Translate this page into:
Protective effect of probiotic bacteria and its nanoformulation against cadmium-induced oxidative stress in male Wistar rat
⁎Corresponding author. bvirk@ksu.edu.sa (Promy Virk)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study assessed the ameliorative potential of a commercial probiotic, Protexin® and its nanoformulation against cadmium (Cd) toxicity in Wistar rats. The ‘green’ synthesized nanoparticles had a size of 170 nm (PDI 0.527) with spherical and tubular bacillary shapes. Rats were exposed to 70 ppm of cadmium chloride hydrate for 35 days. Group I and V were negative and positive control respectively. Groups II, III, and IV were exposed to Cd. The Cd exposed groups, III and IV were treated with the probiotic and nanoprobiotic (1 ml containing 2 × 108cfu) respectively. Cd intoxication led to significant bioaccumulation of cadmium in liver and kidney. A significant increase in the serum malondialdehyde (MDA) and 8 -hydroxydeoxyguanosine (8-OHdG) levels was observed. The metallothionien (MT) and reduced glutathione (GSH) levels in the liver were significantly increased. The advance oxidation protein products (AOPP) and methylglyoxal (MG) levels were also elevated. Treatment with probiotic and its nanoparticles markedly reversed the Cd induced alterations in the parameters. The nanoformulation was more profound in reducing the (GSH) and MT levels in the liver and 8-OHdG levels in the serum. The key findings suggest that a nutritional intervention in the form of probiotic supplementation is a safe and efficacious remediation against heavy metal toxicity/oxidative stress.
Keywords
Cadmium
Probiotics
Nanoparticles
Oxidative stress
1 Introduction
Cadmium (Cd) is the seventh most toxic heavy metal as per the Agency for Toxic Substances and Disease Registry (ATSDR) ranking (Jaishankar et al., 2014). Human exposure to the metal is primarily by inhalation and ingestion and can thus lead to acute and chronic intoxications. Cd is well recognized for its adverse influence on the enzymatic systems of cells, and oxidative stress which subsequently induces oxidative damage of biomolecules (Espín et al., 2014). Cysteine-rich metal-binding proteins such as metallothioneins are involved in the detoxification of toxic metals. Most of Cd in the body is bound to metallothioneins (Jaishankar et al., 2014). In addition, non-enzymatic reaction between reducing sugars and proteins, known as glycation, has been in the forefront of nutritional and medical research lately. The proposed role of Cd in inducing advanced glycation end products (AGEs) that further alter the production of reactive oxygen species and the activation of several inflammatory pathways (Suhartono et al., 2014).
This concept of food being the medicine has been reintroduced in the recent times as 'functional foods' (Chow, 2002). One of the functional foods which has garnered attention in the field of nutrition and medicine over the past few decades is the probiotic (Toma and Pokrotnieks, 2006). Currently, the best-studied probiotics are Lactobacilli, Bifidobacterium and Saccharomyces (Feher, 2012). The antioxidant potential of probiotics against oxidative stress in pathogenesis of diseases as well as metal toxicity has been reported in several experimental studies(Spyropoulos et al., 2011; Jama et al., 2012; Ghenioa et al., 2015). Nanoformulation of various nutraceuticals and functional foods have been explored due to their enhanced bioavailability. A range of different novel methods of nano formulations have been introduced lately, keeping environmental sustainability as the cornerstone of the recent advances in nanotechnology (Maurya and Singh, 2019).
With this premise, the aim of the present study was to synthesize ‘green’ nanoparticles of a commercially available probiotic mixture (Lactobacilli and Bifidobacterium) and to further evaluate its role in alleviating Cd associated toxicity in male Wistar rat.
2 Materials and methods
2.1 Chemicals and kits
Cadmium chloride (Cd Cl2 1/2 H2O) was purchased from Techno Pharmchem, Haryana (India). Commercial ELISA (enzyme-linked immunosorbent assay) kits for Methylglyoxal (MG), Metallothionein (MT), Advanced oxidation protein products (AOPP), Reduced glutathione (GSH) ,Malondialdehyde (MDA), 8-hydroxydeoxyguanosine were purchased from My Bio Source (USA).
A commercial Probiotic (PROTEXIN®) was procured from a local pharmacy.
2.2 Synthesis and characterization of nanoparticles
Nanoparticles were prepared according to the method by Virk et al. (2019).
