characteristics (Park et al., 2014). RT oral treatment may protect against paclitaxel-induced harm to the liver and cytotoxicity by enhancing the antioxidant defense system, lowering oxidative stress, along with apoptosis (Ali et al., 2023). It can reduce CCl4-induced liver damage by blocking TNF-α and NF-κB along with COX-2 pathways (Domitrović et al., 2012). According to Khalil et al. (2021), TNF-α activates NF-κB through the canonical route, leading to increased expression of inflammatory response genes (Khalil et al., 2021). In rats treated with carbon tetrachloride, RT decreased the levels of IL-6 as well as TNF-β in the liver (Lee et al., 2013). It was also discovered to drastically lower caspase-3 immunopositivity in rats that underwent treatment 5-FU. (Gelen et al., 2017). In light of Hozayen et al., (2014), RT pretreatment may safeguard the liver from doxorubicin’s hepatotoxic effects by reducing increased ALP, ALT, and AST activities (Hozayen et al., 2014). This is due to rutin’s hepatoprotective properties (Abdel-Ghaffar et al., 2019). The current investigation demonstrated that livers from the MSG group had lower levels of Bcl-2 content than the CNT group. So, the current findings imply that Bcl-2 protein has a significant role in MSG-induced apoptosis in rat hepatocytes. There is a considerable increase in Bcl-2 protein content following RT therapy as compared to the MSG group. The results revealed that RT can reduce MSG-triggered apoptosis in the liver tissue of male rats via the mitochondrial route. Various studies have indicated that RT has a liver-protective effect. Patil et al. (2013) demonstrated that pre-treatment of gamma-irradiated mice with RT for five days successivelyi exhibited radioprotective potential and increased the animals’ survival. This protective impact might be linked to many mechanisms and involves managing intracellular levels of antioxidants, anti-lipid peroxidative actions, as well as free radical scavenging capacity (Patil et al., 2013). RT can be utilized as an adjuvant medication to reduce hepatic adverse effects associated with methotrexate therapy in many different therapeutic settings (Erdogan et al., 2015). Glutathione (GSH) levels were markedly increased by rutin treatment, as was the involvement of the antioxidant defense system, which includes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx), in addition to liver function tests like alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST).
Moringa oleifera leaves contain variable phytochemicals, including tannins, saponins, alkaloids, carotenoids, flavonoids, polyphenols, and phenolic acids. According to Ghimire et al. 2021, the leaves’ antioxidant qualities are also attributed to the high concentration of beta-carotene, chlorogenic acid, kaempferol, and quercetin (Ghimire et al., 2021). It has been observed that giving MOE to experimental rats dramatically lowers MDA levels in oxidative stress caused by acetaminophen; MOE caused a significant (p<0.05) increase in catalase activity (Sharifudin et al., 2013). It inhibited the cellular changes brought on by MSG by raising the concentrations of both non-enzymatic (GSH, GST) and enzymatic (SOD, CAT) antioxidants, as well as decreasing MDA activity in liver tissues. The findings of this investigation are in line with those of Uma et al., 2010, who discovered that Moringa oleifera counteracted the hepatotoxic impact of paracetamol by increasing and enhancing the action of antioxidant enzymes, hence enhancing hepatic antioxidant status (Uma et al., 2010). Furthermore, our findings are consistent with those of Sharifudin et al. (2013), who examined whether MOE might counteract the hepatotoxic outcomes of acetaminophen using rat models (Sharifudin et al., 2013). As per the current study’s findings, four weeks of co-administration allowed MOE, which functions as a ROS scavenger, to reverse the oxidative damage linked to MSG-induced liver degeneration. Moringa oleifera is a part of the Moringaceae plant family and possesses potent antioxidant qualities. Numerous therapies are linked to Moringa oleifera, such as those for diabetes, cancer, inflammation, oxidative free radicals, high blood pressure, lipoprotein elevation, along with inflammation (Stohs and Hartman, 2015). By focusing on free radicals and oxidants, Moringa oleifera appears to have a protective impact on cell membrane permeability, which may be shown in the reduction of raised AST and ALT levels (Onyema et al., 2006). According to Upadhyay et al. (2015), there was a substantial protective impact of Moringa oleifera’s ethanolic floral