Translate this page into:
Protective effect Spirulina against Monosodium glutamate-induced hepatic dysfunction: A biochemical, molecular, and histopathological study
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
This study aimed to investigate the role of spirulina against Monosodium glutamate (MSG) induced hepatic dysfunction as an in vivo study in a rat model.
Methods
In this study, 28 rats were divided into four groups (i) controls, (ii) spirulina, (iii) MSG and (MSG + spirulina). Each group consists of 7 rats. The first group is considered as a normal group and consists of the availability of food and water without any restrictions. The second group involves 1000 mg/kg bw spirulina extract daily for ten days. The third group is the positive intoxicated group, given orally 4 g/kg bw monosodium glutamate (MSG) for ten days. The final group is orally administered Spirulina and MSG for ten days. Within each of the four groups, serum, qPCR, histology, and immunohistochemistry were examined.
Results
Biochemical and antioxidant analysis showed a significant association with MSG and MSG + spirulina, and molecular analysis revealed a significant association with Nrf2, HO-1, BCl2, and β-actin genes. Histological analysis showed modest congestion of the central vein, intact hepatocytes organized in cords, and mild sinusoidal dilatation in the Centro lobular area of the liver of the spirulina-treated group. The H&E stain was documented to be 50–200 m bar. The liver section analysis in NF-κB, TGF-β and COX-2 showed a protective effect.
Conclusion
This study concludes as the combination of MSG and spirulina has a protective effect. However, MSG has shown an elevated role among all groups.
Keywords
Spirulina
Biochemical
Monosodium glutamate
Histology and quantitative polymerase chain reaction
1 Introduction
Cyanobacterium blue-green algae (Spirulina) is a microscopic undifferentiated filamentous, spiral-shaped cyanobacterium that may grow spontaneously in alkaline settings and quadruple its biomass every 2–5 days. The cyanobacteria Spirulina platensis and Spirulina maxima go by the term spirulina, which is used to characterize both species (Trotta et al., 2022). Two primary categories of Microcoleaceae cyanobacterium species have been intensively studied for their potential in pharmacological and food applications: Arthrospira platensis and Arthrospira maxima, two species of arthrospira (sometimes known more generically as Spirulina for their spiral structure) (Fais et al., 2022). The flavor enhancer monosodium glutamate (MSG) is widely utilized in commercial foods worldwide. Many foods, including mushrooms, algae, soy, various kinds of cheese like Roquefort and Parmesan, and some vegetables, including tomatoes and broccoli, naturally contain the sodium salt of the essential amino acid L-glutamic acid. MSG may be found in packaged and processed foods such as frozen foods, potato chips, salty snacks, sauces, sausages, and candies. Known as umami, brothy or savory, it has a distinct flavor and aroma. FDA allowed MSG, although pre-clinical research found that it was linked to numerous health concerns, including cancer-induced obesity, diabetes, and asthma, when used repeatedly and in excess (El-Hashash, 2021). The high protein content (up to 70 %) and vitamins (B12/β-carotene), and minerals (notably iron) of spirulina have been supported by several scientific studies, which show that it possesses antiviral, anticancer, and immune-supporting qualities. Tocopherols, phenolic acids, and gamma-linolenic acid are found in high concentrations in this food. Furthermore, because it lacks cellulose cell walls, it is easily digestible. In light of these facts, spirulina has a wide range of beneficial elements that can protect the human body from diseases sweeping the globe (Kerna et al., 2022). Spirulina's nutritional benefit was the primary focus of previous studies. Maya, Toltec, and Kanembu people in Mexico ate spirulina during the Aztec civilization, over 400 years ago (Ciferri and Tiboni, 1985). For sustenance, dried Spirulina was obtained from Lake Texcoco's Spirulina algae. In Central Africa, the Chadians have long consumed it. Spirulina from Lake Kossorom was harvested in Chad, used to manufacture cakes and broths, and sold on the market (Abdulqader et al., 2000). Since Spirulina contains a significant amount of protein (60–70 % of its dry weight), and numerous vitamins, minerals, and vital fatty acids, its nutritional significance is well-known (Vonshak, 1997; Iyer et al., 2008).