The synthesized nanoparticles were characterized via transmission electron microscopy (TEM) (JEM-1400plus, JEOL, Japan) and Zetasizer, Nano series, HT Laser, ZEN3600 (Molvern Instrument, UK) for the size, shape and morphology.
2.3 Experimental design
All experiments were performed in accordance with the requirements of the local animal ethics committee of the University of Prince Sattam bin Abdulaziz University (PSAU) (IRB number: PSAU −2018-Para-830/PI). Adult male Wistar rats (n = 60), weighing 150 ± 10 g, were procured from the animal house facility at King Saud University, Riyadh. Rats were acclimated to the laboratory conditions for a week at 22 ± 2 °C and a 12 h light/dark cycle and were fed commercial diet and given tap water ad libitum. After acclimatization, rats were randomly allocated into five groups of 6 rats each in replicates. The exposure period was 35 days. The five experimental groups were as follows.
Group I – control group was administered physiological saline. Group II – received CdCl2 at a dose of 70 ppm in saline (Cd). Group III – received both CdCl2 (70 ppm) and probiotic (1 ml containing 2 × 108 cfu) in saline (Cd + PRO). Group IV – received both CdCl2 (70 ppm) and nanoprobiotic (1 ml containing 2 × 108 cfu)) in saline(Cd + NPRO). Group V – received only nanoprobiotic (1 ml containing 2 × 108 cfu) in saline (NPRO). After the exposure period the blood samples were collected for the preparation of serum and plasma to assess the biochemical variables. Thereafter, the animals were sacrificed and the liver and kidney were excised out for determination of the cadmium concentration. A set of liver samples were kept for biochemical assays. All samples of serum, plasma and tissue were stored at −80 °C till further analysis.
2.4 Determination of cadmium concentration
The tissue samples of liver and kidney were wet digested with 3.5 ml of 65% HNO3 and 0.5 ml of H2O2 30% in a microwave digester (Milestone, Italy). The concentration of Cd in digested tissue samples was analyzed in an atomic absorption spectrophotometer (220FS Varian, Australia) at wavelength of 228.8 nm (detection limit 0.005 µg/ml) with 4.0 mA current. A calibration curve with standard solution was plotted. The average reading of blanks was subtracted from standard, test sample and then final concentration (ng/g) was calculated
2.5 Preparation of plasma and serum
The blood collected in heparinized tubes was centrifuged at 1000 × g for 15 min at 2–8 °C within 30 mins of collection.
A serum separator tube (SST) was used to collect blood and samples were allowed to clot for 30 mins before centrifugation for 15 min at approximately 1000 × g. The plasma and serum were stored in vials at –80 °C until further analysis.
2.6 Determination of lipid peroxides
The MDA levels in in the serum were determined using the Alliance Waters High performance Liquid Chromatography (HPLC) 2695 system and a multi fluorescence detector (Model 2475, USA). This system was operated by a Dell Optiplex GX1 computer and Empower software. The MDA levels in the samples were expressed as nmol/ml of the serum.
2.7 Determination of reduced glutathione (GSH), methyl glyoxal (MG) and Metallothionein
For these assays, liver homogenates were prepared. The samples were then analyzed in accordance with the manufacture’s protocol provided with the ELISA kits.
2.8 Determination of advance oxidation protein products (AOPPs), 8-OHdG levels.
The plasma and serum samples were used for the assays. The samples were analyzed in accordance with the protocol provided with the ELISA kits from My Bio Source, USA.
2.9 Statistical analysis
All presented data are expressed as mean values ± standard error (SE). One-way analysis of variance (ANOVA) was performed followed by an unpaired Student’s t-test to analyze group differences using SPSS 22.0 statistical software (Chicago, IL, USA). The significance level was set to p ≤ 0.05.