extract on the liver. This notable outcome was primarily ascribed to the extract’s high flavonoid content, specifically quercetin. Furthermore, hepatoprotective Moringa oleifera has characteristics as proven by AST, ALP, and ALT level reduction (Upadhyay et al., 2015). According to Alhakmani et al. (2013), this species’ anti-inflammatory properties are its most intriguing traits (Alhakmani et al., 2013). According to Ibrahim et al.’s (2024) laboratory findings, Moringa oleifera significantly (p < 0.05) inhibited pro-inflammatory mediator levels (TNF and IL-1B) and notably increased the levels of anti-inflammatory cytokines (IL-10, IL-6, and COX-2) in both treated groups (Ibrahim et al., 2024). Thus, it is hypothesized that Moringa oleifera has a positive anti-inflammatory array and can inhibit the induction and spread of the bodily release of inflammatory mediators. In the meantime, the CNT group had very little COX-2 expression. The group that consumed MSG nevertheless showed significant dye expression. The imaging analysis of both dyes’ area fractions revealed the suppressed immunoreactivity in the Moringa oleifera extract. Furthermore, because COX-2 is a recognized producer of prostaglandins, the inhibition of COX-2 expression via MO extracts suggested that these substances have anti-inflammatory properties (Al-Rasheed et al., 2017). According to Yamamoto and Gaynor, oxidative stress triggers the generation of markers of inflammation by inflammatory cells, which subsequently fosters the production of COX-2 and iNOS, as well as inflammatory cytokines such as TNF-α, IL-1b, and IL-6 (Yamamoto and Gaynor, 2001). In lipopolysaccharide-induced macrophages, research by Arulselvan et al. (2016) revealed that extract derived from Moringa oleifera leaves may decrease the growth of COX-2, iNOS, and TNF-α, along with IL-1-β (Arulselvan et al., 2016).
5. Conclusion
In conclusion, RT and MOE protect the liver from MSG-induced damage through anti-inflammatory, anti-apoptotic, and antioxidant mechanisms, as confirmed by biochemical, histological, and ultrastructural evidence. There are potential limitations of the study, such as the short duration of the study and use of only male rats, which may affect the generalizability of the results. This study gave pharmacological insight into the possible utility of RT and MOE in treating common medical diseases due to their antioxidant capabilities, raising the likelihood of clinical applicability. However, more research into its pharmacotoxicity is required before clinical recommendations can be given.
Acknowledgement
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R39), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Zakiah Almohawes: Conceptualization, methodology; Wafa Al-Megrin: Investigation; Mona Ibrahim: Writing—original draft; Ayah Fathalla and Sherif Mohamed: Writing—review and editing; Doaa Soliman: Statistical analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Data availability
Available upon request.
References
- Histological Study on the Protective Effect of Green Tea Extract on the Liver of Rats Exposed to Ketamine. J. Cytol. Histol 2015:06. https://doi.org/10.4172/2157-7099.1000349
- [CrossRef] [Google Scholar]
- An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem. Cell Biol.. 2017;95:524-530. https://doi.org/10.1139/bcb-2016-0256
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective Effect of Rutin Against Oxidative Stress of Isoniazid in Albino Rats. Int. J. Pharmacol.. 2019;13:516-528. https://doi.org/10.3923/ijp.2017.516.528
- [Google Scholar]
- Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev.. 2014;15:8571-8576. https://doi.org/10.7314/apjcp.2014.15.20.8571
- [CrossRef] [PubMed] [Google Scholar]
- Catalase in vitro. Methods Enzymol.. 1984;105:121-126. https://doi.org/10.1016/s0076-6879(84)05016-3
- [CrossRef] [PubMed] [Google Scholar]
- Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49
- [Google Scholar]
- Estimation of total phenolic content and in vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed.. 2013;3:623-627; discussion 626-627. https://doi.org/10.1016/S2221-1691(13)60126-4
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rutin and hesperidin evoke the hepatotoxicity induced by Paclitaxel in male Wistar rats via their antioxidant, anti-inflammatory, and antiapoptotic activities. Evid. Based Complement Alternat. Med.. 2023;2023:2738351. https://doi.org/10.1155/2023/2738351
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of monosodium glutamate and acrylamide on the liver tissue of adult Wistar rats. Life Sci. J.. 2013;10:35-42.