In 1999, Saudi Arabia's Agricultural Service Company (ARASCO) established a tiny Spirulina farm, sparking a surge in interest in the microalga (Al-Homaidan, 2002). The effects of dry powders on the phagocytic activity of chicken macrophages and nitrite production were studied (Al-Batshan et al., 2001). The hypolipidemic effects of Spirulina on rats and rats have been studied repeatedly since the first study was published in various experimentally produced situations. Wistar rats were fed a diet high in fructose (68 %), leading to hyperlipidemia. Spirulina (5 %, 10 %, and 15 %) considerably improved the hyperlipidemic profiles of those who incorporated it into their diets. Furthermore, Spirulina consumption resulted in an increased lipoprotein lipase activity as well as hepatic triglyceride lipase. As a result, it was speculated that Spirulina could treat hyperlipidemia resulting from a high fructose diet. Rats with fatty livers were induced with carbon tetrachloride (CCl4). Thus, liver total cholesterol and triacylglycerols rose as a result (Deng and Chow, 2010). Few in-vivo studies that provide significant evidence for Spirulina's antiviral effects. Spirulina has been shown to have a dose-dependent effect on the intestinal microbiota and physiological states when taken orally once daily for 24 consecutive days. An earlier study, showed a relative abundance in increase in Clostridium XIVa and Desulfovibrio, Eubacterium, Barnesiella and Bacteroides in the colonic microbial population (Hu et al., 2019). Another study found that Spirulina platensis effected on fecal samples from rats on a high-fat diet (HFD) in terms of the Proteobacteria and Firmicute/Bacteroids ratios (Yu et al., 2020). S. platensisis water extracts contain a sulfated polysaccharide, calcium spirulina, as its primary active ingredient (Ca-Sp). Ca-Sp inhibits the in vitro reproduction of numerous enveloped viruses, such as Herpes simplex type I, human cytomegalovirus, measles and mumps virus, influenza A virus, and human immunodeficiency virus-1 virus (Hayashi et al., 1996). Aqueous extract of S. platensis inhibited T-cells, peripheral blood mononuclear cells, and Langerhan cells (Ayehunie et al., 1998). It is possible to use antiviral herbs and algae products that have been shown to have antiviral capabilities to modulate the immune system after the infection has already occurred. Of course, more study on animals and humans is needed before we can draw any explicit judgments regarding these possible outcomes. Based on the above issues, this study was designed. The current study will examine the biochemical, molecular, and histological functions identified in vivo experiments on selected rats to investigate the role of spirulina against MSG induced hepatic dysfunction.
2 Materials and methods
2.1 Experimental animals and study design
King Abdel-Aziz University's College of Pharmacy in Saudi Arabia provided the experimental rats. For this investigation, randomly, 28 male rats weighing 150 g and aged ten weeks were employed. Rats were handled manually for ten days to achieve complete adaption and to prevent handling stress. Four animal groups were formed:
(i) Group I: Negative control (CNT) with full access to food and water.
(ii) Group II: Spirulina extract was administered orally every day for ten days at a dosage of 1000 mg/kg bw.
(iii) Group III: A positive intoxicated group was given MSG orally for ten days at a dose of 4 g/kg bw.
(iv) Group IV: Spirulina and MSG were administered orally for ten days as mentioned in groups ii and iii.
2.2 Sample collection
Rats were anaesthetized with dimethyl ether inhalation at the end of the experiment. Following that, tissue and blood samples were collected. The blood was centrifuged for 5 min at 4000 rpm to separate the serum (Lowry et al., 1951), which was then kept at −20 °C until required for biochemical analysis. After the animals were sacrificed liver tissues were immediately collected and divided into two packages. The first package was stored at −20 °C in ice phosphate buffer until it was utilized to determine gene expression. The second package was fixed in 10 % neutral formalin for H&E histopathology. Sample size calculation was performed based on this study (El-Naggar et al., 2018).
2.3 Biochemical measurements
The separated serum was used for the determination of various biochemical parameters like gamma-glutamyl transferase (GGT), glutamic oxaloacetic transaminase (GOT/sGOT), glutamic pyruvic transaminase (GPT/sGPT), glutathione (GSH), Malondialdehyde (MDA), superoxide dismutase (SOD), Catalase (CAT), Tumor necrosis factor- (TNF-α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), uric acid, serum creatinine, total proteins and urea.
