Protective effects of herbacetin against polystyrene microplastics-instigated liver damage in rats
⁎Corresponding author. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polystyrene microplastics (PS-MPs) are raising global concerns as they have tendency to induce adverse effects on organisms. Herbacetin (HBN) is a natural flavone that shows diverse biological activities. Therefore, the current study was designed to evaluate the curative role of HBN against PS-MPs provoked hepatic damage. Forty-eight rats were divided into 4 groups, control, PS-MPs-intoxicated (0.01 mg/kg), PS-MPs + HBN co-treated (0.01 mg/kg + 40 mg/kg) and HBN only treated (40 mg/kg) group. The experiment was conducted for 30 days. PS-MPs administration reduced the expressions of Nrf-2 as well as anti-oxidants genes and increased Keap-1 expression. It also lessened the activities of superoxide dismutase (SOD), glutathione reductase (GSR), catalase (CAT), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and heme oxygenase-1 (HO-1) and glutathione (GSH) level, while elevating the levels of MDA and ROS. Additionally, PS-MPs exposure augmented the levels of alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine transaminase (ALT). Furthermore, there was an upsurge in the levels of inflammatory indices in PS-MPs treated group. PS-MPs also upregulated Caspase-3 and Bax expression whereas, decreased Bcl-2 expression. Nevertheless, HBN treatment recovered all the impairments due to its anti-apoptotic, anti-oxidant and anti-inflammatory and hepatoprotective nature. Therefore, it is deduced that HBN could be used as a potential therapeutic agent to counter PS-MPs induced hepatic toxicity.
Keywords
Polystyrene microplastics
Herbacetin
Reactive oxygen species
Inflammation
Apoptosis
Hepatotoxicity
1 Introduction
Plastic pollution is a major global issue due to an exponential increase in the usage of plastic items. Plastics are widely used in healthcare, agriculture and many other industries owing to its excellent physical and chemical characteristics (Rochman and Hoellein, 2020). It is predicted that by 2050, there will be 33 billion tons of plastic products on the planet (Phillips and Bonner, 2015). Environmental abiotic and biotic weathering degrade and fragment plastics into smaller elements (<5mm) known as microplastics (MPs) (Wang et al., 2021). MPs are present in a variety of food items, i.e., drinking water, salt, fruits and vegetables as well as seafood and aquatic products (Zhang et al., 2020). MPs impose severe threat to all organisms due to their slow rate of degradation, small diameter and large surface area, as they can easily penetrate into the cells resulting in cellular toxicity (Wright and Kelly, 2017).
Polystyrene (PS) is a primary form of MPs. PS-MPs are widely used in insulatory materials, beakers, lamp covers and food packaging (Ahmad et al., 2023; Hu et al., 2022). They are prevalent in terrestrial and aquatic environments (Wang et al., 2021). Animals are exposed to PS-MPs via dermal contact, ingestion and inhalation (Yang et al., 2019). According to previous studies, PS-MPs cause multiple organ damage, including kidney, brain, heart, testes and liver (Banerjee and Shelver, 2021). Previous literature revealed that the major culprit behind PS-MPs instigated toxicity are inflammation and oxidative stress (OS) related damage (Qiao et al., 2019). PS-MPs can build up in various tissues and they are directly responsible for cell death, immunological response and antioxidant dysfunction (Lu et al., 2022). Liver is one of the major site to encounter potentially toxic environmental compounds, absorbed by the intestine (Yu et al., 2017). PS-MPs administration is reported to affect the physiological and biochemical functions of liver (Zhao et al., 2020). PS-MPs exposure instigates OS, lipid peroxidation (LP), inflammation as well as apoptosis (Mu et al., 2022).
Flavonoids are polyphenolic phytochemical compounds that serve as secondary metabolites in plants (Alvi et al., 2022). They are used as dietary supplements as well as in the pharmaceutical industry due to their wide range of properties (Jucá et al., 2020). Herbacetin (HBN) is a novel flavone, present in plants such as flaxseed, Rhodiola rosea and Ephedrae herba (Ma et al., 2013). HBN displays neuroprotective, anti-viral, anti-inflammatory and anti-oxidant nature (Kim et al., 2016). Therefore, the present study was designed to evaluate the attenuative potential of HBN against PS-MPs-induced liver damage in rats.
