Translate this page into:
Protective effect of gallic acid against thioacetamide-induced metabolic dysfunction of lipids in hepatic and renal toxicity
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. iftekharhassan2002@gmail.com (Iftekhar Hassan) ihassan@ksu.edu.sa (Iftekhar Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Gallic acid (GA) has significant antioxidant bioactivity and can prevent diet-induced hypertriglyceridemia by reducing adipocytes. GA was also observed to enhance cell glucose uptake.
Methods
The current study looked at the effect of gallic acid (GA) (100 mg, 200 mg/kg orally) on liver and kidney damage caused by thioacetamide (TAA; 100 mg/Kg via intraperitoneal route). TAA was treated thrice weekly for eight weeks, whereas gallic acid was administered daily.
Results
GA alleviated the thioacetamide-induced decreases in hepatic or renal reduced glutathione (GSH) or increases in malondialdehyde (MDA, an indication of lipid peroxidation). TAA treatment significantly increased plasma inflammatory markers, tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP), liver enzymes, Gamma-glutamyltransferase (GGT), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), alkaline phosphatase (ALP), and kidney function parameters (creatinine and urea) and uric acid. However, these values decreased after GA treatment in a dose-dependent manner. Furthermore, GA mitigated the considerable decrease in plasma protein caused by TAA. GA also reduced hepatic fibrosis or histological abnormalities in the liver and kidney.
Conclusion
Our results suggest that GA may attenuate TAA-induced liver and kidney toxicity via suppression of oxidative stress and inflammatory markers. Moreover, the hypolipidemic effect of GA may be considered another route for protection.
Keywords
Gallic acid
Thioacetamide hepatic injury
Kidney injury
Oxidative stress
Inflammatory markers
1 Introduction
Thioacetamide (TAA) is a commonly used additive in the food, leather processing, laboratory, beverage, textile, paper, and motor fuel industries (Choubey et al., 2018). TAA has been classified as an established human carcinogen (Gholamine et al., 2021). TAA is a well-known liver toxicant that requires oxidative bioactivation to become hepatotoxic, affecting amine lipids and proteins (Lu et al., 2006). A single dose of TAA causes centrilobular necrosis in liver cells, and increases in plasma transaminases and bilirubin levels cause acute hepatic injury. Chronic exposure, on the other hand, induces hepatic cirrhosis, leading to liver tumorogenesis and cytomegaly (Lu et al., 2006; Hussein et al., 2020). TAA-treated rats showed changes in the structure of renal corpuscles like Bowman's capsule and glomerular degeneration (Owumi et al., 2020). The deleterious effects of TAA on the rat’s kidney include DNA damage, oxidative stress, cytokine release and abnormal renal function (Dehghani et al., 2020). TAA causes cell death in the proximal renal tubules. TAA bioactivation generates thioacetamide S-oxide, peroxide radicals, and reactive oxygen species (ROS). The free radicals disperse throughout the body’s organs (Olayinka et al., 2015). Humans are exposed to TAA in two ways: inhaling/ingesting harmful fumes or absorbing them through the skin (Dehghani et al., 2020).