2.4 Quantitative real time-PCR and gene expression
The livers of all the rats were used to extract the RNA. DEPC water was used to dissolve the RNA pellets. At 260/280 OD, the RNA concentration was determined spectrophotometrically (Saif and Khan, 2022). Three micrograms of RNA were used in semi-quantitative reverse transcription PCR (RT-PCR), which required 5 min of denaturation in a PCR thermal cycler (Bio-Rad T100TM) at 70 °C with 0.5 ng of oligo dT primers. cDNA was synthesized using a mixture of 2 L 10X RT-buffer, 2 µL of 10 mM dNTP, and 1 µL of 100 M reverse transcriptase in a total volume of 20 µL after incubation at 42 °C for 1 h, followed by 10 min at 70 °C to assure enzyme inactivation. Table 1 lists the primers and gene names used in the PCR process. Table 1 shows the PCR genes and reactions in a total volume of 25 µL using 2x Master Mix, as specified (Promega Corporation, Madison, WI, USA). In order to determine the densitometric expression levels of the genes under study, mRNA expression of β-actin was employed as a standard. Table 1 shows the genes involved in apoptosis and anti-apoptosis. The 2- ΔΔCT technique was used to measure the expression levels of these genes in real time-PCR. As an internal standard gene, actin was employed to normalize against the studied genes. Comparative cycle threshold (CT) values were used to analyze changes in gene intensity and mRNA expression.
Gene
Accession number
Product size (bp)
Direction
Primer Sequence
Nrf2
NM_010902.4
140 bp
Sense
CGCCTGGGTTCAGTGACTCG
Antisense
AGCACTGTGCCCTTGAGCTG
HO-1
NM_010442.2
126 bp
Sense
CGCCTCCAGAGTTTCCGCAT
Antisense
GACGCTCCATCACCGGACTG
Bcl2
NM_009741
153 bp
Sense
AGCCTGAGAGCAACCCAAT
Antisense
AGCGACGAGAGAAGTCATCC
β-actin
NM_007393.5
140 bp
Sense
CCAGCCTTCCTTCTTGGGTA
Antisense
CAATGCCTGGGTACATGGTG
2.5 Histopathological examination
After 24 h of fixation in 10 % formalin, liver specimens were washed, soaked in alcohol from low to high concentrations, cleaned in xylene, and finally embedded and cast in soft and hard paraffin. Hematoxylin and eosin (H&E) stain was performed after sectioning. Five microns thick tissue were prepared, stained with hematoxylin and eosin stains (Slaoui and Fiette, 2011), mounted in Canada Balsam, and examined by light microscope for recording any histological change.
