Translate this page into:
Protective effect of oregano and sage essentials oils against the effect of extracellular H2O2 and SNP in Tetrahymena thermophila and Tetrahymena pyriformis
⁎Corresponding author. ab.soukri@gmail.com (Abdelaziz Soukri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oxidative stress results from an imbalance between the intracellular antioxidants and the radicals ROS or RNS, in favor of the latter. Oxidative stress is associated with several human diseases and causes significant cellular damage to the structure, nucleic acids (DNA, RNA), proteins and lipids. These consequences can compromise the health and viability of the living cell by causing cell death by necrosis or apoptosis.
A variety of antioxidant supplements have been developed in recent years to enrich the endogenous defense systems of living organisms. However, the prevention of chronic diseases through the use of antioxidant supplements remains controversial until now. However, natural products with regard to essential oils are very much in demand in biology research because of their antimicrobial and antioxidant properties.
We investigated the protective effect of oil of oregano and sage against the oxidative stress induced by hydrogen peroxide (H2O2) and sodium nitroprusside (SNP) in two cellular models Tetrahymena thermophila and Tetrahymena pyriformis. The results of our study showed that oxidative stress inhibits growth, mobility and changes in cell shape of both species. The application of essential oils has shown that both oils protect cells treated with H2O2 and SNP. However, the intracellular mechanism of SNP inhibition is longer than that of H2O2. Therefore, the use of these essential oils could be important to the prevention of chronic diseases related to oxidative stress in higher organisms.
Keywords
Oxidative stress
Hydrogen peroxyde
Sodium nitroprusside
Tetrahymena
Essentials oils
1 Introduction
The redox reactions are an important process in fundamental biology. Maintaining redox homeostasis is essential for cell survival and among the regulating factors are free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). These species are generated as by-products of cellular metabolism and are biologically important molecules, for example superoxide anion (O2–), hydrogen peroxide (H2O2), nitric oxide (NO) and peroxynitrite (ONOO–). It is described that ROS/RNS, at a moderate level of production, are considered as signal products regulating a series of physiological processes, while excessive production of these products, to the detriment of antioxidant defenses, is the main cause of oxidative stress in living organisms (Kurutas, 2016).
Oxidative stress is a process that is involved in the deterioration of several cellular components such as structure, nucleic acids, proteins and lipids (Errafiy and Soukri, 2012; Maes et al., 2009), which can compromise health and cell viability or induce a variety of cell response by generating secondary reactive species leading to cell death by necrosis or apoptosis (Flora, 2009; Valko et al., 2007). For this purpose, several defense mechanisms are developed by humans (Kurutas, 2016), bacteria (Tonner et al., 2015), archaea (Mishra and Imlay, 2012) and eukaryotes (Sies, 1993) to fight against oxidative stress by producing enzymatic and non-enzymatic antioxidants (Ames et al., 1993; Sardesai, 1995). In addition to these endogenous defense mechanisms, a panoply of research is based on the use of antioxidant supplements for the prevention of oxidative stress-related diseases. However, many large-scale controlled trials have yielded inconsistent and disappointing results in the prevention of chronic diseases (Kurutas, 2016).
The use of natural antioxidant substances is a growing area of research. During the last decade, many researchers have focused on aromatic and medicinal plants because of their extracts and essential oils that correspond to natural sources of antimicrobial compounds and antioxidants (Sagdic et al., 2003). Essential oils are oily, volatile and odoriferous products formed from aromatic plants as secondary metabolites. Their use is related to their antibacterial (Canillac and Mourey, 2001), antifungal (Nielsen and Rios, 2000), antiviral (Bishop, 1995), antiparasitic (Pessoa et al., 2002) and insecticidal (Konstantopoulou et al., 1992) properties. Essential oils have a broad spectrum of biological activities (Paster et al., 1990) including their antiseptic, anti-inflammatory, spasmolytic, sedative, analgesic, anesthetic and anti-tumor potential (Bakkali et al., 2008; Kumar et al., 2008).
This work is part of the objective of studying the protective effect of two essential oils, oregano and sage, against oxidative stress in two eukaryotic cell models Tetrahymena thermophila and Tetrahymena pyriformis following kinetic growth, morphology, mobility and cell density. These two species are widely used in physiological and toxicological studies (Darcy et al., 2002) and have led to the acquisition of a great deal of knowledge in fundamental biology (Leick et al., 1996; Tiedtke, 2001). The protozoan Tetrahymena combines the biological complexity of eukaryotes and the accessibility of unicellular organisms. It is very early introduced to the laboratory because of its ease of culture in axenic medium and its ideal length (50 μm) to studies under optical and electronic microscope.
2 Material and methods
2.1 Strains and culture conditions
The Tetrahymena thermophila SB 1969 and Tetrahymena pyriformis strains were used in this study. Both species were maintained in growth in an axenic medium, the PPYE medium composed of 1.5% (w / v) proteose peptone and 0.25% (w / v) yeast extract (Rodrigues-Pousada et al., 1979). The culture medium was inoculated with 1% (v/v) of a preculture of Tetrahymena thermophila (approximately 1.5 × 105 cells/ml) in Tetrahymena pyriformis (approximately 104 cells/ml). The protozoa were incubated without shaking at 32 ° C (Tetrahymena thermophila) or 28 ° C (Tetrahymena pyriformis).