2 Materials and methods
2.1 Chemicals
PS-MPs (CAT No. 9003–53-6) and HBN (CAT No. 527–95-7) were acquired from Sigma-Aldrich (Germany).
2.2 Animals
Forty-eight rats (6–8 weeks old) were housed in the animal house of University of Agriculture, Faisalabad and kept in steel enclosures. Standard temperature (23–25 ◦C) and 12 h dark/light cycle were maintained. Standard feed and water were given to the rats during the whole trial. The animals were handled by following the protocol of the European Union of Animal Care and Experimentation (CEE Council 86/609) that was further approved by the ethical committee of UAF.
2.3 Experimental design
The experiment was conducted on forty-eight albino rats by dividing them into 4 groups. Control, PS-MPs administered (0.01 mgkg−1). PS-MPs (0.01 mgkg−1) + HBN (40 mgkg−1) co-administered and only HBN (40 mgkg−1) administered group. All the doses were given orally through oral gavage. The dose of PS-MPs was selected in compliance with the previous study conducted by Hamza et al. (2023a). HBN dose was selected according to the study of Ijaz et al. (2022a). The trial was accomplished in 30 days. The rats were anesthetize with the help of ketamine (60 mg/kg) and xylazine (6 mg/kg), decapitated and blood was collected in sterile tubes. Plasma was separated via centrifuging blood for 20 min at 3000 rpm, which was later stored at −20 °C until further assessment. The liver was removed and homogenized in Na3PO4 buffer solution at 12000 rpm for 14–15 min. Finally, various parameters were evaluated by using the supernatant.
2.4 Anti-oxidant enzymes analysis
CAT activity was determined by using the technique described by Aebi (1984). SOD activity was appraised according to the method demonstrated by Sun et al. (1988). The methodology of Sedlak and Lindsay was used to examine GSH level (Sedlak and Lindsay, 1968). GPx activity was appraised according to the protocol of Lawrence and Burk, (1976) whereas GSR activity was appraised via the method outlined by Factor et al. (1998). GST activity was measured following the technique of Couri and Abdel-Rahman (1979). HO-1 activity was measured according to the method of Magee et al. (1999).
2.5 Evaluation of ROS and MDA
MDA concentration was measured by using the technique of Ohkawa et al. (1979). The method described by Hayashi et al. was applied to measure ROS contents (Hayashi et al., 2007).
2.6 Real-time polymerase chain reaction (qRT-PCR)
The expressions of Nrf-2/Keap-1, anti-oxidant genes and apoptotic markers were appraised by qRT-PCR. Total RNA was changed into cDNA by using TRIzol reagent and Fast Quant RT kit (Takara, China). Alterations in their expressions were determined by 2-ΔΔCT, considering β-actin as an internal control. Table 1 shows the primer sequences of the genes (Hamza et al., 2023b; Ijaz et al., 2022b).
| Gene | Primers 5′ −> 3′ | Accession number |
|---|---|---|
| Nrf-2 | F: ACCTTGAACACAGATTTCGGTG | NM_031789.1 |
| R: TGTGTTCAGTGAAATGCCGGA | ||
| Keap-1 | F: ACCGAACCTTCAGTTACACACT | NM_057152.1 |
| R: ACCACTTTGTGGGCCATGAA | ||
| CAT | F: TGCAGATGTGAAGCGCTTCAA | NM_012520.2 |
| R: TGGGAGTTGTACTGGTCCAGAA | ||
| SOD | F: AGGAGAAACTGACAGCTGTGTCT | NM_017051.2 |
| R: AAGATAGTAAGCGTGCTCCCAC | ||
| GPx | F: TGCTCATTGAGAATGTCGCGTC | NM_030826.4 |
| R: ACCATTCACCTCGCACTTCTCA | ||
| GSR | F: ACCAAGTCCCACATCGAAGTC | NM_053906.2 |
| R: ATCACTGGTTATCCCCAGGCT | ||
| GST | F: TCGACATGTATGCAGAAGGAGT | NM_031509.2 |
| R: CTAGGTAAACATCAGCCCTGCT | ||
| HO-1 | F: AGGCTTTAAGCTGGTGATGGC | NM_012580.2 |
| R: ACGCTTTACGTAGTGCTGTGT | ||
| Bax | F: GGCCTTTTTGCTACAGGGTT | NM_017059.2 |
| R: AGCTCCATGTTGTTGTCCAG | ||
| Bcl-2 | F: ACAACATCGCTCTGTGGAT | NM_016993.1 |
| R: TCAGAGACAGCCAGGAGAA | ||
| Caspase-3 | F: ATCCATGGAAGCAAGTCGAT | NM_012922.2 |
| R: CCTTTTGCTGTGATCTTCCT | ||
| β-actin | F: TACAGCTTCACCACCACAGC | NM_031144 |
| R: GGAACCGCTCATTGCCGATA |
2.7 Evaluation of hepatic serum markers
The levels of ALT (ab285264), AST (ab263883) and ALP (ab287823) were assessed by using ELISA kits. All the assays were accomplished following the company’s protocols (Abcam, MA, USA).