3 Results
3.1 Characterization of nanoparticles of probiotic
The particle size distribution of the prepared probiotic nanoparticles (ProN) is shown in Fig. 1.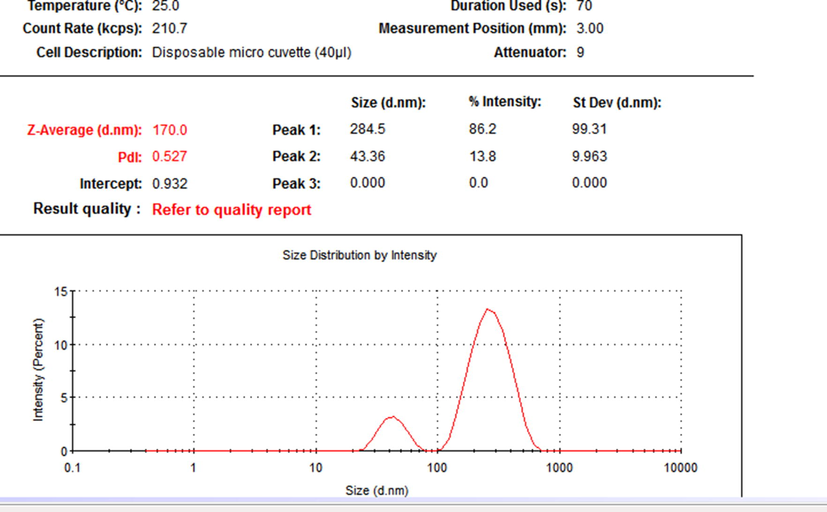
Particle size distribution of the nanoparticles as obtained from Dynamic Light Scattering.
Mean particle size observed was 170 nm with a poly dispersity index (PDI) of 0.527, with two contiguous peaks. The TEM analysis showed the presence of distinct variable forms of the nanoparticles, cluster widespread, spherical accumulated and tubular bacillary as shown in Fig. 2.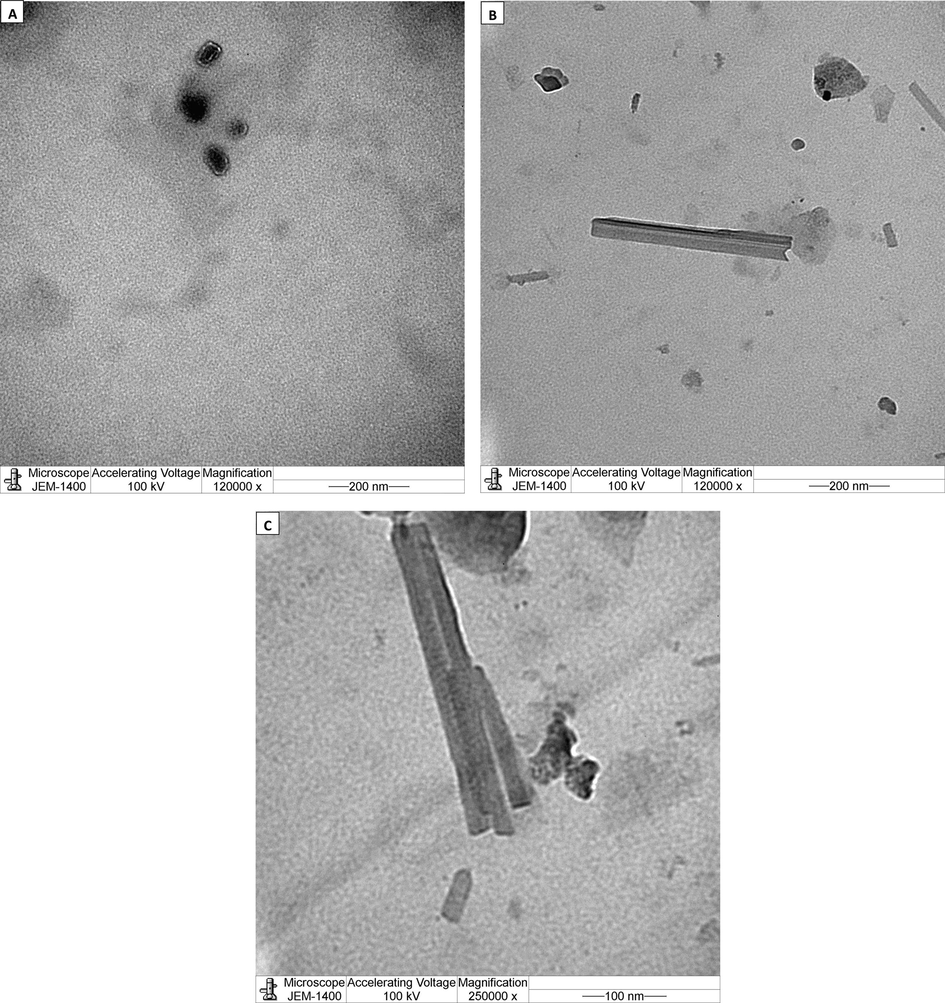
Electronmicrographs of the nanoparticles of probiotic with methanol. Nanoparticles had distinct variable forms (a) spherical accumulated (b) tubular bacillary (c) cluster tubular bacillary.