- [Google Scholar]
- Herbal antioxidants in clinical practice: A review. Asian Pac. J. Trop. Biomed.. 2014;4:78-84. https://doi.org/10.1016/S2221-1691(14)60213-6
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hepatoprotective role of α-lipoic acid and thymoquinone in acetaminophen-induced liver injury: Down-regulation of COX-2 and FLT-1 expression. Braz. Arch. Biol. Technol.. 2017;60:e17160703. https://doi.org/10.1590/1678-4324-2017160703
- [Google Scholar]
- Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res.. 2007;21:17-25. https://doi.org/10.1002/ptr.2023
- [CrossRef] [PubMed] [Google Scholar]
- Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev.. 2016;2016:5276130. https://doi.org/10.1155/2016/5276130
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules. 2018;23:664. https://doi.org/10.3390/molecules23030664
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Disulfiram moderately restores impaired hepatic redox status in rats subchronically exposed to cadmium. J. Enzyme Inhib. Med. Chem.. 2017;32:478-489. https://doi.org/10.1080/14756366.2016.1261132
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term effect of monosodium glutamate in the liver of albino mice after neonatal exposure. Nepal Med. Coll. J.. 2011;13:11-16.
- [PubMed] [Google Scholar]
- Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med.. 2007;43:645-657. https://doi.org/10.1016/j.freeradbiomed.2007.04.026
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathophysiological and toxicological aspects of monosodium glutamate. Toxicol. Mech. Methods. 2019;29:389-396. https://doi.org/10.1080/15376516.2018.1528649
- [CrossRef] [PubMed] [Google Scholar]
- Ultrastructural pathology: The comparative cellular basis of disease (Second Edition). 2009. p. :1-973. https://doi.org/10.1002/9780813810379
- Kidney and liver functions and stress oxidative markers of monosodium glutamate-induced obese rats. Food Public Health. 2012;2:168-177.
- [CrossRef] [Google Scholar]
- Certification procedures for sirius red F3B (CI 35780, Direct red 80) Biotech. Histochem.. 2011;86:133-139. https://doi.org/10.3109/10520295.2011.570277
- [CrossRef] [PubMed] [Google Scholar]
- Cadmium uptake kinetics in rat hepatocytes: Correction for albumin binding. Toxicol. Sci.. 2003;72:19-30. https://doi.org/10.1093/toxsci/kfg009
- [CrossRef] [PubMed] [Google Scholar]
- Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res.. 2019;33:2870-2903. https://doi.org/10.1002/ptr.6475
- [CrossRef] [PubMed] [Google Scholar]
- Differential hepatoprotective mechanisms of rutin and quercetin in CCl(4)-intoxicated BALB/cN mice. Acta Pharmacol. Sin.. 2012;33:1260-1270. https://doi.org/10.1038/aps.2012.62
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of monosodium glutamate on the liver of male adult albino rat and the possible protective role of vitamin C (light and electron microscopic study) Medi. J. Cairo Uni.. 2018;86:3407-3418. https://doi.org/10.21608/mjcu.2018.60313
- [CrossRef] [Google Scholar]
- Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed. Pharmacother.. 2018;108:799-808. https://doi.org/10.1016/j.biopha.2018.09.093
- [CrossRef] [PubMed] [Google Scholar]
- Tissue sulfhydryl groups. Arch. Biochem. Biophy.. 1959;82:70-77. https://doi.org/10.1016/0003-9861(59)90090-6
- [CrossRef] [Google Scholar]
- Rutin ameliorates methotrexate-induced hepatic injury in rats. Acta Cir. Bras. 2015;30:778-784. https://doi.org/10.1590/S0102-865020150110000009
- [CrossRef] [PubMed] [Google Scholar]
- Histochemical studies of the effects of monosodium glutamate on the liver of adult wistar rats. Ann. Med. Health Sci. Res.. 2011;1:21-29.