2.6 Statistical analysis
Seven separate rats from each group were used in the experiments, and the results are shown as means ± standard error of the means (SEM). The data were analyzed using SPSS software for Windows with one-way ANOVA and Dunnett's post hoc descriptive test (SPSS, IBM, Chicago, IL, USA). Statistical significance was defined as a p-value of 0.05 or less (Khan et al., 2019). Figures were created using Graphpad prism software.
3 Results
3.1 Biochemical analysis
In this study, 28 male rats were created into four groups, and serum sample was collected from each. All 14 biochemical indicators were subjected to one-way ANOVA analysis in four groups: control, spirulina, MSG, and MSG + spirulina. In GSH (p = 0.02) and MDA levels, controls were shown to be significant with MSG and MSG + spirulina, spirulina was associated with MSG and MSG + spirulina, and MSG was associated with MSG + spirulina (p = 0.02). Both Catalase (p = 0.02) and total protein levels showed controls vs MSG, controls vs MSG + spirulina, spirulina vs MSG, and spirulina vs MSG + spirulina showed significant association (p = 0.02). The other parameters such as TNF-α (p = 0.01; p = 0.02), IL-1β (p = 0.01; p = 0.02), IL-6 (p = 0.01; p = 0.02), GGT (p = 0.01; p = 0.02), sGOT (p = 0.01; p = 0.02), sGPT (p = 0.01; p = 0.02), uric acid (p = 0.01; p = 0.02), serum creatinine (p = 0.01; p = 0.02) and urea (p = 0.01; p = 0.02) showed string association between controls vs MSG, controls vs MSG + spirulina, spirulina vs MSG, spirulina vs MSG + spirulina and MSG vs MSG + spirulina (p = 0.01; p = 0.02). Figs. 1-2 depict the biochemical data.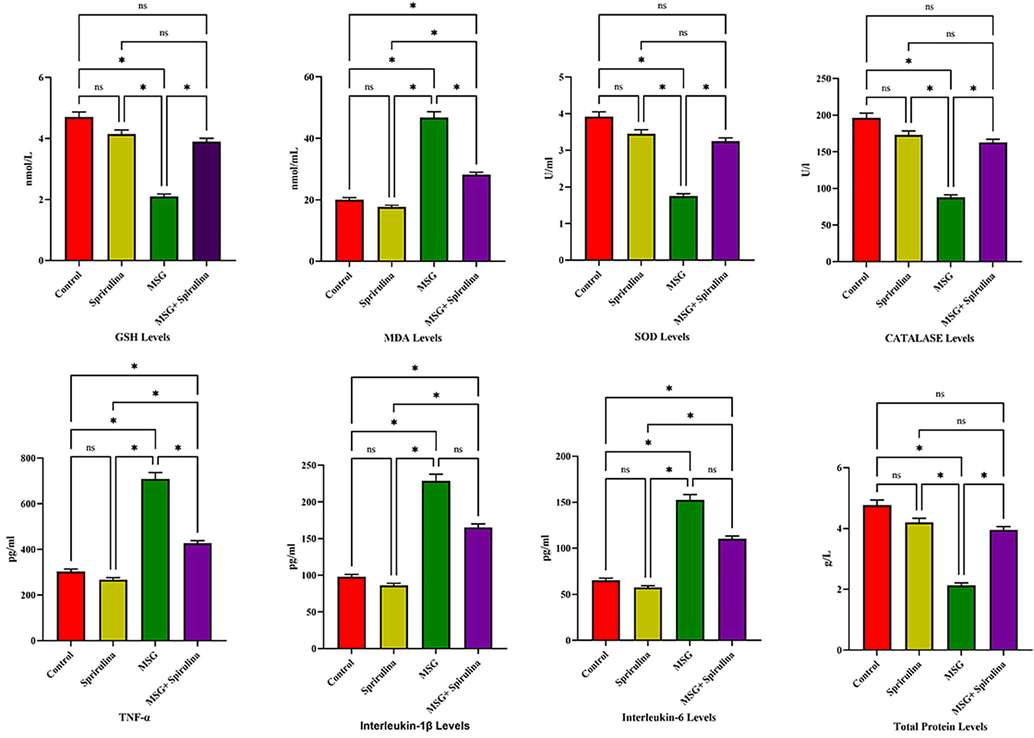
Anova analysis revealed the significant association between GSH, MDA, SOD, CAT, TNF-α, IL-1β, IL-6 and TP levels in 4 groups of rats.
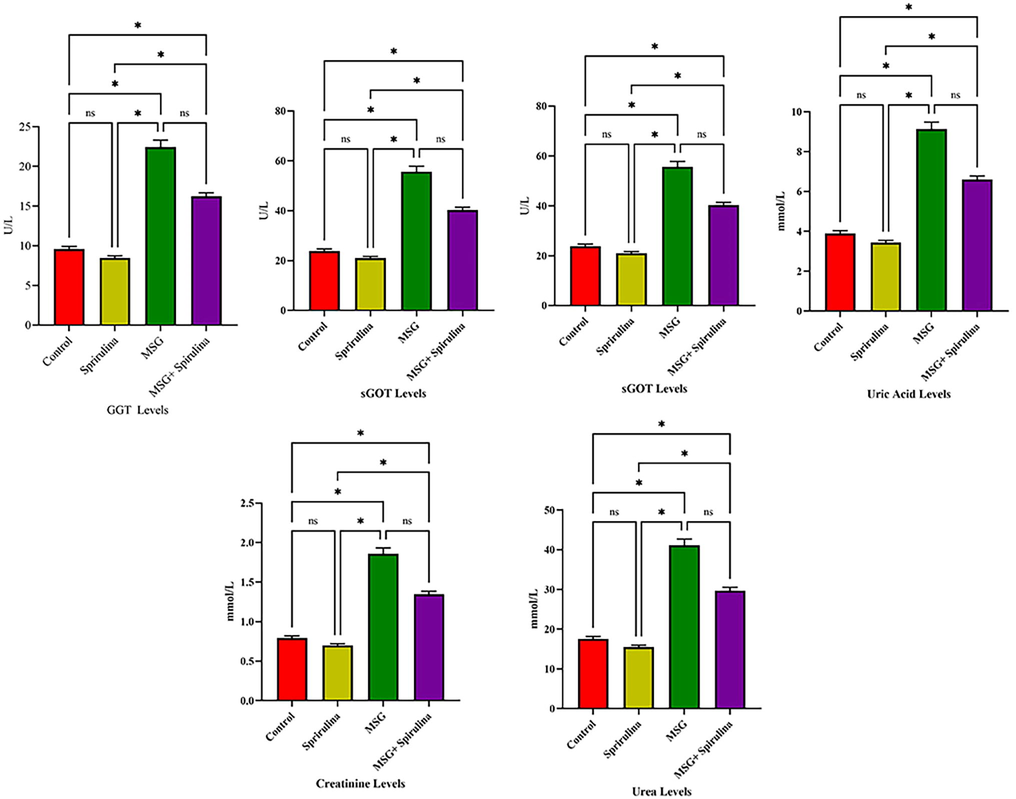
Anova analysis revealed the significant association between GGT, sGOT, sGPT, uric acid levels, serum creatinine and urea levels in 4 groups of rats.