2.8 Inflammatory indices analysis
The levels of NF-κB (CSB-E13148r), TNF-α (CSB-E07379r), IL-1ß (CSB-E08055r), IL-6 (CSB-E04640r) and COX-2 activity (CSB-E13399r) were evaluated by using ELISA kit (YL Biotech Co. Ltd., Shanghai, China). The analyses were performed with the help of ELISA Plate reader in compliance with the company's guidelines (BioTek, Winooski, USA).
2.9 Statistical analysis
Mean ± SEM were used to display all the results. Data were interpreted by using one-way ANOVA and Tukey’s test to assess significant difference with the help of Minitab software. The normal distribution of the data was tested by Shapiro–Wilk test. Significance level was adjusted at P<0.05.
3 Results
3.1 Impact of PS-MPs and HBN on Nrf-2 and Keap-1 expression
PS-MPs treatment induced a significant (P<0.05) decrease in the expressions of Nrf-2 and anti-oxidant genes on the other hand elevated Keap-1 expression as compared to the control rats. Nevertheless, the administration of HBN escalated the expressions of Nrf-2 and anti-oxidant genes and down-regulated the expression of Keap-1. Additionally, HBN alone administered group presented these expressions close to the control group (Figs. 1, 2).
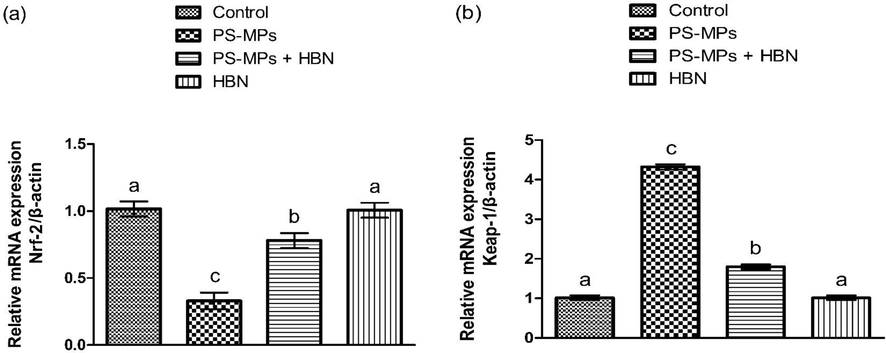
- Impact of PS-MPs and HBN on the expression of Nrf-2 and Keap-1. Bars are are shown on the basis of Mean ± SEM. Bars with different superscripts are significantly (P<0.05) different from others. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
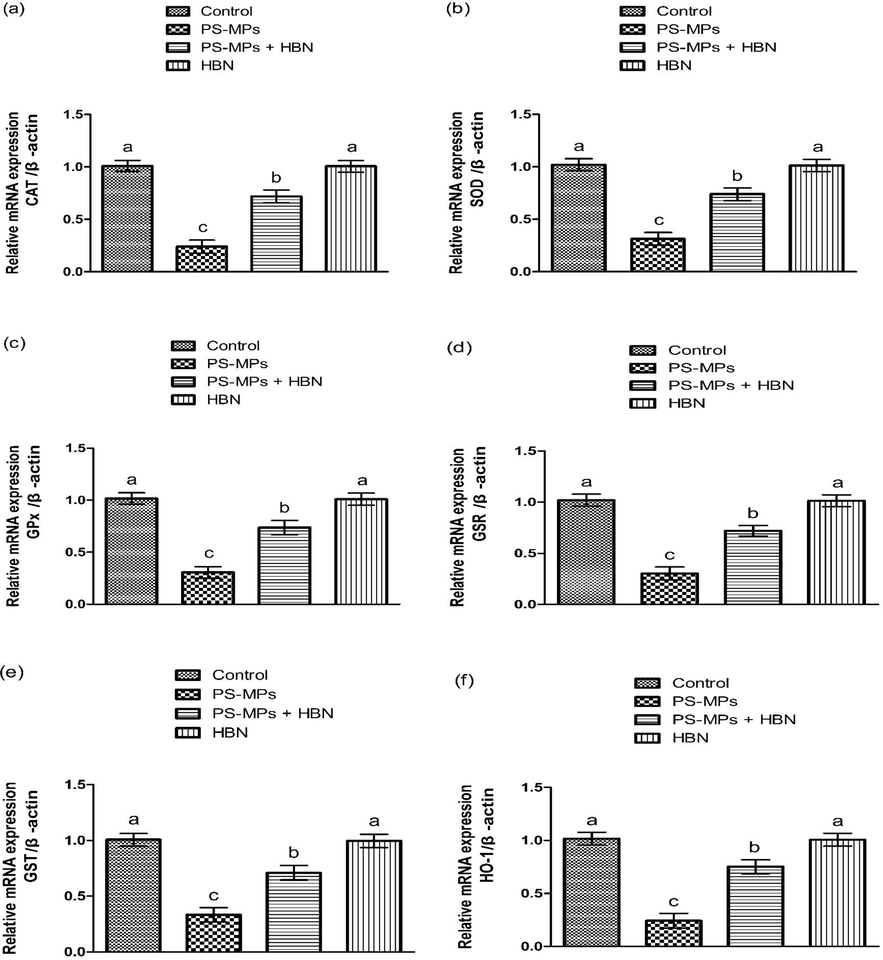
- Impact of PS-MPs and HBN on the expression of anti-oxidant genes. Bars are are shown on the basis of Mean ± SEM. Bars with different superscripts are significantly (P<0.05) different from others. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
3.2 Impact of PS-MPs and HBN on antioxidant profile