Fourier -transform infrared spectroscopy (FTIR) measurement was carried out to identify the possible biomolecules present both in the bulk and nano probiotic. Prominent IR bands were observed for the probiotic at 600.93 cm−1, 834.57 cm−1, 930.55 cm−1, 1060.43 cm−1, 1133.50 cm−1, 1422.61 cm−1, 1647.50 cm−1, 2114.54 cm−1, 2927.12 cm−1, 3250.21 cm−1. Similar bands were also observed for the nanoprobiotic. The bands at 599.88 cm−1 and 600.93 cm−1 may be attributed to C-I stretching indicating a halo compound. The bands at 834.57 cm−1 and 833.99 cm−1 indicate strong C–H bending. The band at 930.55 cm−1 and 929.99 cm−1 was identified as as an alkene with a strong C = C bending. The bands between 1053.95 and 1133.96 were identified as alcohol and ester groups with strong C-O stretching. The bands at 1647.50 cm−1 and 1642.55 cm−1 indicate C = C stretching due to the presence of alkenes. Further, the bands at 1647.50 cm−1 and 1642.55 cm−1 suggest the presence of imine group with medium C = N stretching. The bands at 2930.20 cm−1 and 2927.12 cm−1 are attributed to the alkane group due to the medium C–H stretching. Finally, a broad peak located at 3250.21 cm−1, which could be assigned to the O–H stretching vibrations, indicates the presence of hydroxyl groups(Fig. 3).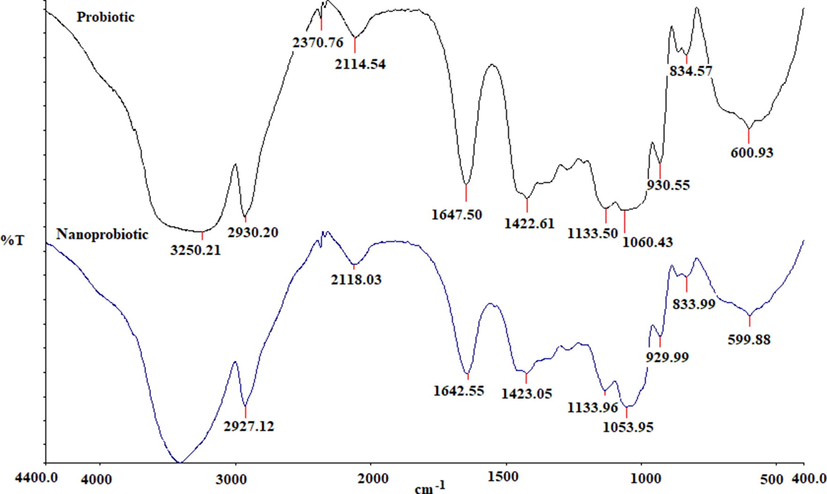
Fourier-transform infrared spectroscopy spectra of the probiotic and nano probiotic powder.
The synthesized nanoparticles and the probiotic powder were characterized by X-ray diffractometer (XRD). The XRD patterns of the of the probiotic and the nanoparticles are shown in Fig. 4. The powder diffraction pattern indicates the amorphous structure of nanoparticles by the intense peak at about 2θ = 22°. This indicates that the probiotic and its nanoformulation exhibits a more or less amorphous structure.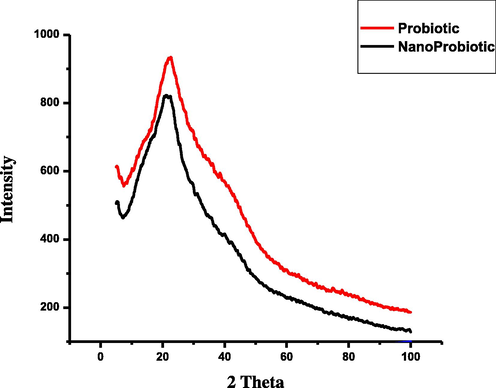
X-ray diffractograms of the probiotic and nano probiotic powder.