2.2 Induction of oxidative stress
The two species Tetrahymena thermophila and Tetrahymena pyriformis were cultured in PPYE medium as previously described for one week (168 h). Hydrogen peroxide H2O2 (from Fluka) and sodium nitroprusside SNP (form Sigma Aldrich) were the stressors used in our study. The stressors were added, after 24 h of protozoa culture, to their concentration inhibiting half of the growth of protozoa determined from previous studies carried out in our laboratory (table 1) (Errafiy et al., 2013; Fourrat et al., 2007). H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis.
Stressors
Tetrahymena thermophila
Tetrahymena pyriformis
H2O2
0.7 mM
0.3 mM
SNP
1.8 mM
0.5 mM
Protozoan growth was monitored by sterile sampling every 3H and protozoan absorbance was measured at 600 nm using the Jenway 7315 UV-Visible Spectrophotometer. The optical zero corresponds to the culture medium which did not contain protozoa.
The shape and mobility of protozoa were also observed under an optical microscope. Samples were collected sterilely every 24 h, prepared between slide and cover slip and observed at objective ×10 of A.KRÜSS Optronic optical microscope.
2.3 Preparation of essential oils and determination of the MIC
Sage (salvia officinalis) and oregano (oreganum vulgare) oils were obtained directly from leaves. Plant material was harvested randomly, then washed and dried in a well-ventilated place at room temperature for ten days before their use. Essential oils were obtained by hydrodistillation using the standard Clevenger apparatus. The essentials oils were extracted from the distillate with hexane and dehydrated by passing through anhydrous sodium sulfate. After filtration, the solvent was removed by distillation under reduced pressure in a rotary evaporator at 35 °C, and the pure oils stored in an amber vial at 4 °C, until their use.
The determination of the minimum inhibitory concentration of essential oils was based on the serial dilution method (Baron and Fingold, 1990). Each essential oil was dissolved in a sterile 0.2% (w/v) agar solution and a dilution series ranging from 10−1 to 10−9 was prepared. Each concentration was added at 0.1‰ (v/v) in a tube containing 5 ml of PPYE culture medium and 1% (v/v) of a preculture. The MIC was the lowest concentration that showed no turbidity in the tube after 72 h incubation of protozoa. The non-lethal concentration of essential oils was the concentration that did not affect the growth and shape of the cells.
2.4 Protective effect of essential oils
To evaluate the protective effect of essential oils, the latter were added to their non-lethal concentration at the same time as the protozoa (1%, v/v) were introduced into the culture medium. After 24 h of protozoan culture, the H2O2 and SNP stressors were added to their concentration inhibiting half the growth of the protozoa. H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis (Table 1). Thus, the growth and form of the protozoa were monitored during 168 h of culture by measuring the optical density and visualization of the cells under optical microscope.
2.5 Cell count
The number of living cells was counted parallel to the kinetic growth monitoring. Thus the number of cells was determined by in counting the malassez slide (0.0025 mm2, 0.2 mm deep). Cell viability was verified by the trypan blue exclusion method by adding 0.2% (v/v) of the product. The cells were fixed in 0.2% formaldehyde (prepared in Phosphate Buffered Saline at pH 7), 10 μl of sample was deposited on the malassez slide and the counting was performed on 100 fields. Statistical significance was determined by Student’s t-test. Differences were considered significant if p < 0.05; and highly significant if p < 0.01 when compared to control.
3 Results
3.1 Effect of extracellular H2O2 and SNP on protozoa
Tetrahymena thermophila and Tetrahymena pyriformis were grown in the presence of H2O2 and SNP stressors as described in materials and methods. During 168 h, the growth, mobility and form of protozoa were studied. As shown in Fig. 1, there was a difference in growth in both species depending on the stress agent used. The growth of Tetrahymena thermophila was completely inhibited in the presence of H2O2 while the latter slowed the growth of Tetrahymena pyriformis. In the presence of the SNP, there was a significant decrease in the growth of both species and in particular a longer latency than the unstressed cells (control). The growth of Tetrahymena thermophila was more sensitive to H2O2, while the growth of Tetrahymena pyriformis was more sensitive to SNP.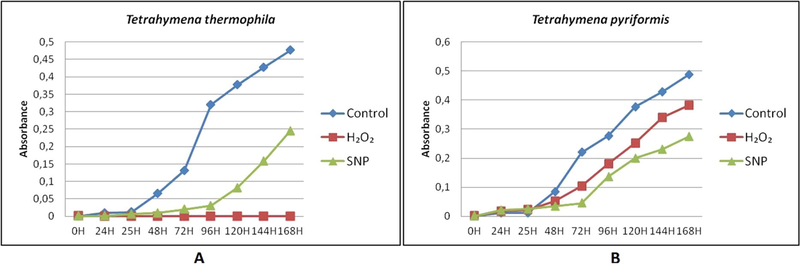
H2O2 and SNP effects on Tetrahymena thermophila and Tetrahymena pyriformis growth during 168 h. A: Tetrahymena thermophila; B: Tetrahymena pyriformis. Cell absorbance was determined at 600 nm each 24 h of growth at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) in PPYE medium containing H2O2 at 0.7 mM (T. thermophila) or 0.3 mM (T. pyriformis); or SNP at 1.8 mM (T. thermophila) or 0.5 mM (T. pyriformis) after 24 h of protozoa growth. Cells grown in absence of stressors were taken as control.