3.2 RT-PCR analysis
The RT-PCR analysis was performed in four groups with Nrf2, HO-1, BCl-2 and β-actin genes. The obtained data is visible in Fig. 3. In this study the β-actin gene showed significant association with controls vs MSG, spirulina vs MSG and MSG vs MSG + Spirulina (p = 0.008; p = 0.01 and p = 0.02). Bcl-2 (p = 0.05) and HO-1 genes showed significant association with controls vs Spirulina, controls vs MSG + Spirulina, Spirulina vs MSG and MSG vs MSG + Spirulina (p = 0.004; p = 0.01). In Nrf2 gene, control vs MSG, controls vs MSG + Spirulina, spirulina vs MSG and Spirulina vs MSG + Spirulina showed the signiifcant association (p = 0.003; p = 0.01). Fig. 3 depicts the predominance of four categories among the genes examined in this study.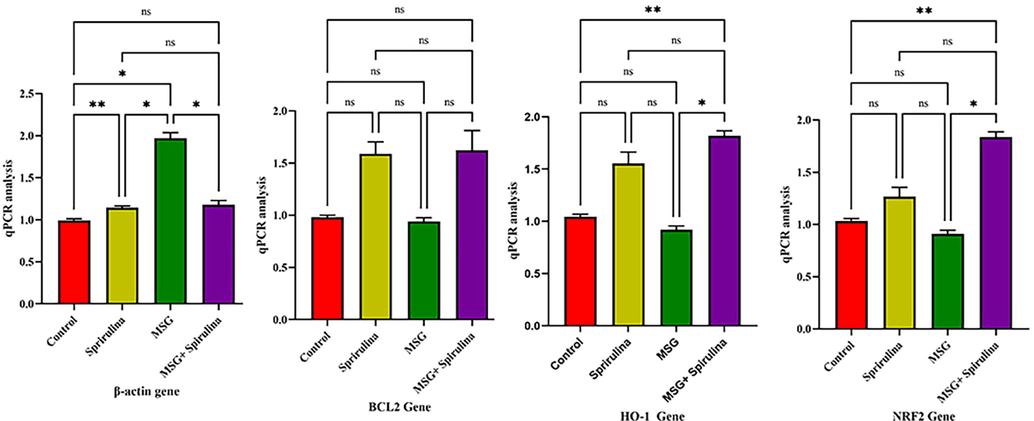
Anova analysis revealed the significant association between β-actin, BCL2, HO-I and NRF2 genes in 4 groups of rats.
3.3 Histopathological analysis of liver
Fig. 4 depicts a histopathological study of the liver. The H&E slides of control group indicate the typical architecture of the hepatic parenchyma; hepatocytes arranged in a cord-like pattern separated by blood sinusoids (blue arrow heads) radiating from an intact central vein and the H&E staining was at a level of 200 m bar. However, spirulina treated group had slight centro venal congestion, intact hepatocytes organized in cords, and mild sinusoidal dilatation in the liver’s centro lobular region. The H&E stain was measured at 50 m bar. The MSG-treated group's liver showed moderately congested blood vessels, intact hepatocytes in the central lobular region, and hepatocytes degradation towards the lobular periphery. The H&E staining was confirmed to be 200 m Bar. In the H&E stain the MSG + Spirulina group displayed a 50 µL bar confirmed by minor central vein congestion, intact hepatocytes spreading from the central vein in a fan-shaped pattern, and mild sinusoidal dilatation and congestion.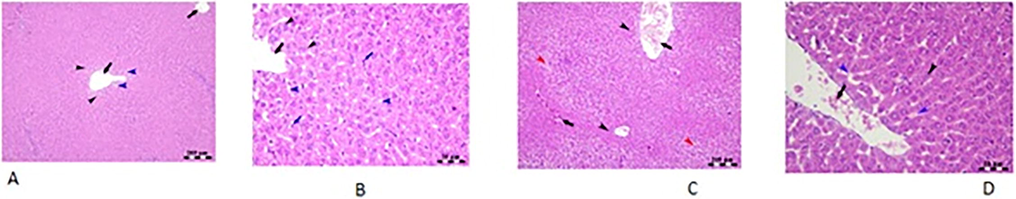
Histopathological analysis of liver tissue of rat.