PS-MPs exposure notably (P<0.05) reduced the anti-oxidants activities, while augmenting MDA and ROS contents as compared to the control rats. Contrarily, in PS-MPs + HBN treated group anti-oxidant enzymes activities were notably increased, besides ROS and MDA contents were decreased as compared to PS-MPs administered rats. Moreover, HBN administered rats showed these activities near to the control rats (Table 2).
|
Parameters |
Groups | |||
|---|---|---|---|---|
| Control | PS-MPs | PS-MPs + HBN | HBN | |
| CAT (Umg−1 protein) | 8.48 ± 0.25a | 4.57 ± 0.11c | 7.20 ± 0.23b | 8.53 ± 0.26a |
| SOD (Umg−1 protein) | 6.55 ± 0.19a | 2.11 ± 0.24c | 5.07 ± 0.26b | 6.59 ± 0.19a |
| GSR (nM NADPH oxidized/min/mg tissue) | 0.23 ± 0.13a | 1.52 ± 0.36c | 4.16 ± 0.12b | 5.61 ± 0.22a |
| GPx (Umg−1 protein) | 29.37 ± 1.38a | 7.33 ± 0.29c | 21.18 ± 1.48b | 29.59 ± 1.41a |
| GSH (µM/g tissue) | 17.37 ± 0.56a | 5.21 ± 0.26c | 12.51 ± 0.69b | 17.51 ± 0.64a |
| GST (nM/min/mg protein) | 35.77 ± 2.04a | 11.27 ± 0.54c | 25.77 ± 1.11b | 36.06 ± 2.13a |
| HO-1 (pmoles bilirubin/mg protein/h) | 328.33 ± 8.51a | 68.87 ± 3.05c | 241.25 ± 4.82b | 332.41 ± 8.76a |
| MDA (nmol/mg protein) | 0.62 ± 0.06a | 5.54 ± 0.15c | 1.94 ± 0.05b | 0.60 ± 0.06a |
| ROS (Umg−1 tissue) | 1.32 ± 0.12a | 8.71 ± 0.19c | 2.54 ± 0.21b | 1.29 ± 0.12a |
Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (P<0.05) different from other groups. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
3.3 Impact of PS-MPs and HBN on hepatic serum enzymes
The levels of ALT, AST and ALP were markedly (P<0.05) increased in PS-MPs animals in contrast to the control animals. Nevertheless, HBN+PS-MPs supplementation decreased the level of these enzymes compared with PS-MPs exposed rats. Additionally, these levels in only HBN administered and control groups were comparable (Table 3).