It was also noted that the form of the protozoa was different in both species (Fig. 2). In the presence of H2O2, Tetrahymena thermophila cells had a rounded shape and were immobile. In contrast, Tetrahymena pyriformis cells were normal in appearance with several dark vacuoles appearing and moving faster than untreated cells. In the presence of the SNP, the morphology of the protozoa was more affected. The cells had a narrow form in both species and moved slowly relative to normal cells.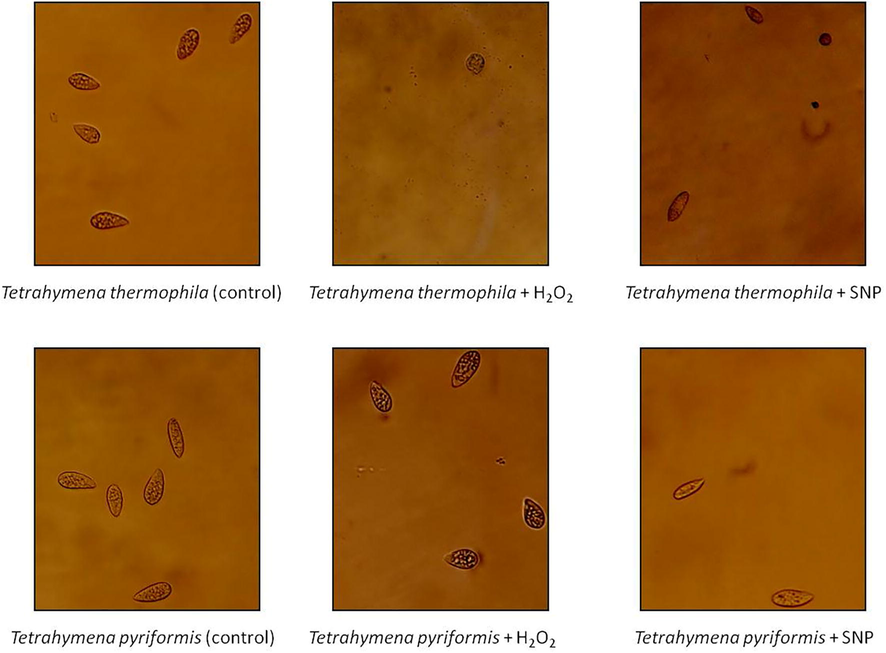
Microscopic images of Tetrahymena thermophila and Tetrahymena pyriformis treated with H2O2 and SNP stressors (at 168 h). Microscopic images were taken at ×10 objective of cells grown in PPYE medium at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) containing H2O2 (0.7 mM for T. thermophila or 0.3 mM for T. pyriformis) or SNP (1.8 mM for T. thermophila or 0.5 mM for T. pyriformis). Cells grown in absence of stressors were taken as control.
3.2 Determination of the MIC and non-lethal concentration of essential oils
The protozoa were cultured in the presence of the different concentrations of the essential oils mentioned above. The results show that essential oils were toxic at high doses (Table 2) lead to cell death, in this case stopping the growth of protozoa. The effect of essential oils on protozoa did not show a significant difference in the two species Tetrahymena thermophila and Tetrahymena pyriformis. The non-lethal concentration (10−3) of the essential oils was used to evaluate the anti-stress effect of these oils.
Essentials oils
Oregano
Sage
MIC
10−1 (0.1‰, v/v)
Pure oil (0.1‰, v/v)
Non-lethal concentration
10−3 (0.1‰, v/v)
10−3 (0.1‰, v/v)
3.3 Protective effect of essential oils against oxidative stress
The protozoa were cultured in the presence of essential oils; the H2O2 and SNP stressors were added after 24 h of culture. Three important parameters are monitored for 168 h: the kinetic growth, the cell density and the protozoa form.
3.3.1 Kinetic growth of protozoa
When the cells were treated with oregano and stressors, there was a marked improvement in the growth of protozoa treated with H2O2 in both species (Fig. 3A and B), including Tetrahymena thermophila which was very sensitive to this reagent (Figs. 3A and 4A). However, oregano had no effect on the growth of Tetrahymena thermophila treated with SNP (Fig. 3C) while there was a slight improvement in the growth of Tetrahymena pyriformis (Fig. 3D).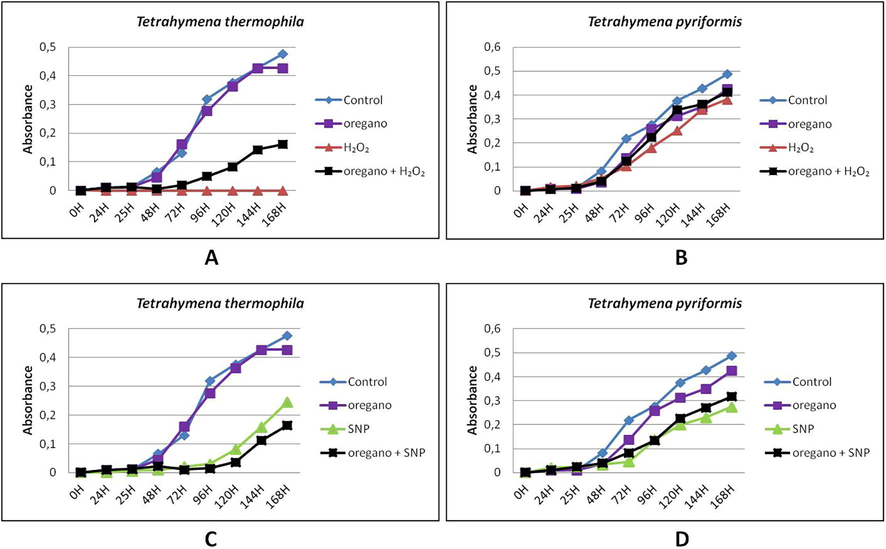
Effect of oregano on the growth of Tetrahymena thermophila and Tetrahymena pyriformis treated with H2O2 and SNP during 168 h. A: Tetrahymena thermophila treated with H2O2; B: Tetrahymena pyriformis treated with H2O2; C: Tetrahymena thermophila treated with SNP; D: Tetrahymena pyriformis treated with SNP. Cell absorbance was determined at 600 nm each 24 h of growth at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) in PPYE medium containing 0.1% of 10−3 diluted oregano oil. Stressors agent were added after 24 h of cell culture, H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis. Cells grown in absence of stressors were taken as control.