3.4 The immunohistochemica expression of NF-B, TGF-β, and COX-2 expression in the liver of rats
The expression of NF-κB in the control group indicated hepatocytes in the centro lobular area (arrowheads). The NF-κB immunostaining was positive in the spirulina-treated group. The NF-κB immunostaining was negative in the MSG-treated group. However, the NF-κB immunostaining in the fourth group (MSG + Spirulina) was consistent with the control group (Fig. 5).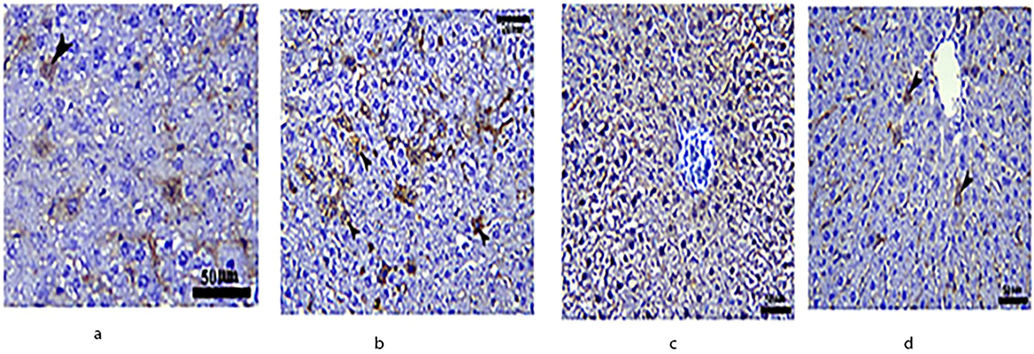
Immunohistochemical expression of NF-κB in the liver of rats.
Fig. 6 depicts Transforming Growth Factor beta (TGF-β) expression in the liver. TGF-β expression was negative in the control group's centro-lobular hepatocytes. Spirulina treatment resulted in modest TGF-β immunostaining in hepatocytes (arrowhead). TGF-β expression in hepatocytes is enhanced in the MSG-treated group (arrows up). TGF-β expression in hepatocytes was reduced in the MSG + spirulina group (arrows up).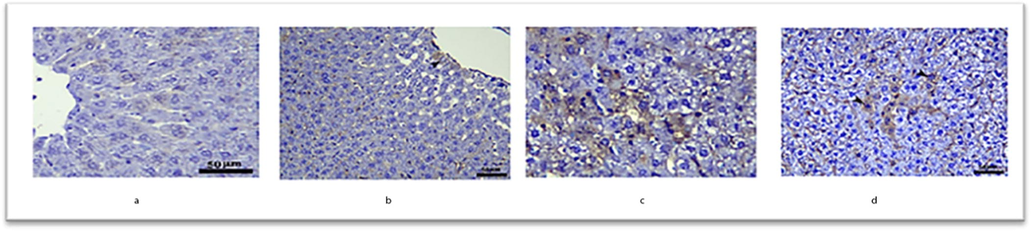
Immunohistochemical expression of TGF-β in the liver of rats.
Fig. 7 depicts the expression of cyclooxygenase (COX-2) in four groups. COX-2 expression in Centro lobular hepatocytes is modest in the Control group (arrowhead). COX-2 immunostaining in hepatocytes is negative in the spirulina-treated group. MSG treatment increases COX-2 expression in hepatocytes. MSG + spirulina-treated hepatocytes, on the other hand, exhibited COX-2 expression comparable to the control group.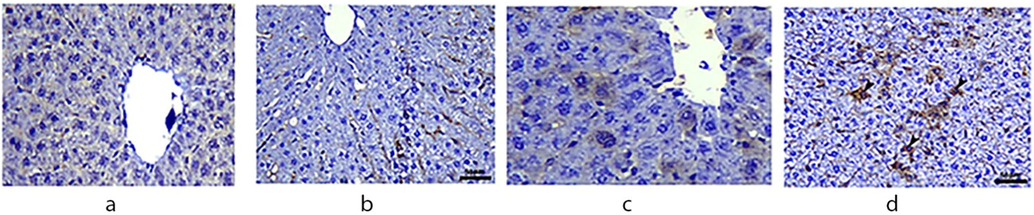
Immunohistochemical expression of COX-2 in the liver of rats.