3.2 Cadmium concentration in tissues
A significant (p ≤ 0.05) increase in Cd concentration was observed in the liver, in comparison to control group. Treatment with the probiotic and nano probiotic did reduce the cadmium concentration significantly (p ≤ 0.05). Similarly, a significant (p ≤ 0.05) increase in the Cd concentration was observed in the kidneys in comparison to the control. Treatment with both probiotic and nano probiotic significantly (p ≤ 0.05) reduced the concentration of Cd. However, within the treatments, there was no significant difference observed (Figs. 5 and 6).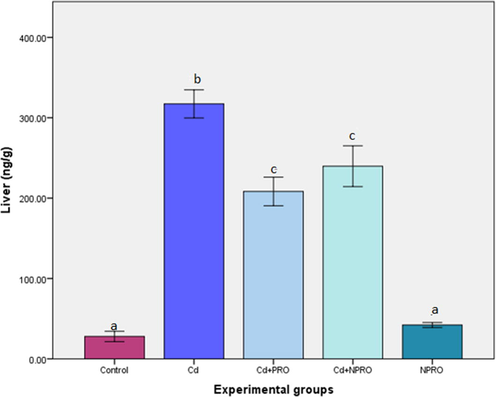
Mean (±SE) cadmium concentration (ng/g) in liver of rats exposed to 70 ppm CdCl2·H2O and treated with probiotic (Cd + Pro) and nanoprobiotic (Cd + NPro). Different letters indicate significant differences between experimental groups (p ≤ 0.05).
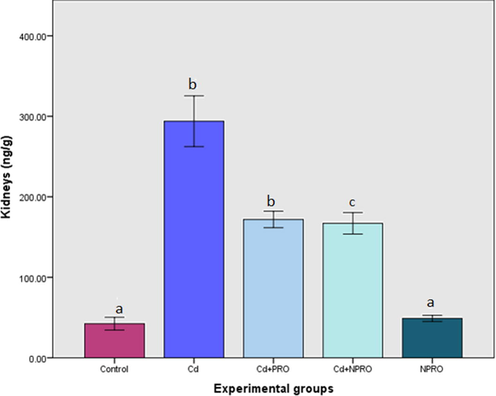
Mean (±SE) cadmium concentration (ng/g) in kidneys of rats exposed to 70 ppm CdCl2·H2O and treated with probiotic (Cd + Pro) and nanoprobiotic (Cd + NPro). Different letters indicate significant differences between experimental groups (p ≤ 0.05).
4 Biochemical analysis
4.1 Lipid peroxidation
The serum MDA levels were significantly (p ≤ 0.05) higher in the group exposed to Cd only, in comparison to the control. Treatment with the probiotic and nanoprobiotic significantly (p ≤ 0.05) reduced the MDA levels in comparison to the group exposed to Cd only (Table 1).
Experimental groups
Serum MDA(nmol/ml) levels
GSH (mmol/L) levels in liver
Serum 8-OHdG levels (ng/ml)
Plasma AOPP levels (μmol/L)
Serum methylglyoxal levels (ng/ml)
Control
0.7960 ± 0.01085a
151.9694 ± 2.71380a
25.7412 ± 0.92705a
23.5030 ± 0.44638a
4.6630 ± 0.37867a
Cd
1.5074 ± 0.08762b
175.3968 ± 2.74542b
28.8832 ± 1.58224b
32.3400 ± 2.69710b
9.0746 ± 0.70370b
Cd + PRO
0.7762 ± 0.02164a
159.6618 ± 6.68666b
27.1994 ± 0.58263b
23.4100 ± 2.17051a
3.8710 ± 0.70170a
Cd + NPRO
0.7516 ± 0.05208a
156.0316 ± 3.02353a
26.0116 ± 1.16281a
24.3680 ± 1.30898a
4.8010 ± 0.47595a
NPRO
0.6157 ± 0.10249a
162.5148 ± 4.01480a
25.6338 ± 0.77956a
23.3500 ± 0.60000a
4.5380 ± 0.46420a
4.2 Methylglyoxal (MG) levels in liver
The levels of the advanced glycation end products such MG in the serum were significantly (p ≤ 0.05) higher in the group exposed to Cd only, in comparison to the control. In the Cd exposed groups, treatment with probiotic and nano probiotic significantly (p ≤ 0.05) reduced the MG levels in comparison to the group exposed to Cd only. However, within the treatments there was no significant difference observed (Table 1).