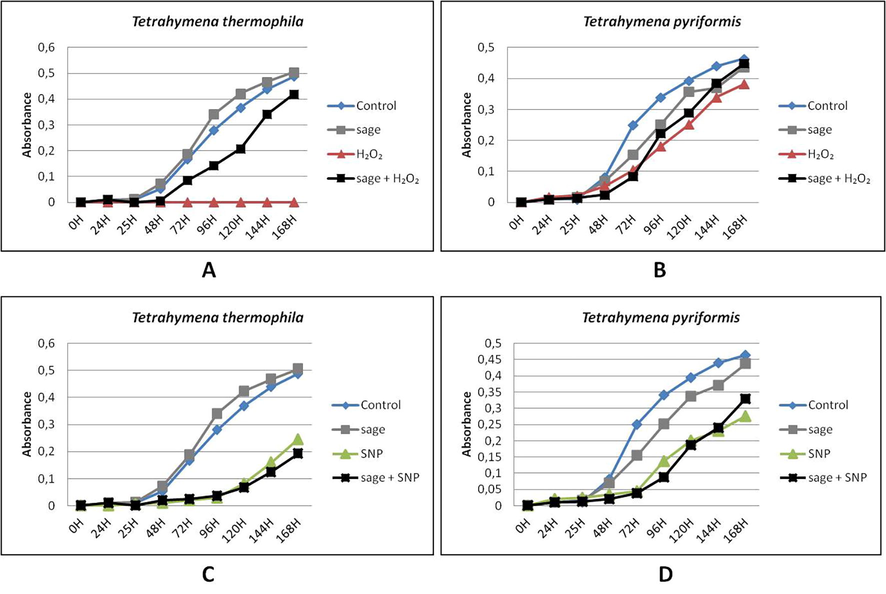
Effect of sage on the growth of Tetrahymena thermophila and Tetrahymena pyriformis treated with H2O2 and SNP during 168 h. A: Tetrahymena thermophila treated with H2O2; B: Tetrahymena pyriformis treated with H2O2; C: Tetrahymena thermophila treated with SNP; D: Tetrahymena pyriformis treated with SNP. Cell absorbance was determined at 600 nm each 24 h of growth at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) in PPYE medium containing 0.1% of 10−3 diluted sage oil. Stressors agent were added after 24 h of cell culture, H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis. Cells grown in absence of stressors were taken as control.
In the presence of sage, there was a significant improvement in the growth of protozoa treated with H2O2, similar to the normal cell growth (Fig. 4A and B). However, sage had no significant effect on the growth of both species treated with SNP (Fig. 4C and D).
3.3.2 Cell density of protozoa
During the kinetic growth of protozoa, the number of viable cells was counted in three essential phases of the normal growth cycle of Tetrahymena: the latent phase (24 h), the exponential phase (72 h) and the stationary phase (168 h) (Fig. 5). Cell viability was determined by the trypan blue exclusion test, which accumulates and stains non-viable cells (Fig. 6). The results showed that the number of cells treated with essential oils had no significant difference with the number of untreated cells (control), ranging between 200·106–250·106 cells/ml in Tetrahymena thermophila and 150·106–200106 cells/ml in Tetrahymena pyriformis during the stationary phase. The number of cells treated with H2O2 decreased significantly in Tetrahymena thermophila (p < 0.01) while it decreased slightly in Tetrahymena pyriformis. In the presence of the SNP agent, the number of cells during the stationary phase decreased by half in Tetrahymena pyriformis (p < 0.5) whereas in Tetrahymena thermophila the number of cells decreased considerably (4 times less than the number of normal cells, p < 0.05).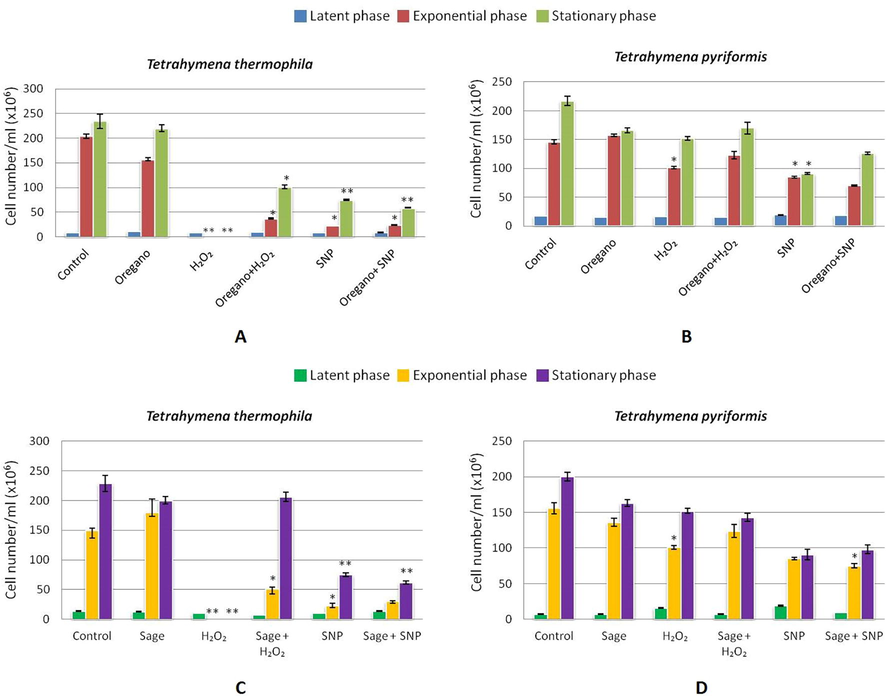
Enumeration of protozoa during the different phases of cell growth. A: Oregano effect on Tetrahymena thermophila treated with H2O2 or SNP; B: Oregano effect on Tetrahymena pyriformis treated with H2O2 or SNP; C: Sage effect on Tetrahymena thermophila treated with H2O2 or SNP; D: Sage effect on Tetrahymena pyriformis treated with H2O2 or SNP. Cell numbers were determined after 24, 