- [PubMed] [PubMed Central] [Google Scholar]
- Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem.. 1998;273:15846-15853. https://doi.org/10.1074/jbc.273.25.15846
- [CrossRef] [PubMed] [Google Scholar]
- Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E, and quercetin. Hum. Exp. Toxicol.. 2006;25:251-259. https://doi.org/10.1191/0960327106ht621oa
- [CrossRef] [PubMed] [Google Scholar]
- Glutamic acid, twenty years later. J. Nutr.. 2000;130:901S-9S. https://doi.org/10.1093/jn/130.4.901S
- [CrossRef] [PubMed] [Google Scholar]
- The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pacific J. Tropical Biomed.. 2017;7:647-653. https://doi.org/10.1016/j.apjtb.2017.06.013
- [Google Scholar]
- Moringa oleifera: A tree of life as a promising medicinal plant for neurodegenerative diseases. J. Agric. Food Chem.. 2021;69:14358-14371. https://doi.org/10.1021/acs.jafc.1c04581
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional and medicinal applications of Moringa oleifera Lam. Review of current status and future possibilities. J. Herbal Med.. 2018;11:1-11. https://doi.org/10.1016/j.hermed.2017.07.003
- [Google Scholar]
- Natural products as safeguards against monosodium glutamate-induced toxicity. Iran J. Basic Med. Sci.. 2020;23:416-430. https://doi.org/10.22038/IJBMS.2020.43060.10123
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721-724. https://doi.org/10.1016/s0140-6736(94)92211-x
- [CrossRef] [PubMed] [Google Scholar]
- Monosodium glutamate-induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Rep.. 2014;1:1037-1045. https://doi.org/10.1016/j.toxrep.2014.10.002
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Does Commiphora extract have an effect on carbon tetrachloride-induced liver injury in adult albino rats? Histological and biochemical study. Egyptian J. Histology. 2013;36:528. https://doi.org/10.1097/01.EHX.0000431736.49629.8d
- [CrossRef] [Google Scholar]
- Protective effects of ruitn and/or hesperidin against doxorubicin-induced hepatotoxicity. Int. J. Clinical Nutrition. 2014;2:11-17. https://doi.org/10.12691/ijcn-2-1-2
- [Google Scholar]
- Regulation of obesity and fatty liver by moringa oleifera: Insights into inflammatory pathways. 2024 https://doi.org/10.1101/2024.04.28.591562
- [Google Scholar]
- Protective effect of myricetin on nonylphenol-induced testicular toxicity: Biochemical, steroidogenic, hormonal, spermatogenic, and histological-based evidence. Environ. Sci. Pollut Res Int. 2021;28:22742-22757. https://doi.org/10.1007/s11356-020-12296-5
- [CrossRef] [PubMed] [Google Scholar]
- Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via the mTOR/Ulk1 pathway. Biomed. Pharmacother.. 2018;99:583-590. https://doi.org/10.1016/j.biopha.2018.01.067
- [CrossRef] [PubMed] [Google Scholar]
- Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65-87. https://doi.org/10.1016/j.tox.2011.03.001
- [CrossRef] [PubMed] [Google Scholar]
- Effect of different doses of monosodium glutamate on the thyroid follicular cells of adult male albino rats: aA histological study. Int. J. Clin. Exp. Pathol.. 2015;8:15498-15510.