4 Discussion
The in vivo investigation included 28 rats divided into four groups of seven: control (group-i), Spirulina (group-ii), MSG (group-iii), and MSG + Spirulina (group-iv). Blood samples from each group were obtained for biochemical parameters examination, and liver tissue was for RT-PCR and histological investigation. The study results confirmed MSG group was strongly associated with elevated levels compared to the control, Spirulina and MSG + Spirulina groups. The only difference between the four groups was that group I had no food or water restrictions, group II had extract orally in a dose of 1000 mg/kg bw daily for ten days, group III had MSG orally at a dose of 4 g/kg bw for ten days, and finally, group IV had as discussed on one group II and III orally for ten days. Studied serum analysis such as GSH and MDA levels were significant between controls, MSG, and MSG + Spirulina (p = 0.01; p = 0.02). Catalase and total protein levels were significantly different between controls and MSG, controls and MSG + Spirulina, and Spirulina and MSG. TNF-β, IL-1, IL-6, GGT, sGOT, sGPT, uric acid, serum creatinine, and urea revealed significant associations between controls vs MSG, controls vs MSG + Spirulina, Spirulina vs MSG, and MSG vs MSG + Spirulina (p = 0.01; p = 0.02). The elevated uric acid levels will lead to hyperuricemia which further causes GOUT, which leads to arthritic pain and then to formation of kidney stones in the kidney. GGT, sGOT and sGPT are connected with liver diseases.MSG has also been shown to have a negative impact on ovarian tissue antioxidant enzymes (SOD, GPx, and CAT) and gene expressions (Abdel-Aziem et al., 2018). One of the previous study (Seiva et al., 2012) observed that MSG reduced the SOD, GPx, and CAT, which is consistent with these findings. On the other hand, oral administration of Cv and Sp brought them back under reasonable control. In rats, Abdel-Daim et al. (Abdel-Daim et al., 2016) found that Sp reduced oxidative stress indicators in the brain, liver, and kidneys. Many biochemical events have been linked to the presence of flavonoids and β-carotene in Cv or Sp extracts as well as their potent antioxidant properties and ability to activate free radical-scavenging enzymes.
Many flavor enhancers, including MSG, have been correlated to genotoxicity, according to documented reports. MSG has also been related to genotoxicity and cytotoxicity in vitro (animal) studies. Human peripheral blood lymphocytes have been proven in vitro to be genotoxic to MSG, which has also been shown by other studies to be genotoxic. In rats and rats, the oral median fatal dose of MSG was 15 and 18 g/kg bw, respectively. It was found that the palatal mucosa of rats exposed to MSG at dosages of 20 and 40 mg/kg bw for two months (equal to 1 and 2 g/person) developed genotoxicity. Increases in LPO in the liver, kidney, and brain of rats indicate that ROS and oxidative stress are implicated in MSG-induced cytotoxicity and genotoxicity. Some studies, on the other hand, feel that MSG is not a carcinogen (Hajihasani et al., 2020). In this study, there is no genotype effect which was performed with RT-PCR in β-actin, Bcl2, HO-1 and Nrf2 genes.
The liver is a key organ in the xenobiotic’s metabolism, making it vulnerable to xenobiotic damage. Paracetamol and other hepatotoxic medications can induce liver damage (Buraimoh et al., 2011). It is well known that Diclofenac sodium (DFS) has hepatotoxic effects on people and animals. DFS is known to cause liver damage, as seen by changes in indicators for liver function, lipid profile, and the liver's endogenous antioxidant status. When hepatocytes are damaged, releasing intracellular enzymes is one of the most striking signs. The antioxidant defense system's enzyme SOD has been identified as a critical player (Lia Longodor et al., 2021).New product concepts incorporating spirulina and quercetin are advocated since they have positively impacted consumers in creating potentially profitable consumer-oriented items. Therefore, we will conduct more investigations with rats treated with MSG to determine whether quercetin has any beneficial benefits. If so, we will use the data from these two trials to guide our future study.
In this in vivo studies biochemical parameter was performed which is one of the strengths of this study and skipping of female rats were major limitation of this study.