Oxidative stress is caused by the overproduction and accumulation of free radicals. It is one of the major causes of various degenerative diseases such as atherosclerosis, cancer, aging, cardiovascular, and inflammatory disorders. Natural products are potent antioxidants for managing several diseases, such as diabetes (Choubey et al., 2018; Gholamine et al., 2021), and slow diabetic wound healing (Lu et al., 2006; Hussein et al., 2020). Toxic insults to the kidney (Owumi et al., 2020; Dehghani et al., 2020), liver (Olayinka et al., 2015) and brain (Nabavi et al., 2013) can increase antioxidant enzymatic activity by scavenging ROS. Polyphenols are naturally occurring antioxidants with a wide range of biological actions, including antibacterial, anticancer, antiviral, antifungal, and anticholesterol (Albus, 2012). Gallic acid (GA) (3, 4, 5-trihydroxy benzoic acid), a low molecular triphenolic molecule, is highly antioxidant with a strong ability to induce apoptosis (Choubey et al., 2018). GA is a phenolic molecule that protects against oxidative stress-induced damage to many tissues (Gholamine et al., 2021). The potent antioxidant activity and suitable hydrophobicity of GA are very efficient in reducing oxidative damage causing neurodegenerative disorders (Lu et al., 2006). It is documented that GA has a protective capacity against toxic insults to the liver and kidney, attributed to decreasing the oxidative burden on the tissues (Hussein et al., 2020). In addition, TAA-induced liver fibrosis in rats has been reported to be alleviated by GA because of antioxidant and hepatoprotective properties (Hussein et al., 2020). By increasing catalase and glutathione S-transferase activity, the chemical can also protect diabetic rats' livers from oxidative stress-induced damage. It decreases the number of nuclei while increasing the size of the core in hepatic tissue and increases the glomerular area in renal tissue (Owumi et al., 2020). In acute nephrotoxicity caused by cisplatin in rats, the pure GA nanoparticles reduce oxidative stress, inflammation, and mitochondrial dysfunction (Dehghani et al., 2020). This protective effect is thought to be due to GA's antioxidant and anti-inflammatory properties (Olayinka et al., 2015). Sodium fluoride caused nephrotoxicity and oxidative stress in renal tissues, but daily treatment with GA for 1 week previous to intoxication reduced toxicity and oxidative stress (Nabavi et al., 2013).
This study focuses on the influence of gallic acid on TAA-induced liver and kidney damage in male rats. The study involved using liver and kidney function tests, a lipid profile, inflammatory markers, and oxidative stress parameters.
2 Material and methods
2.1 Chemicals
Gallic acid (GA) and thioacetamide (TAA) were bought from Sigma, USA. Other chemicals used in these experiments were of analytical grade obtained from commercial suppliers.
2.2 Animals
Adult male Wistar rats (150-160 g) were procured from the animal house of NRC, Egypt. The animals were fed a standard pellet diet and water ad libitum and kept at an adjusted temperature (22 ± 2 °C) with a 12 h light–dark cycle. Animal handling was carried out following the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Albus, 2012).
2.3 Grouping
Twenty-four rats were sorted into four groups in a random fashion. Group 1: A control group was administered orally at the same volume as the vehicle. Group II: TAA100 mg/kg intraperitoneally (IP) was given to rats (de David et al., 2011). Group III: Rats were pre-treated with 100 mg/kg of gallic acid orally (Kalantar et al., 2018), followed by TAA intraperitoneally after 30 min. Both gallic acid and TAA were dissolved in distilled water. Group IV: Rats pre-treated with 200 mg/kg GA orally (Kalantar et al., 2018), followed by treatment with TAA intraperitoneally after 30 min. The rats were treated with TAA three times weekly and treated with GA for eight weeks.
2.4 Samples collection
After eight weeks, three milliliters of blood were drawn from the retro-orbital plexus in heparinized tubes under anesthesia. Animals were euthanized via cervical dislocation. Centrifugation at 3000 g for 15 min was used to separate the plasma. The liver and kidney samples were quickly extracted and cleaned in ice-cold saline. A weighed portion of each tissue was homogenized with ice-cold saline (0.9 % NaCl) for homogenate. The homogenate was centrifuged for 10 min at 4 °C at 3000 rpm using a cooled centrifuge (Laborzentrifugen, Sigma, Germany). The collected supernatant was employed in many analyses. The remainder of the liver or kidney was promptly fixed in 10 % neutral buffered formalin.
2.5 Biochemical analysis in plasma
All the routine bio kits for liver, kidney functions, and lipid profiles were procured from an Egyptian company for biotechnology.
2.6 Plasma inflammatory markers
Tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) were assayed by enzyme-immunoassay using a kit manufactured by R & D Systems, USA.
Hepatic and renal MDA and GSH.
MDA and GSH were determined colorimetrically in liver and kidney homogenates using Biodiagnostic kits (Egypt).