72 and 168 h corresponding to the latent, exponential and stationary phases respectively. Cells were grown in PPYE medium containing 0.1% of 10−3 diluted oregano (or sage) oil. Stressors agent were added after 24 h of cell culture, H2O2 was added at 0.7 mM for T.thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis. For significance comparisons, Student’s t-test was used (*significant at p < 0.05 and **highly significant at p < 0.01).
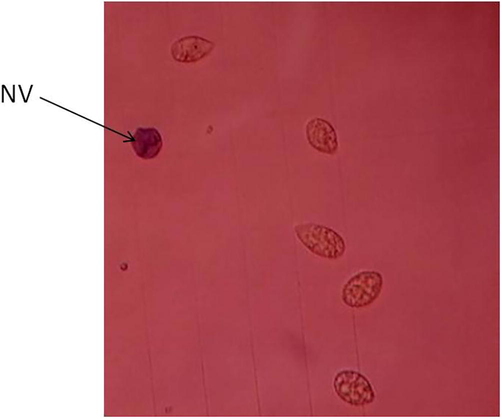
Trypan blue exclusion test. Microscopic image was taken at x10 objective of Tetrahymena pyriformis growth at 28 °C in PPYE medium containing 0.5 mM of H2O2. Blue stained cells were non viable cells (NV).
In the presence of oregano, the number of cells treated with H2O2 increased significantly in Tetrahymena thermophila (p < 0.5) and slightly in Tetrahymena pyriformis (Fig. 5A and B). The number of SNP-treated cells remained low in both species (Fig. 5A and B).
In the presence of sage, there was a significant increase in the number of Tetrahymena thermophila cells treated with H2O2, similar to the number of normal cells after 168 h. However, the number remained low in the presence of the SNP (p < 0.5). In Tetrahymena pyriformis, sage had no significant effect on the number of cells treated with both stressors (Fig. 5C and D).
3.3.3 Morphology of protozoa
The shape of the protozoa was correlated with the kinetics of growth. Each 24 h, samples were collected sterilely and observed under optical microscope. In the standard culture medium (control), the cells had a pear shape which characterizes the normal form of protozoa. The cells multiply throughout the exponential phase and stop multiplying at the end of the stationary phase, by acquiring their maximum size. When the protozoa were treated with the essential oils at their non-lethal concentration, the shape of the treated cells was identical to the shape of the normal cells (Figs. 7 and 8).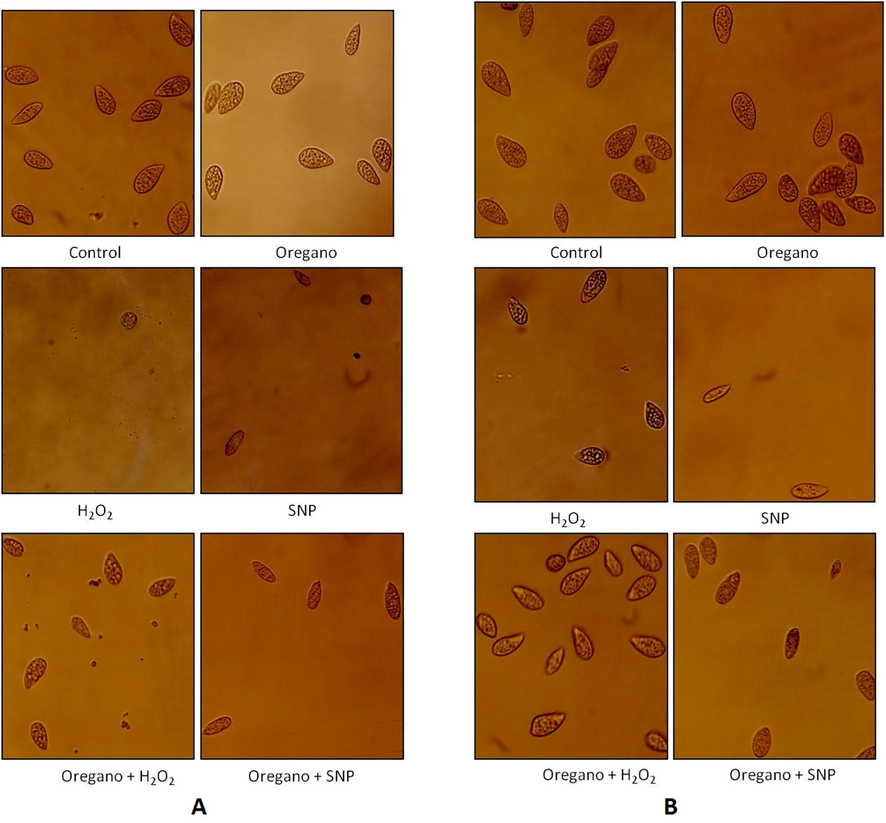
Microscopic images of protozoa showing the protective effect of oregano against H2O2 and SNP agents at 168 h. A: Tetrahymena thermophila; B: Tetrahymena pyriformis. Microscopic images were taken at ×10 objective of cells grown in PPYE medium at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) containing 0.1% of 10−3 diluted oregano oil. Stressors agent were added after 24 h of cell culture, H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis. Cells grown without stressors were taken as control.