5 Conclusion
It is concluded that the combination of MSG and spirulina has a protective effect. However, MSG has shown an elevated role among all groups. MSG effectively countered biochemical, oxidative, and inflammatory effects as a therapeutic solution containing several active ingredients, probably by restoring biochemical parameters, enhancing antioxidant defence mechanisms, and decreasing the generation of inflammatory mediators.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of the alleviative role of Chlorella vulgaris and Spirulina platensis extract against ovarian dysfunctions induced by monosodium glutamate in mice. J. Genet. Eng. Biotechnol.. 2018;16(2):653-660.
- [Google Scholar]
- Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed. Pharmacother.. 2016;77:79-85.
- [Google Scholar]
- Harvest of Arthrospira platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol.. 2000;12(3):493-498.
- [Google Scholar]
- Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacol. Immunotoxicol.. 2001;23(2):281-289.
- [Google Scholar]
- Large-scale cultivation of Spirulina in Saudi Arabia. Saudi J. Biol. Sci.. 2002;8(2):13-23.
- [Google Scholar]
- Inhibition of HIV-1 replication by an aqueous extract of Spirulina platensis (Arthrospira platensis) J. Acquired Immune Deficiency Syndromes Human Retrovirol.: Official Publication Int. Retrovirol. Assoc.. 1998;18(1):7-12.
- [Google Scholar]
- Hepatoprotective effect of ethanolic leave extract of Moringa oleifera on the histology of paracetamol induced liver damage in Wistar rats. Int. J. Anim. Vet. Adv.. 2011;3(1):10-13.
- [Google Scholar]
- The biochemistry and industrial potential of Spirulina. Annu. Rev. Microbiol.. 1985;39(1):503-526.
- [Google Scholar]
- Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther.. 2010;28(4):e33-e45.
- [Google Scholar]
- Spirulina as Anti-Obesity and Hepato-Renal Protective Agent in MSG-Exposed Female Rats. Alexandria Sci. Exchange J.. 2021;42(2):547-557.
- [Google Scholar]
- Pretreatment with the micro-alga, Spirulina platensis ameliorates cyclophosphamide-induced hematological, liver and kidney toxicities in male mice. Ain Shams J. Forensic Med. Clin. Toxicol.. 2018;30(1):1-7.
- [Google Scholar]
- Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs. 2022;20(5):299.
- [Google Scholar]
- Natural products as safeguards against monosodium glutamate-induced toxicity. Iran. J. Basic Med. Sci.. 2020;23(4):416.
- [Google Scholar]
- Comparative analysis of p16/CDKN2, p53 and ras gene alterations in human non-small cell lung cancers, with and without associated pulmonary asbestosis. Int. J. Oncol.. 1996;8(1):85-90.
- [Google Scholar]
- Dose effects of orally administered Spirulina suspension on colonic microbiota in healthy mice. Front. Cell. Infect. Microbiol.. 2019;243
- [Google Scholar]
- Iyer, U., Dhruv, S., Mani, I., et al., 2008. Spirulina in human nutrition and health. Spirulina in Human Nutrition and Health, CRC Press Boca Raton: 312.
- J. “Spirulina Consumption: Concerns Regarding Contaminants and Uncommon but Possible Adverse Reactions and Interactions”. EC Pharmacol. Toxicol.. 2022;10:69-78.
- [Google Scholar]
- Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab. Syndr.. 2019;13(1):688-694.
- [Google Scholar]
- Protective Effects of Dietary Supplement Spirulina (Spirulina platensis) against Toxically Impacts of Monosodium Glutamate in Blood and Behavior of Swiss mouse. Separations.. 2021;8(11):218.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Association of genetic variants of the vitamin D receptor gene with vitiligo in a tertiary care center in a Saudi population: a case-control study. Ann. Saudi Med.. 2022;42(2):96-106.
- [Google Scholar]
- Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem. Toxicol.. 2012;50(10):3556-3561.
- [Google Scholar]
- Slaoui, M., Fiette, L., 2011. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. (Clifton, N.J.) 691, 69–82. https://doi.org/10.1007/978-1-60761-849-2_4.
- Beneficial effects of spirulina consumption on brain health. Nutrients. 2022;14(3):676.
- [Google Scholar]
- Spirulina platensis arthrospira: physiology, cell-biology and biotechnology. CRC Press; 1997.
- Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J. Cell Mol. Med.. 2020;24(15):8603-8613.
- [Google Scholar]