2.7 Histological analysis
For one week, the testis was immersed in 10 % formal saline. The samples were subjected to routine histology and stained with hematoxylin and eosin.
2.8 Statistical analysis
Statistical analysis of the data was performed using the SPSS program. One-way analysis of variance with Tukey’s test. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. The data are provided as mean SEM, with significance levels set at P < 0.05, 0.005, and 0.001.
3 Results
3.1 Liver function tests
The results presented in Fig. 1, indicated a significant increase (P ≤ 0.05) in plasma liver enzymes (ST, ALT, GGT, ALP) of rats treated with TAA. The liver enzymes were significantly stored to values close to normal in rats treated with GA + TAA (low and high doses: 100, 200 mg/kg) compared to the TAA group. The effect of GA on liver enzymes was dose-dependent.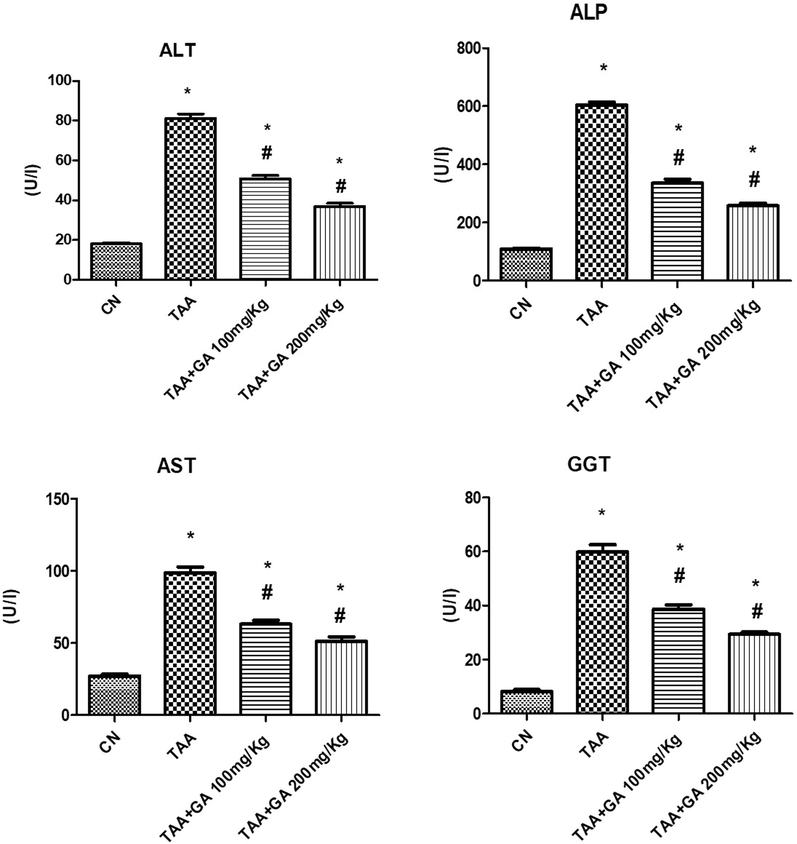
The levels of liver function enzymes (ALT, ALP, AST and GGT) in plasma from different rat groups. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. Values in the histogram are the mean ± SEM, and significance was set at P < 0.05, 0.005, and 0.001.