|
Parameters |
Groups | |||
|---|---|---|---|---|
| Control | PS-MPs | PS-MPs + HBN | HBN | |
| ALT (U/I) | 42.92 ± 1.13a | 92.16 ± 1.86c | 58.81 ± 2.38b | 42.80 ± 1.12a |
| AST (U/I) | 85.12 ± 1.51a | 177.04 ± 3.38c | 114.57 ± 3.61b | 84.38 ± 1.75a |
| ALP (U/I) | 144.31 ± 1.84a | 331.49 ± 2.59c | 195.72 ± 4.75b | 143.14 ± 1.56a |
Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (P<0.05) different from other groups. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
3.4 Impact of PS-MPs and HBN on apoptotic proteins
The treatment with PS-MPs considerably (P<0.05) upregulated Bax and Caspase-3 expressions, while reducing the Bcl-2 expression as compared to the control group. Contrarily, PS-MPs + HBN administration significantly reduced the expressions of Bax and Caspase-3 and escalated Bcl-2 expression as compared to PS-MPs intoxicated rats. However, HBN alone treated group was compareable to the control group (Fig. 3).
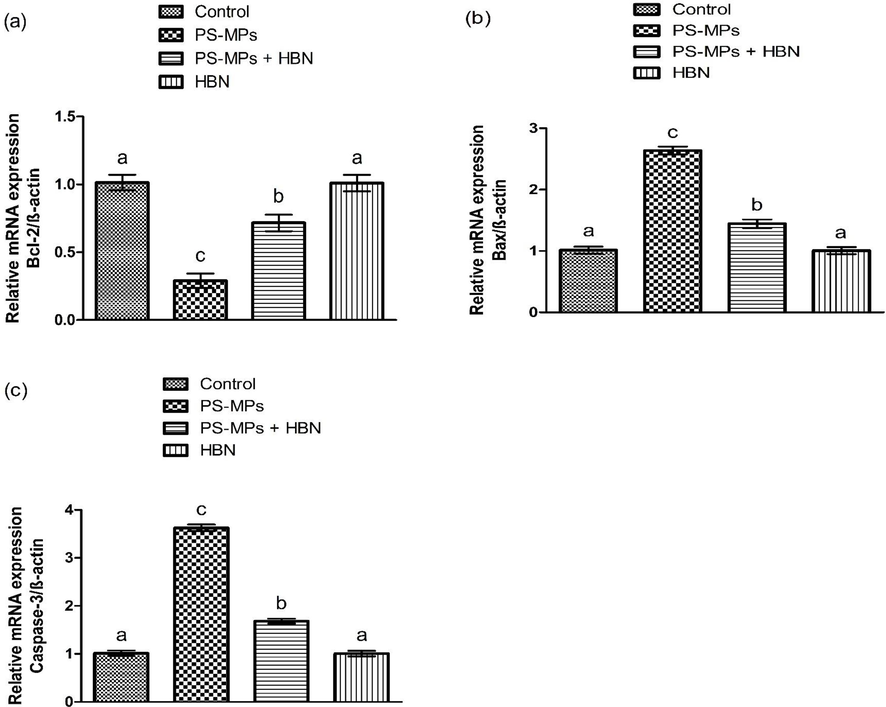
- Impact of PS-MPs and HBN on the expression of apoptotic markers. Bars are are shown on the basis of Mean ± SEM. Bars with different superscripts are significantly (P<0.05) different from others. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
3.5 Impact of PS-MPs and HBN on inflammatory markers
The levels of inflammatory markers were significantly (P<0.05) increased in PS-MPs exposed group as compared to the control rats. Contrarily, HBN+PS-MPs co-treatment decreased the levels of these markers in HBN+PS-MPs supplemented rats as compared to PS-MPs rats. However, HBN alone treated group was compareable to the control group (Table 4).