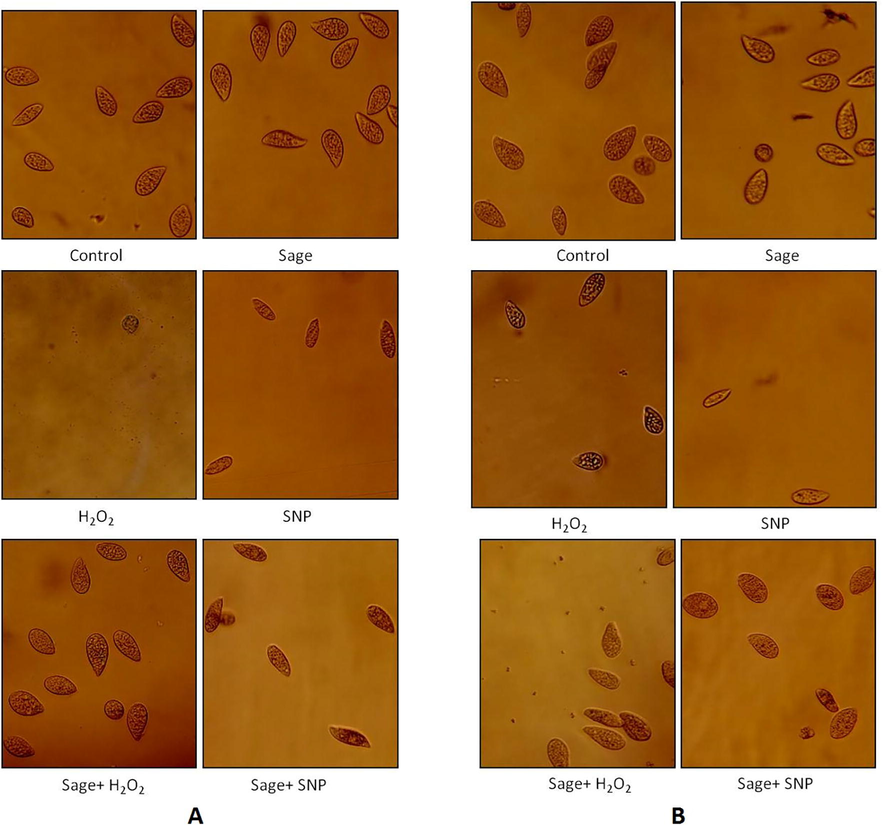
Microscopic images of protozoa showing the protective effect of sage against H2O2 and SNP agents at 168 h. A: Tetrahymena thermophila; B: Tetrahymena pyriformis. Microscopic images were taken at x10 objective of cells grown in PPYE medium at 32 °C (Tetrahymena thermophila) or 28 °C (Tetrahymena pyriformis) containing 0.1% of 10−3 diluted sage oil. Stressors agent were added after 24 h of cell culture, H2O2 was added at 0.7 mM for T. thermophila or 0.3 mM for T. pyriformis, SNP was added at 1.8 mM for T. thermophila or 0.5 mM for T. pyriformis. Cells grown without stressors were taken as control.
Fig. 7 showed that in the presence of oregano oil, the majority of Tetrahymena thermophila cells treated with H2O2 and SNP had a normal form at the end of the stationary phase. In Tetrahymena pyriformis, the addition of H2O2 in the presence of oregano oil did not affect the shape of the cells, just as the addition of SNP showed that most cells had a normal form. In the presence of sage, it was found that the majority of the cells of both species also had a normal form after 168 h (Fig. 8).
4 Discussion
The results of this work show that oxidative stress agents induce a change in several physiological parameters in protozoa Tetrahymena thermophila and Tetrahymena pyriformis including growth, shape, density and cell mobility. Under normal conditions, the protozoa have a growth characterized by three essential phases: a latent phase which lasts 24 h followed by an exponential phase and a stationary phase which approaches 168 h. When protozoa are treated with stressors, there is a disruption in protozoan growth that varies with the species and stress agent used. Hydrogen peroxide completely inhibits the growth of Tetrahymena thermophila while it slows the growth of Tetrahymena pyriformis, while nitroprusside sodium slows the growth of both species. However, the protozoan Tetrahymena thermophila is more sensitive to hydrogen peroxide and less sensitive to SNP, while Tetrahymena pyriformis is more sensitive to SNP than to hydrogen peroxide. Similar results have been reported in the work of our laboratory (Errafiy et al., 2013; Fourrat et al., 2007). Other studies have shown that hydrogen peroxide inhibits the growth of Yarrowia lipolytica yeast (Biryukova et al., 2006). Similarly, the stress induced by RNS inhibits the growth of yeasts Saccharomyces cerevisae and Rhodotorula mucilginosa (Sahoo et al., 2003).