3.2 Kidney function tests
Statistical analysis showed a significant increase in plasma urea, uric acid and creatinine concentrations in the rats treated with TAA. Interestingly, these parameters tended to be recovered to their normal values after treatment with the GA in GA + TAA rat group in a dose-dependent manner compared to the TAA group (Fig. 2).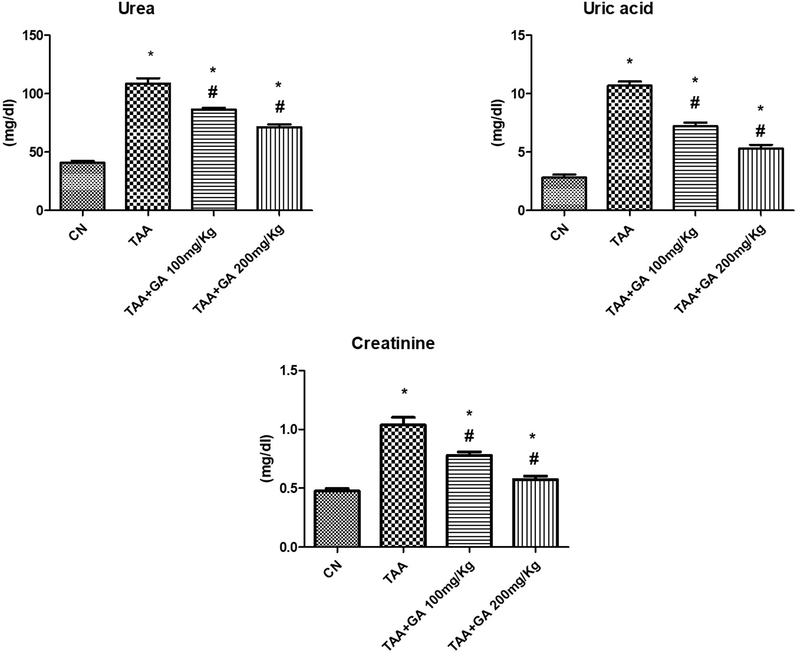
The levels of kidney functions parameters (urea, uric acid and creatinine) in plasma from different rat groups. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. Values in the histogram are the mean ± SEM, and significance was set at P < 0.05, 0.005, and 0.001.
3.3 Lipid profile and protein
Fig. 3 demonstrated that the TAA-treated rats' plasma cholesterol and triglyceride values were significantly (P ≤ 0.05) higher than those of control rats. TAA and GA adminstration together resulted in a substantial decrease in cholesterol or triglycerides levels when compared to the TAA group. Moreover, GA was found to prevent the decrease of plasma protein produced by TAA significantly.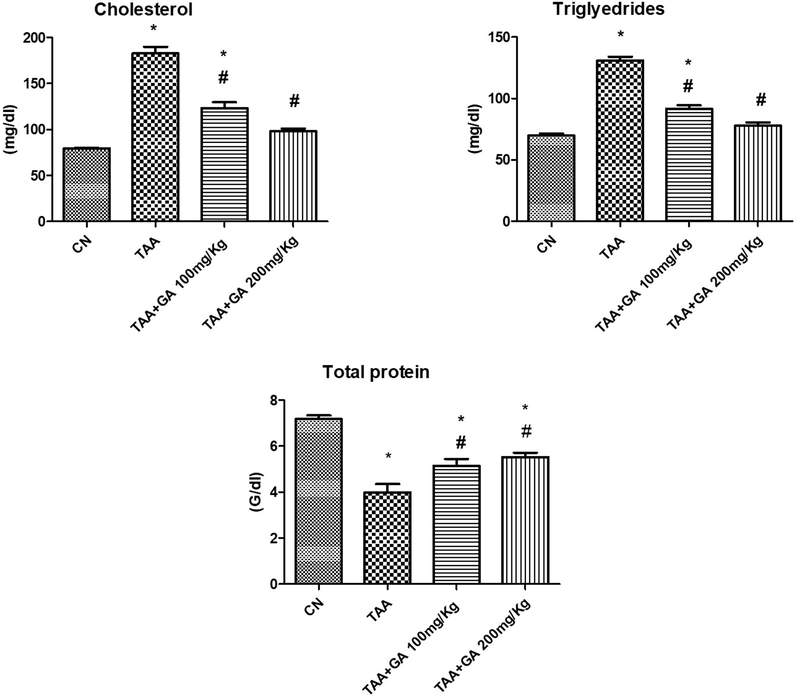
The concentrations of lipids (cholesterol and triglycerides) and plasma proteins from different rat groups. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. Values in the histogram are the mean ± SEM, and significance was set at P < 0.05, 0.005, and 0.001.