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | PS-MPs | PS-MPs + HBN | HBN | |
| NF-kB (ng/g tissue) | 13.24 ± 0.94a | 75.50 ± 1.57c | 23.56 ± 1.12b | 13.12 ± 1.06a |
| TNF-α (ng/g tissue) | 7.81 ± 0.22a | 61.23 ± 1.31c | 14.10 ± 1.39b | 7.73 ± 0.24a |
| 1L-1β (ng/g tissue) | 25.72 ± 1.04a | 95.52 ± 1.60c | 43.92 ± 1.47b | 25.19 ± 1.22a |
| IL-6 (ng/g tissue) | 7.59 ± 0.48a | 63.23 ± 1.29c | 25.48 ± 1.11b | 7.56 ± 0.48a |
| COX-2 (ng/g tissue) | 15.92 ± 0.75a | 81.96 ± 1.57c | 25.68 ± 0.98b | 15.82 ± 0.73a |
Values are shown on the basis of Mean ± SEM. The values with different superscripts in a row are significantly (P<0.05) different from other groups. All the values in the table are based on 12 biological replicates per group with 3 technical replicates of each.
4 Discussion
PS-MPs are receiving attention due to their persistence, ubiquitous nature and toxic effects on organisms (Jin et al., 2022). Animals and human are exposed to PS-MPs via seafood, packaged food and water (Hwang et al., 2019). PS-MPs have tendency to increase OS and inflammatory response in the animals (Kim et al., 2021). PS-MPs administration disturbs the balance of oxidants and anti-apoptotic proteins. PS-MPs intoxication also increases the levels of liver serum indices (Hamza et al., 2023b). According to previous literature plant based anti-oxidants can be used to cure the organs's damage induced by various toxicants (Rizwan et al., 2023). HBN, a novel flavone is the major constituent of Ephedrae herba L., Rhodiola rosea L. and flaxseed (Ma et al., 2013). Previous studies have shown that HBN has a considerable protective effect against OS-triggered toxicities owing to its free radical scavenging potential (Kim et al., 2016). Therefore, the current study was planned to evaluate the attenuative role of HBN on PS-MPs provoked hepatic damage.
PS-MPs treatment reduced the expressions of Nrf-2 and anti-oxidant genes, while increasing the Keap-1 expression. Vomund et al. (2017) stated that Nrf-2 performs crucial function in the regulation of OS, while Keap-1 acts as the inhibitor of Nrf-2 and controls its stability. Nrf-2 separates from Keap-1 during ROS production via some physical modifications and translocates into the nucleus. Small MAF proteins interact with Nrf-2 in the nucleus (Telkoparan-Akillilar et al., 2019). Nrf-2 and MAF proteins induce the expressions of anti-oxidant genes. Therefore, Nrf-2 has a critical role in inducing the expression of anti-oxidant genes. However, during extreme OS, the expression of Nrf-2 gets decreased, while increasing the Keap-1 expression. Consequently, reduced Nrf-2 lowers the expressions of anti-oxidant genes (Yang et al., 2022). Nevertheless, HBN administration increased the expressions of Nrf-2 as well as anti-oxidant genes, whereas lowered the expression of Keap-1. Therefore, it is inferred that HBN has potential to regulate Nrf-2 and Keap-1 expressions.
PS-MPs treatment noticeably (P<0.05) decreased the activities of anti-oxidant enzymes (CAT, SOD, GPx, GSR GST and HO-1) and GSH level, while elevating the production of MDA and ROS. According to Mezynska and Brzóska (2018), PS-MPs intoxication induces adverse effects by producing ROS i.e., hydrogen peroxide (H2O2), superoxide ions (O2•-) and hydroxyl radical (HO•). The entire anti-oxidant system is dysregulated when the accumulation of ROS exceeds the antioxidant capacity. The endogenous anti-oxidants are the first line of defense against ROS. SOD transfers O2•- into H2O2. CAT facilitates the transformation of H2O2 into H2O and O2. GPx lowers the levels of H2O2 and lipid peroxide to counter oxidative stress. GST promotes the binding of GSH to xenobiotic, therefore playing a central role in hepatic detoxification (Ighodaro and Akinloye, 2018). However, excessive ROS production causes lipid peroxidation (LP) by decreasing the activities of anti-oxidants enzymes. HO-1 is involved in the breakdown of heme and plays a pivotal function in cellular homeostasis (Bai et al., 2017). MDA is a biomarker of LP and the amount of MDA is directly proportional to LP. However, HBN supplementation significantly elevated the anti-oxidants activity, whereas it reduced MDA and ROS contents due to its anti-oxidant nature. Moreover, it was reported that the 8-OH groups in the chemical structure of HBN contribute to its anti-oxidant nature as well as ROS scavenging ability (Choe et al., 2012). Therefore, the potential of HBN in preventing PS-MPs-instigated LP and disruption of the hepatic antioxidant defense system might be ascribed to its ROS salvaging activity.