Oxidative stress also causes a change in the morphology of protozoa. The results showed that the shape of both species is more affected in the presence of the SNP. An earlier study revealed that the SNP induced a significant change in the structure of the protozoan Tetrahymena thermophila (Errafiy et al., 2013). Hydrogen peroxide is recognized in oxidative stress as a messenger molecule that diffuses through cells to initiate intermediate cellular effects such as changes in the shape and recruitment of immune cells (Sies, 2017). SNP is known as a donor of nitric oxide (NO). Excessive NO production as a radical can react rapidly with ROS such as superoxide anion (O2–) to form peroxynitrite (ONOO–) (Beckman et al., 1990). The latter acts as a biological oxidant by affecting mitochondrial functions and triggering cell death by apoptosis (Sies et al., 2017).
Living organisms have a wide range of enzymatic and non-enzymatic antioxidant defense to combat oxidative stress. Aerobic cells use multiple enzymes to control the excessive production of ROS/RNS, such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) (Halliwell and Gutteridge, 1999). In the presence of the H2O2 and SNP stressors, it is shown that the level of SOD and CAT produced is higher than the normal rate in the protozoan Tetrahymena thermophila (Errafiy et al., 2013). Catalase plays an important role in oxidative stress by breaking down hydrogen peroxide into a molecule of water and an oxygen molecule. Superoxide dismutase also plays a crucial role in oxidative stress by catalyzing the conversion of superoxide free radicals to hydrogen peroxide, which is easily degraded by catalase or glutathione peroxidase (Inal et al., 2001).
Essential oils are widely used in biological studies due to their broad spectrum of action (Cimanga et al., 2002; Cowan, 1999). They are used in several fields such as aromatherapy, perfumery, pharmacy, food preservation and cosmetics. Several studies have shown that essential oils and their constituents have considerable potential as an antimicrobial (Başer et al., 2002; Dorman and Deans, 2000) and antiprotozoal (Mojica et al., 2004) agent. To remedy the damage caused by oxidative stress, we used two essential oils: oregano and sage. Oregano oil has several properties, such as antimicrobial, antimutagen and antioxidant (Sarikurkcu et al., 2015). Sage oil also has important antibacterial and antioxidant properties (Bouajaj et al., 2013). However, it is necessary to test the toxic effect of oils against the protozoa Tetrahymena. The results obtained in our study showed that the essential oils have a toxic effect on the cells according to the concentration used, and totally inhibit the protozoa growth at their respective MIC (Table 2). However, the non-lethal concentration (10−3) does not affect the growth or morphology of the protozoa.
The non-lethal concentration of the two essential oils is used to evaluate their protective effect against stressors. The results showed that oregano and sage protect protozoa against H2O2-induced stress, significantly increasing growth and maintaining the normal protozoan form. Studies have shown that Oregano Oil protects porcine small intestine epithelial cells against H2O2-induced oxidative stress by increasing the production of SOD and glutathione peroxidase (Zou et al., 2016). Sage oil was reported to have significant antioxidant activity through its phenolic compounds (Schwarz and Ternes, 1992). For this purpose, we can say that the oils used in our study can have a direct effect on the activity of ROS by trapping them or an indirect effect by increasing the production of intracellular antioxidant enzymes.
In the presence of SNP (NO donor), it is found that Oregano and Sage oils have no immediate effect on protozoan growth, while they protect normal cell morphology. Protozoan growth retardation can result from excessive NO production that can induce a reversible (or irreversible) modification of cellular proteins (Wink and Mitchell, 1998; Zou et al., 1999). NO-induced stress inhibits several enzymes essential for metabolism, including the specific activity of GAPDH by S-nitrosylation correlated with a significant decrease in the growth of protozoan Tetrahymena thermophila (Errafiy et al., 2013). For this purpose, we can deduce from these parameters that both oils have a protective effect against the toxic effects of reactive species. The slow growth of protozoa is due to the regulation of nitric oxide-induced cell metabolism. An improvement in protozoan growth was observed after two weeks of culture (results not shown).
By comparing the effect of both oils on the morphology and growth of protozoa in the presence of stressors, we can deduce that sage oil has a higher protective effect than oil of oregano. Similar results have reported that the phenolic extracts of sage (Salvia officinalis and Salvia fructicosa) have on average better antioxidant activity than those of oregano oil (Origanum onites and Origanum intercedens) (Pizzale et al., 2002).
5 Conclusion
Oxidative stress causes significant cellular damage and is the object of ideal antioxidant supplements research for combating this phenomenon. Our study conducted on the Tetrahymena cell model shows that oxidative stress has negative consequences on growth, shape and cell density. The application of essential oils showed a protection of the cells against the oxidative stress induced by hydrogen peroxide in the short-term, by the sodium nitroprusside in the long term. Therefore, the use of these natural compounds may have a beneficial effect in higher organisms and contribute to the prevention of chronic diseases related to oxidative stress.
References
- Oxidants, Antioxidants, and the Degenerative Diseases of Aging. PNAS. 1993;90(17):7915-7922.
- [Google Scholar]
- Biological effects of essential oils – a review. Food Chem. Toxicol.. 2008;46:446-475.