4.3 Reduced glutathione (GSH) levels
The GSH levels in the liver were significantly (p ≤ 0.05) higher in the group exposed to Cd only, in comparison to the control. Treatment with the probiotic did not show any significant effect on the GSH levels. However, treatment with nano probiotic significantly (p ≤ 0.05) reduced the GSH levels compared to the group exposed to Cd only (Table 1).
4.4 8-hydroxy-20-deoxyguanosine (8-OHdG) levels in serum
The 8-OHDG levels in serum were significantly (p ≤ 0.05) higher in the group exposed to Cd only, in comparison to the control. Treatment with probiotic did not show a significant detectable effect on the levels. However, treatment with nano probiotic significantly (p ≤ 0.05) reduced the 8-OHDG levels (Table 1).
4.5 Advanced oxidation protein products (AOPP) levels in plasma
The AOPP levels in the plasma were significantly (p ≤ 0.05) higher in the group exposed to Cd only, in comparison to the control group. Both treatments (PRO, NPRO) significantly (p ≤ 0.05) reduced the AOPP levels in the plasma. In fact, the treatments restored the AOPP levels to that of the control. Within the treatments no significant difference was observed (Table 1).
4.6 Metallothionein (MT) levels in liver
The MT levels in liver were significantly (p ≤ 0.05) enhanced in the group exposed to Cd only, in comparison to the control. The treatment with probiotic did not have significant effect on the MT levels. Thus, the nano probiotic treatment was significantly (p ≤ 0.05) more effective in reducing the MT levels in the liver (Fig. 7).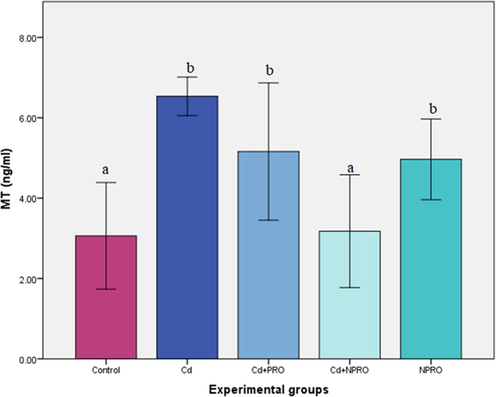
Mean (±SE) MT levels in liver (ng/ml) of rats exposed to 70 ppm CdCl2·H2O and treated with probiotic (Cd + Pro) and nanoprobiotic (Cd + NPro). Different letters indicate significant differences between experimental groups (p ≤ 0.05).
5 Discussion
The novel method of nanonization used in this study was without any stabilizers, surfactants or metals. The results clearly showed the presence of nanoparticles with an average size of 170 nm (PDI = 0.527) with two contiguous peaks which exhibit the stability of the particles. The particles were poly dispersed, a general pattern of distribution exhibited by nanoparticles from biological synthesis (Markus et al., 2016). Almost similar nanoparticle size of 166 nm (PDI = 0.291) have been reported previously for titanium oxide nanoparticles (Okuda-Shimazaki et al., 2010). The nanoparticles clearly showed variable shapes, cluster widespread, spherical and tubular bacillary. Similar spherical and cluster shaped AuNps were reported with probiotic, Lactobacillus kimchicus DCY51T (Markus et al., 2016). The XRD pattern revealed the low degree of crystallinity of the nano probiotic. It seems that the probiotic maintained a low level of crystallinity and it did not change its form during nano formulation. Some previous studies have suggested that stable amorphous forms (low crystallinity) are advantageous as they exhibit better solubility and therefore higher rates of dissolution which possibly enhances drug release and subsequent adsorption and bioavailability (Boateng and Areago, 2014; Vijayalakshmi et al., 2014). The FTIR spectra observed in the present study was similar to the pattern reported for the spectra of AuNps synthesized by Lactobacillus kimchicus DCY51T (Markus et al.,2016) and silver nanoparticles using a probiotic Bacillus licheniformis Dahb1 (Shanthi et al.,2016).
The results of the present study showed that the exposure of rats to Cd significantly increased the concentration of cadmium in the liver and kidneys. This is in line with previous studies which have also reported an elevation in Cd bioaccumulation in test tissues in rats (Klaassen et al., 2009). The high levels of Cd accumulation in both liver and kidney over time could be attributed to the fact that these organs are metabolically active and are involved in detoxification (Klaassen et al., 2009). In the present study, probiotic treatment both as bulk and nanoparticles markedly reduced the Cd bioaccumulation in the liver and kidneys. A similar study on broilers by Ghenioa et al. (2015) reported that a concurrent treatment of Bactosac®, a probiotic showed significant improvement in the accumulation pattern of Pb in target organs.