3.4 Inflammatory markers
TAA significantly increased plasma CRP and TNF-α in the TAA-treated rat group compared to the control rats (Fig. 4). GA was found to substantially reduce the effect of TAA on the previous two parameters, namely CRP and TNF-α. Intestigly, the high dose of GA (200 mg/kg) was found to signicantly restored values of the inflammatory markers, especially in the case of TNF-α in comparison to the low dose (100 mg/kg).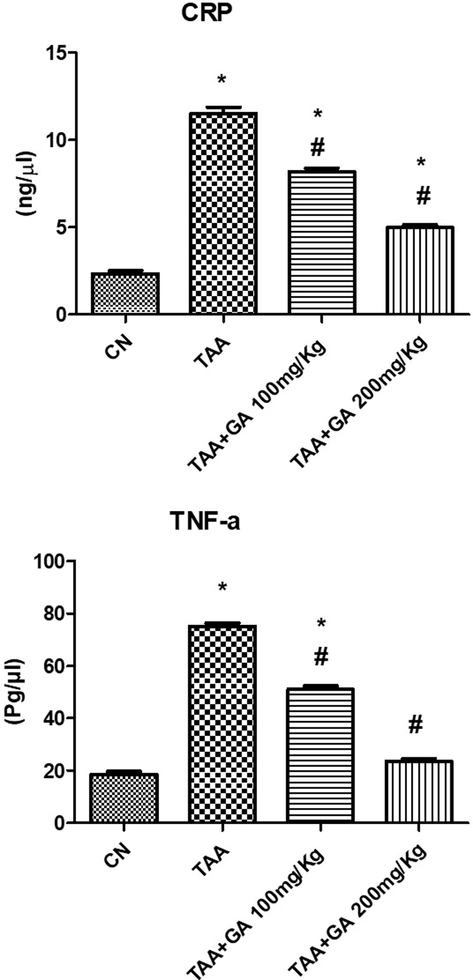
The levels of both plasma CRP and TNF-α in different rat groups. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. Values in the histogram are the mean ± SEM, and significance was set at P < 0.05, 0.005, and 0.001.
3.5 Oxidative stress parameters
The data presented in Fig. 5 showed a significant increase in hepatic or renal MDA content of the TAA-treated group. At the same time, it lowered significantly in the rats given GA + TAA as compared to the TAA group. On the other hand, GA prevented the decrease of hepatic or renal GSH levels (Fig. 5) observed in the TAA group.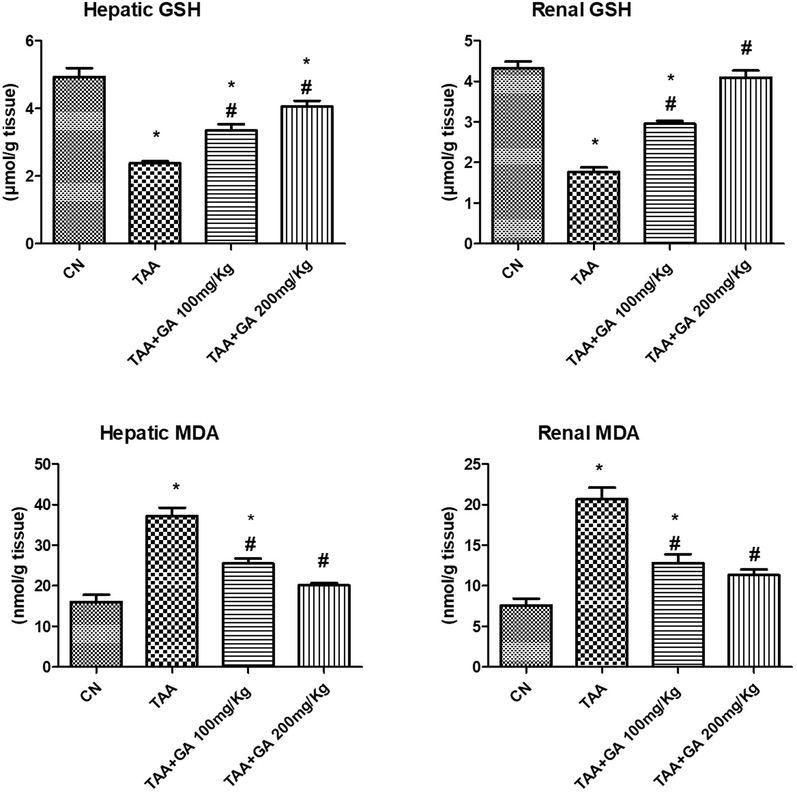
The concentrations of reduced glutathione (GSH) and malondialdehyde (MDA) in both renal and hepatic tissues from different studied rat groups. * indicates the statistical significance compared with the control. # indicates the significance compared with the TAA group. Values in the histogram are the mean ± SEM, and significance was set at P < 0.05, 0.005, and 0.001.