PS-MPs intoxication markedly (P<0.05) augmented the levels of ALT, AST and ALP (Hamza et al., 2023b). Liver serum marker enzymes are reliable indicators of liver function. According to Kandemir et al. (2020) the increased levels of ALT, AST and ALP shows the disorganized hepatic structure and subsequent liver damage. Furthermore, PS-MPs intoxication induces LP, which interrupts the integrity of hepatocytes' plasma membrane, that leads to an excessive discharge of hepatic enzymes into the blood. However, HBN treatment markedly regulated the levels of these enzymes. HBN supplementation lessened LP due to its ROS scavenging ability and repaired the disorganized hepatic structure, which ultimately decreased the discharge of hepatic function enzymes into the blood.
PS-MPs treatment notably increased the expressions of Caspase-3 and Bax, while decreasing Bcl-2 expression. Apoptosis is one of the processes contributing to hepatic dysfunction. Frenzel et al. (2009) reported that Bax and Bcl-2 are linked to the Bcl-2 protein family that regulates the apoptosis. Bax is an apoptotic protein, whereas Bcl-2 is an anti-apoptotic protein (Ehsan et al., 2023). The disturbance in Bax to Bcl-2 ratio provokes the liberation of Cytochrome C into the cytosol from the mitochondria, which results in Caspase-3 activation (McComb et al., 2019). Caspase-3 degrades cellular proteins, alters their structure and results in apoptosis (Kim et al., 2019). Nevertheless, HBN supplementation lowered Caspase-3 and Bax expression while increasing Bcl-2 expression. Our results are further endorsed by the study of Ijaz et al., (2022a), who stated that HBN has the potential to regulate apoptotic markers in the renal tissues of the rats.
The intoxication PS-MPs significantly raised the levels of inflammatory markers. NF-kB instigates gene upregulation of IL-1β, TNF-α, IL-6 and contributes to serious tissues damage (Kim et al., 2019). It is well documented that COX-2 also shows important biological function in inflammatory responses. Moreover, in the current study, COX-2 activity was increased in PS-MPs treated rats, indicating inflammatory reactions. However, HBN supplementation notably reduced the inflammatory indices due to its anti-inflammatory effect.
5 Conclusion
Our findings indicated that HBN showed significant attenuative effects against PS-MPs-induced hepatic damage by regulating the Nrf-2/Keap-1 pathway. HBN treatment effectively improved the levels of hepatic serum markers, anti-oxidants activity, the levels of MDA and ROS, inflammatory as well as apoptotic markers. Thus, it was demonstrated that HBN has an alleviative potential to improve hepatic damage due to its anti-inflammatory, anti-oxidant, hepato-protective and anti-apoptotic properties effects.
CRediT authorship contribution statement
Zainab Rafi: Writing – original draft, Methodology, Investigation, Conceptualization. Muhammad Umar Ijaz: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Ali Hamza: Writing – review & editing, Writing – original draft, Validation, Methodology. Hammad Ahmad Khan: Visualization, Software, Formal analysis, Data curation. Zubair Ahmed: Writing – original draft, Validation, Resources, Funding acquisition. Mian Nadeem Riaz: Writing – review & editing, Visualization, Formal analysis, Data curation.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSPD2024R1113), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effects of rhamnetin against polystyrene microplastics-induced nephrotoxicity in rats. Pak Vet J. 2023;43(3):623-627.
- [CrossRef] [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43(1):189-193.
- [CrossRef] [Google Scholar]
- Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci.. 2017;96(1):74-82.
- [CrossRef] [Google Scholar]
- Micro-and nanoplastic induced cellular toxicity in mammals: A review. Sci. Total Environ.. 2021;755:142518
- [CrossRef] [Google Scholar]
- The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR. Molecules. 2012;17(10):11484-11494.
- [CrossRef] [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol. Oncol.. 1979;3:451-460.
- [Google Scholar]
- Attenuative effects of ginkgetin against polystyrene microplastics-induced renal toxicity in rats. Pak Vet J. 2023;43(4):819-823.
- [CrossRef] [Google Scholar]
- Disruption of redox homeostasis in the transforming growth factor-α/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem.. 1998;273(25):15846-15853.