- [PubMed] [PubMed Central] [Google Scholar]
- Bee venom: From venom to drug. Molecules. 2021;26:4941. https://doi.org/10.3390/molecules26164941
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Influence of selenium on mono sodium glutamate as food additive induced hepatotoxicity and testicular toxicity in adult albino rats. Egyptian J. Forensic Sci. Appl. Toxicol.. 2018;18:1-24. https://doi.org/10.21608/ejfsat.2018.4459.1018
- [CrossRef] [Google Scholar]
- Protective effect of rutin against ultraviolet b-induced cyclooxygenase-2 expression in mouse epidermal cells. Food Sci. Biotechnol. 2013;22:1-6. https://doi.org/10.1007/s10068-013-0233-3
- [CrossRef] [Google Scholar]
- 3.04: Tissue preparation for microscopy and histology. In: Pawliszyn J., ed. Comprehensive sampling and sample preparation. Oxford: Academic Press; 2012. p. :53-93. https://doi.org/10.1016/B978-0-12-381373-2.00070-3
- [Google Scholar]
- Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct.. 2013;4:794-802. https://doi.org/10.1039/c3fo30389f
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr.. 2019;7:1754-1760. https://doi.org/10.1002/fsn3.1015
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed. Pharmacother.. 2018;107:721-728. https://doi.org/10.1016/j.biopha.2018.08.046
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation, and apoptosis in mouse kidneys associated with the ceramide, MAPKs, p53, and calpain activities. Chem. Biol. Interact.. 2018;286:26-33. https://doi.org/10.1016/j.cbi.2018.03.003
- [CrossRef] [PubMed] [Google Scholar]
- Rutin, a bioflavonoid antioxidant, protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int. J. Mol. Med.. 2013;32:235-240. https://doi.org/10.3892/ijmm.2013.1375
- [CrossRef] [PubMed] [Google Scholar]
- The determination of bilirubin with the photoelectric colorimeter. J. Biological Chem.. 1937;119:481-490. https://doi.org/10.1016/S0021-9258(18)74392-5
- [CrossRef] [Google Scholar]
- HPLC analysis of phenolic compounds and flavonoids with overlapping peaks. Food Technol. Biotechnol.. 2020;58:12-19. https://doi.org/10.17113/ftb.58.01.20.6395
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Biological Activities of Black Truffle (TerfeziaClaveryi) Against Dietary Acrylamide-Induced Toxicity in Rats Liver: Biochemical and Histopathological Study. Egypt. Acad. J. Biolog. Sci.. 2022;14(2):147-163.
- [CrossRef] [Google Scholar]
- Rutin ameliorates cyclophosphamide-induced oxidative stress and inflammation in Wistar rats: role of the NFκB/MAPK pathway. Chem. Biol. Interact.. 2015;231:98-107. https://doi.org/10.1016/j.cbi.2015.02.021
- [CrossRef] [PubMed] [Google Scholar]
- Effects of quercetin and rutin on serum and hepatic lipid concentrations, fecal steroid excretion, and serum antioxidant properties. J. Health Sci.. 2000;46:229-240. https://doi.org/10.1248/jhs.46.229
- [Google Scholar]
- Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. J. Autoimmun.. 2008;30:42-50. https://doi.org/10.1016/j.jaut.2007.11.016
- [CrossRef] [PubMed] [Google Scholar]
- Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: A biochemical, inflammatory, apoptotic, and histopathological investigation. Biomed. Pharmacother.. 2022;150:112934. https://doi.org/10.1016/j.biopha.2022.112934
- [CrossRef] [PubMed] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analy. Biochem.. 1979;95:351-358. https://doi.org/10.1016/0003-2697(79)90738-3
- [CrossRef] [Google Scholar]
- Effect of vitamin E on monosodium glutamate-induced hepatotoxicity and oxidative stress in rats. Indian J. Biochem. Biophys.. 2006;43:20-24.
- [PubMed] [Google Scholar]
- Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother.. 2006;60:86-91. https://doi.org/10.1016/j.biopha.2005.07.012
- [CrossRef] [PubMed] [Google Scholar]
- Study of the role of antioxidant (vitamin C) on modulation toxicity of chronic use of monosodium glutamate in liver of albino rats. Ain Shams J. Forensic Med. Clini. Toxi.. 2012;19:75-87. https://doi.org/10.21608/ajfm.2012.19538
- [CrossRef] [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70:158-169.