3.6 Histopathological results
3.6.1 Liver
Control group stained sections indicated normal histological hepatic architecture (Fig. 6A). Histopathological alterations of liver tissues treated with TAA only showed a loss in typical architecture, with hepatocytes' deleterious effect and cirrhotic nodules' formation. Cirrhosis is the histological alteration in response to chronic liver damage. Cirrhosis is regenerative or aberrant nodules surrounded by fibrotic bands. The central and portal veins were coated in dense fibrous bands with many tiny proliferating bile ducts.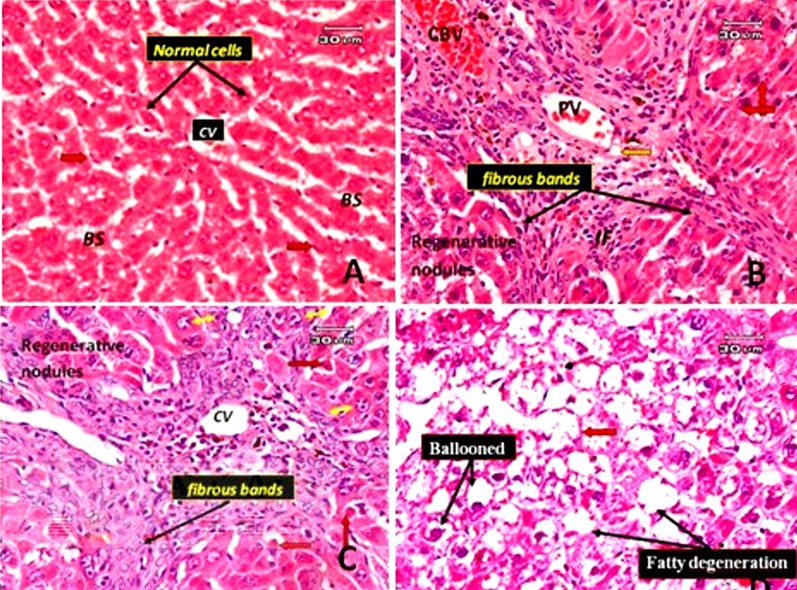
Photomicrographs of rat liver sections. A: Normal hepatic section with classic hepatic lobules containing central vein (CV) and separated by blood sinusoids (BS). The cords of hepatocytes are lined by flattened endothelial cells (arrowhead) and Kupffer cells (red arrow). B: A TAA-treated rat's liver section only shows cirrhotic nodules. Cirrhosis is characterized by fibrotic bands with loss of architecture and nodule formation. The bands intervene between the regenerative hepatocyte nodules, around the portal vein (PV), around congested blood vessels (CBV) and numerous small proliferated bile ducts (orange arrow). Hyperchomatic nuclei with clumping chromatin (red arrow) and a few inflammatory cells are present in fibrous tissue (IF). C: Liver section of rat treated with TAA only (another different filed from that of B) showing dense fibrous bands surrounded the central vein and around perisinusoidal spaces and eventually will progress to cirrhosis, some hepatocyte appeared apoptosis with cells shrinkage and chromatin margination (red arrow), some enlarged hepatocytes with karyomegaly and multiple nuclei are present (Yellow arrow). D: Liver section of rat treated with TAA only showing ballooning degeneration form of cell death, accumulation of fatty changes (steatosis) and blood sinusoids appeared narrow or obliterated (red arrow) (E&H).