The Cd-induced oxidative stress in rats in this study was evident from the increased lipid peroxidation, manifested as the high serum MDA levels. These results are in line with previous experimental studies on Cd- induced oxidative stress (Renugadevi and Prabu, 2009; Haidari et al., 2013; Al-Anazi et al., 2015). The significant induction in MDA levels observed in Cd-exposed rats, mirrored the higher metal in target organs. A profound protective effect of the probiotic was observed as the serum levels of MDA were significantly reduced in the treated groups. These findings are in consensus with a previous studies which reported the antioxidant potential of probiotics (Castex et al., 2010; Zhao et al., 2020).
Liver dysfunction could possibly further lead to dysregulation of the GSH-dependent antioxidant system (Cheng et al., 2017). It has been reported that hepatic GSH levels could initially increase on Cd exposure and then decline with increased exposure duration (de Voogt, 2017). In congruence with this, in the present study the hepatic GSH levels were significantly enhanced on Cd exposure and treatment with the nanoprobiotic did reverse the effect and improved the overall hepatic oxidative stress in line with the study by Zhao et al. (2020). The metal-mediated generation of ROS can generate severe oxidative damage in nucleic acids, such as strand breaks and base oxidation 8-OHdG (Valavanidis et al., 2009). In this study the exposure to Cd showed a marked increase in the serum levels of 8-OHdG which corresponds to the Cd- induced oxidative DNA damage. Treatment with nanoprobiotic significantly reversed the effect of Cd on the oxidative DNA damage. The Cd-induced oxidative damage was further assessed on protein oxidation which leads to the formation of AOPPs. Exposure to Cd significantly increased the AOPP levels in plasma. In consensus to this, Husna et al. (2014) also reported enhanced levels of AOPPs in the ovarian cells of female rats exposed to Cd. Increasing evidence indicates AOPPs as a novel marker of oxidative stress (Sun et al., 2013) which are produced as a result of myeloperoxidase activity in activated neutrophils acting on hypochloric acid and chloramines; reliable markers of oxidative modification of proteins (Husna et al., 2014).
In the present study the levels of α-dicarbonyl compound, MG, an end product of advanced glycation were significantly increased on Cd exposure. Increased concentrations of MG are linked to oxidative stress, apoptosis and increased frequency of DNA strand breaks (Rabbani and Thornalley, 2012). A similar pattern was previously reported in the ovarian cells of female rats, exposed to Cd for a period of 4 weeks (Husna et al., 2014). The proposed mechanism is that metals can catalyze the 2,3-enediol and form MG and hydroperoxide (Husna et al., 2014). Treatment with the probiotic and nanoprobiotic significantly decreased the serum levels of 8-OHdG, AOPPs and MG owing to the antioxidant and chelating property of the probiotics.
A marked increase in the hepatic MT levels was observed in rats exposed to Cd. This corresponds to the increased bioaccumulation observed in the liver and kidneys which could have induced the MT biosynthesis. These proteins play a vital role in the detoxification of heavy metals and metal ion homeostasis, which is due to their high affinity for these metals (Ruttkay-Nedecky et al., 2013). The effect of the nanoprobiotic on lowering the MT levels was more profound. Recent studies in nutritional research have revived the health benefits of probiotics in humans (Wang et al., 2017) with the focus primarily on the Lactobacillus and Bifidobacterium strains commonly found in the probiotic formulations. A recent review by Wang et al. (2017) summarized that probiotics may modulate the redox status of the host via their metal ion chelating ability, antioxidant systems, regulating signaling pathways, controlling the enzyme producing ROS, and intestinal microbiota. The involvement of anionic surface groups on probiotic bacteria (L. rhamnosus and some Bifidobacterium longum strains) have been reported that increase the number of ligands capable of binding cationic metals such as cadmium and lead (Jama et al., 2012).
6 Conclusions
Thus, concurrent treatment with the probiotic and nano probiotic significantly reversed the Cd induced alterations and based on the broad assessment the nano probiotic was more efficacious.
Acknowledgement
The authors extend their appreciation to King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia for funding this project (registration no: 1-0080-001-01-17).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effects of Rosmarinus officinalis leaf extract and Vitamin C on cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus. J. Environ. Biol.. 2015;36(6):1401.