- [PubMed] [Google Scholar]
- Rutin from Dendropanax morbifera Leveille protects human dopaminergic cells against rotenone-induced cell injury through inhibiting JNK and p38 MAPK signaling. Neurochem. Res.. 2014;39:707-718. https://doi.org/10.1007/s11064-014-1259-5
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys.. 2013;38:87-92. https://doi.org/10.4103/0971-6203.111321
- [CrossRef] [PubMed] [Google Scholar]
- Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Mol. Cell Biochem.. 2007;303:161-166. https://doi.org/10.1007/s11010-007-9469-7
- [CrossRef] [PubMed] [Google Scholar]
- Advances in apoptosis research. Proc. Nat. Academy Sci.. 1997;94:12736-12737. https://doi.org/10.1073/pnas.94.24.12736
- [Google Scholar]
- Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob. Agents Chemother.. 2018;63:e01545-18. https://doi.org/10.1128/AAC.01545-18
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clinical Pathology. 1957;28:56-63. https://doi.org/10.1093/ajcp/28.1.56
- [CrossRef] [Google Scholar]
- Editorial: The liver as an endocrine organ: hepatokines and ketone bodies, novel hormones to be acknowledged. Front Endocrinol. (Lausanne). 2023;13:1117773. https://doi.org/10.3389/fendo.2022.1117773
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The chemo-prophylactic efficacy of an ethanol Moringa oleifera leaf extract against hepatocellular carcinoma in rats. Pharm. Biol.. 2017;55:1458-1466. https://doi.org/10.1080/13880209.2017.1306713
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere-packaged raw beef. Food Pack. Shelf Life. 2015;3:31-38. https://doi.org/10.1016/j.fpsl.2014.10.001
- [Google Scholar]
- Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharma. Bio.. 2013;51:279-288. https://doi.org/10.3109/13880209.2012.720993
- [Google Scholar]
- Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: a mini-review. J. Biomed. Sci.. 2015;22:93. https://doi.org/10.1186/s12929-015-0192-5
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ameliorative effect of graviola (annona muricata) on mono-sodium glutamate-induced hepatic injury in Rats: Antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals (Basel). 2020;10:1996. https://doi.org/10.3390/ani10111996
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamins E and C, beta-carotene, and other carotenoids act as antioxidants. Am. J. Clinical Nutri.. 1995;62:1315S-1321S. https://doi.org/10.1093/ajcn/62.6.1315S
- [Google Scholar]
- Effect of monosodium glutamate on lipid peroxidation and certain antioxidant enzymes in cardiac tissue of alcoholic adult male mice. J. Cardiovasc. Dis. Res.. 2012;3:12-18. https://doi.org/10.4103/0975-3583.91595
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histopathology procedures: From tissue sampling to histopathological evaluation. Methods Mol. Biol.. 2011;691:69-82. https://doi.org/10.1007/978-1-60761-849-2_4
- [CrossRef] [PubMed] [Google Scholar]
- Review of the safety and efficacy of moringa oleifera. Phytother. Res.. 2015;29:796-804. https://doi.org/10.1002/ptr.5325
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutrition Sci.. 2012;3:651-659. https://doi.org/10.4236/fns.2012.35089
- [CrossRef] [Google Scholar]
- Moringa oleifera enhances liver antioxidant status via elevation of antioxidant enzyme activity and counteracts paracetamol-induced hepatotoxicity. Malays. J. Nutr.. 2010;16:293-307.
- [PubMed] [Google Scholar]
- Moringa oleifera: A review of the medical evidence for its nutritional and pharmacological properties. Int. J. Res. Pharma. Sci.. 2015;2015:12-16.
- [Google Scholar]
- Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clini. Cancer Res.. 2000;6:2408-2416.
- [Google Scholar]
- The Effect of Monosodium Glutamate (MSG) On rat liver and the ameliorating effect of “Guanidino Ethane Sulfonic Acid (GES)” (histological, histochemical, and electron microscopy studies) Egyptian J. Hos. Med.. 2006;24:524-538. https://doi.org/10.21608/ejhm.2006.17916
- [Google Scholar]
- Nature and mechanisms of hepatocyte apoptosis induced by D-galactosamine/lipopolysaccharide challenge in mice. Int. J. Mol. Med.. 2014;33:1498-1506. https://doi.org/10.3892/ijmm.2014.1730
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest.. 2001;107:135-142. https://doi.org/10.1172/JCI11914
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem. Int.. 2007;50:1014-1027. https://doi.org/10.1016/j.neuint.2007.04.019
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Amelioration of thioacetamide-induced liver toxicity in Wistar rats by rutin. Int. J. Immunopathol. Pharmacol.. 2017;30:207-214. https://doi.org/10.1177/0394632017714175
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Glutamate is a neurotransmitter in the healthy brain. J. Neural. Transm. (Vienna). 2014;121:799-817. https://doi.org/10.1007/s00702-014-1180-8
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