- [CrossRef] [Google Scholar]
- Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584-596.
- [CrossRef] [Google Scholar]
- Rhamnetin alleviates polystyrene microplastics-induced testicular damage by restoring biochemical, steroidogenic, hormonal, apoptotic, inflammatory, spermatogenic and histological profile in male albino rats. Hum. Exp. Toxicol.. 2023;42 0960327123117378
- [CrossRef] [Google Scholar]
- Hepatoprotective effects of astragalin against polystyrene microplastics induced hepatic damage in male albino rats by modulating Nrf-2/ Keap-1 pathway. J. Funct. Foods. 2023;108:105771
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Emerging microplastics in the environment: Properties, distributions, and impacts. Chemosphere. 2022;134118
- [CrossRef] [Google Scholar]
- An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ.. 2019;684:657-669.
- [CrossRef] [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54(4):287-293.
- [CrossRef] [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Saudi Pharm. J.. 2022;30(5):519-526.
- [Google Scholar]
- Ameliorative effect of herbacetin against cyclophosphamide-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Hum. Exp. Toxicol.. 2022;41
- [CrossRef] [Google Scholar]
- Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol.. 2022;19(1):13.
- [CrossRef] [Google Scholar]
- Flavonoids: biological activities and therapeutic potential. Nat. Prod. Res.. 2020;34(5):692-705.
- [CrossRef] [Google Scholar]
- Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem. Toxicol.. 2020;138:111190
- [CrossRef] [Google Scholar]
- Herbacetin is a novel allosteric inhibitor of ornithine decarboxylase with antitumor activity. Cancer Res.. 2016;76(5):1146-1157.
- [CrossRef] [Google Scholar]
- Remission effects of dietary soybean isoflavones on DSS-induced murine colitis and an LPS-activated macrophage cell line. Nutrients. 2019;11(8):1746.
- [CrossRef] [Google Scholar]
- Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater.. 2021;413:125423
- [CrossRef] [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [CrossRef] [Google Scholar]
- Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater.. 2022;421:126770
- [CrossRef] [Google Scholar]
- Separation of four flavonoids from Rhodiola rosea by on-line combination of sample preparation and counter-current chromatography. J. Chromatogr. a.. 2013;1306:12-19.
- [CrossRef] [Google Scholar]
- In Vitroandin VivoImmunomodulatory Effects of RDP1258, a Novel Synthetic Peptide. J. Am. Soc. Nephrol.. 1999;10(9):1997-2005.
- [CrossRef] [Google Scholar]
- Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or-7. Sci. Adv.. 2019;5(7):eaau9433.
- [CrossRef] [Google Scholar]
- Environmental exposure to cadmium—A risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res.. 2018;25:3211-3232.
- [CrossRef] [Google Scholar]
- Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291:132944
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull.. 2015;100(1):264-269.
- [CrossRef] [Google Scholar]
- Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ.. 2019;662:246-253.
- [CrossRef] [Google Scholar]
- Attenuative effect of astilbin on polystyrene microplastics induced testicular damage: Biochemical, spermatological and histopathological-based evidences. Toxicol. Appl. Pharmacol.. 2023;471:116559
- [CrossRef] [Google Scholar]
- The global odyssey of plastic pollution. Science. 2020;368(6496):1184-1185.
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [CrossRef] [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [CrossRef] [Google Scholar]
- Pharmacological applications of Nrf2 inhibitors as potential antineoplastic drugs. Int. J. Mol. Sci.. 2019;20:20-25.
- [CrossRef] [Google Scholar]
- Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci.. 2017;18(12):27-72.
- [CrossRef] [Google Scholar]
- Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater.. 2021;407:124357
- [CrossRef] [Google Scholar]
- Plastic and human health: a micro issue? Environ. Sci. Technol.. 2017;51(12):6634-6647.
- [CrossRef] [Google Scholar]
- Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater.. 2019;366:703-713.
- [CrossRef] [Google Scholar]
- Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol.. 2022;283:114739
- [Google Scholar]
- Silica nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. Int. J. Nanomedicine. 2017;12:6045.
- [CrossRef] [Google Scholar]
- A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ. Sci. Technol.. 2020;54(7):3740-3751.
- [CrossRef] [Google Scholar]
- Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ.. 2020;710:136279
- [CrossRef] [Google Scholar]