- [Google Scholar]
- Composite sodium alginate and chitosan based wafers for buccal delivery of macromolecules. Austin J. Anal. Pharm. Chem.. 2014;1(5):1022-1027.
- [Google Scholar]
- Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol.. 2010;28(4):622-631.
- [Google Scholar]
- Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS One. 2017;12(1):e0170016
- [CrossRef] [Google Scholar]
- de Voogt,P., 2017. Reviews of Environmental Contamination and Toxicology. Vol.244.Springer, ISBN; 3319668757, 9783319668758
- Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus) Environ. Res.. 2014;14(129):59-68.
- [CrossRef] [Google Scholar]
- Feher, J.,2012. Methods for preparing probiotic nanoparticles. , 2012.
- Protective effect of probiotic bactosac® against induced sub chronic lead toxicity in broiler chicks. AJVS. 2015;47:53-64.
- [Google Scholar]
- Green Tea (Camellia sinensis) supplementation to diabetic rats improves serum and hepatic oxidative stress markers. Iran. J. Pharm. Res.. 2013;12(1):109-114.
- [Google Scholar]
- The Role formation of methylglyoxal, carbonyl compound, hydrogen peroxide and advance oxidation protein product induced cadmium in ovarian rat. IJCEA. 2014;5(4):319-323.
- [Google Scholar]
- Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol.. 2014;7(2):60-72.
- [CrossRef] [Google Scholar]
- Protective effect of probiotic bacteria against cadmium-induced genotoxicity in rat hepatocytes In vivo and In Vitro. Arch. Biol. Sci. Belgrade. 2012;64(3):1197-1206.
- [Google Scholar]
- Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol.. 2009;238:215-220.
- [Google Scholar]
- Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb. Technol.. 2016;95:85-93.
- [Google Scholar]
- Maurya, P.K., Singh,S., 2019. Nanotechnology in Modern Animal Biotechnology: Concepts and Applications. Elsevier, ISBN0128188243, 9780128188248
- Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int. J. Mol. Sci.. 2010;11(6):2383-2392.
- [Google Scholar]
- Dicarbonyls (Glyoxal, Methylglyoxal, and 3-Deoxyglucosone) Uremic Toxins.. 2012;10:177-192.
- [Google Scholar]
- Naringenin protects againts cadmium –induced oxidative renal dysfunction in rats. Toxicol.. 2009;256(1):128-134.
- [Google Scholar]
- The role of metallothionein in oxidative stress. Int. J. Mol. Sci.. 2013;14(3):6044-6066.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using a probiotic Bacilluslicheniformis Dahb1 and their antibiofilm activity and toxicity effects in Ceriodaphnia cornuta. Microb. Pathog.. 2016;93:70-77.
- [Google Scholar]
- Spyropoulos, B., G., Misiakos, E., P., Fotiadis, C., and Stoidis, C., N. (2011). Antioxidant Properties of Probiotics and Their Protective Effects in the Pathogenesis of Radiation-Induced Enteritis and Colitis. Dig. Dis. Sci. 56, 285–294.
- Suhartono ,E., Triawanti, Setyo Leksono, A., Sasmito Djati, M.,2014. The Role of Cadmium in Proteins Glycation by Glucose: Formation of Methylglyoxal and Hydrogen Peroxide in Vitro. J Med Biol Eng. 3(1),59-62. doi: 10.12720/jomb.3.1.59-62
- Effect of advanced oxidation protein products on the proliferation and osteogenic differentiation of rat mesenchymal stem cells. Int. J. Mol. Med.. 2013;32:485-491.
- [CrossRef] [Google Scholar]
- Toma M.M.,Pokrotnieks, J.,2006. Probiotics as functional food: microbiological and medical aspects. Acta Univ. Latviensis.710, 2006, Biology,117–129.
- 8-hydroxy-2' - deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev.. 2009;27:120-139.
- [Google Scholar]
- Preparation and characterization of nanochitosan/sodium alginate/microcrystalline cellulose beads. Der Pharmacia Lett.. 2014;6(4):65-77.
- [Google Scholar]
- Effects of probiotic administration on hepatic antioxidative parameters depending on oxidative stress models: A meta-analysis of animal experiments. J. Functional Foods. 2020;71:103936
- [Google Scholar]