- [CrossRef] [Google Scholar]
- Methods for testing antimicrobial effectiveness, in. In: Mosby C.V., ed. Diagnostic Microbiology. Baltimore; 1990. p. :171-194.
- [Google Scholar]
- Composition and antimicrobial activity of the essential oil of achillea multifida. Planta Med.. 2002;68:941-943.
- [CrossRef] [Google Scholar]
- Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci.. 1990;87:1620-1624.
- [CrossRef] [Google Scholar]
- Tolerance of the yeast Yarrowia lipolytica to oxidative stress. Microbiology. 2006;75:243-247.
- [CrossRef] [Google Scholar]
- Antiviral activity of the essential oil of melaleuca alternifolia (Maiden & Betche) cheel (Tea Tree) against tobacco mosaic virus. J. Essent. Oil Res.. 1995;7:641-644.
- [CrossRef] [Google Scholar]
- Antibacterial, allelopathic and antioxidant activities of essential oil of Salvia officinalis L. growing wild in the Atlas Mountains of Morocco. Nat. Prod. Res.. 2013;27:1673-1676.
- [CrossRef] [Google Scholar]
- Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol.. 2001;18:261-268.
- [CrossRef] [Google Scholar]
- Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol.. 2002;79:213-220.
- [Google Scholar]
- The effect of lofepramine and other related agents on the motility of Tetrahymena pyriformis. Toxicol. Lett.. 2002;128:207-214.
- [CrossRef] [Google Scholar]
- Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol.. 2000;88:308-316.
- [Google Scholar]
- Protective effect of some essential oils against oxidative and nitrosative stress on Tetrahymena thermophila growth. J. Essent. Oil Res.. 2013;25:339-347.
- [CrossRef] [Google Scholar]
- Purification and partial characterization of glyceraldehyde-3-phosphate dehydrogenase from the ciliate Tetrahymena thermophila. Acta Biochim. Biophys. Sin.. 2012;44:527-534.
- [CrossRef] [Google Scholar]
- Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev.. 2009;2:191-206.
- [Google Scholar]
- Effects of oxidative and nitrosative stress on tetrahymena pyriformis glyceraldehyde-3-phosphate dehydrogenase. J. Eukaryot. Microbiol.. 2007;54:338-346.
- [CrossRef] [Google Scholar]
- Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1999. pp. 936
- Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta. 2001;305:75-80.
- [CrossRef] [Google Scholar]
- Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia. 1992;48:616-619.
- [CrossRef] [Google Scholar]
- An essential oil and its major constituent isointermedeol induce apoptosis by increased expression of mitochondrial cytochrome c and apical death receptors in human leukaemia HL-60 cells. Chem. Biol. Interact.. 2008;171:332-347.
- [CrossRef] [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J.. 2016;15:71.
- [CrossRef] [Google Scholar]
- ConcanavalinA receptors and the chemosensory behaviour of Tetrahymena thermophila. Exp. Biol. Online. 1996;1:1-12.
- [CrossRef] [Google Scholar]
- Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol. Lett.. 2009;30:715-722.
- [Google Scholar]
- Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch. Biochem. Biophys.. 2012;525:145-160.
- [CrossRef] [Google Scholar]
- Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol.. 2000;60:219-229.
- [Google Scholar]
- Inhibitory effect of oregano and thyme essential oils on moulds and foodborne bacteria. Lett. Appl. Microbiol.. 1990;11:33-37.
- [CrossRef] [Google Scholar]
- Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet. Parasitol.. 2002;109:59-63.
- [Google Scholar]
- Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric.. 2002;82:1645-1651.
- [CrossRef] [Google Scholar]
- Characterization of preribosomal ribonucleoprotein particles from tetrahymena pyriformis. Eur. J. Biochem.. 1979;102:389-397.
- [CrossRef] [Google Scholar]
- Note: Effect of some spice extracts on bacterial inhibition. Food Sci. Technol. Int.. 2003;9:353-358.
- [CrossRef] [Google Scholar]
- Nitrosative stress on yeast: inhibition of glyoxalase-I and glyceraldehyde-3-phosphate dehydrogenase in the presence of GSNO. Biochem. Biophys. Res. Commun.. 2003;302:665-670.
- [CrossRef] [Google Scholar]
- Role of antioxidants in health maintenance. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr.. 1995;10:19-25.
- [CrossRef] [Google Scholar]
- Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. Complete 2015:178-184.
- [CrossRef] [Google Scholar]
- Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z. Lebensm. Unters. Forsch.. 1992;195:99-103.
- [Google Scholar]
- Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol.. 2017;11:613-619.
- [CrossRef] [Google Scholar]
- Biotechnology with Protozoa. In: Rehm H.-J., Reed G., eds. Biotechnology Set. Wiley-VCH Verlag GmbH; 2001. doi: 10.1002/9783527620999.ch6k
- [Google Scholar]
- A regulatory hierarchy controls the dynamic transcriptional response to extreme oxidative stress in archaea. PLOS Genet.. 2015;11:e1004912.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39:44-84.
- [CrossRef] [Google Scholar]
- Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med.. 1998;25:434-456.
- [CrossRef] [Google Scholar]
- Peroxynitrite inactivates prostacyclin synthase by heme–thiolate-catalyzed tyrosine nitration. Drug Metab. Rev.. 1999;31:343-349.
- [CrossRef] [Google Scholar]
- Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxid. Med. Cell. Longev.. 2016;2016
- [CrossRef] [Google Scholar]