Hyperchromatic nuclei with dense chromatin clumping and some apoptotic cells (acidophilia) note the condensation and dark eosinophilic cytoplasm, the absence of nucleus, and a few inflammatory infiltrates in fibrous tissue were observed. Hepatocellular, karyomegaly, and multiple nuclei are present. The blood vessel was dilated. Ballooning degeneration: in this form of cell death, accumulation of fatty changes (steatosis) and blood sinusoids appear narrow or obliterated (Fig. 6. B, C and D). The histological sections in rats treated with TAA and subjected to a low dose of GA showed some improvement in the changes induced by TAA in the form of no thick bands of fibrous tissue, no apoptotic cells, no hyperchromatic cells. The liver tissues still suffer from pathological changes in the form of hepatocyte regenerative nodules separated by thin fibrous bands. The fibrous bands surround the nodules and around the dilated, congested blood vessels and cellular infiltration around them. Dilated and congested portal vein and bile duct hyperplasia are associated with the deposition of thin collagen fibers mixed with a few inflammatory cells around the portal tract. Fig. 7A and B showed a red blood vessel in the dilated blood sinusoidal space. In the case of rats treated with TAA and subjected to a high dose of GA, there was a more significant decrease in degenerative changes and hepatic lesions (such as cirrhosis, fibrosis and cell death). The liver lobules regained their regular architecture, although pyknotic cells, dilated, congested portal veins, thickened portal vein vascular wall and bile duct hyperplasia remained. There are still congested central veins and red blood cells in the sinusoidal space (Fig. 7C & D).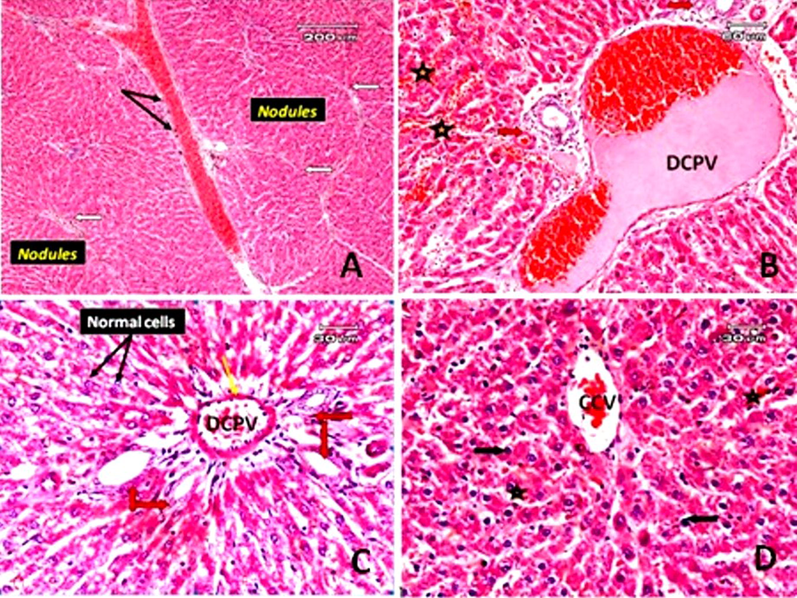
Photomicrographs of sections of rat liver. A: Liver section of a rat treated with TAA and subjected to gallic acid at a dose of 100 mg/kg (low dose) demonstrating micronodular liver cirrhosis, regenerative nodules separated by thin fibrous bands, fibrous bands (white arrow) surrounding the nodules and around the dilated congested blood vessels, and cellular infiltration around them (black arrow). B: Liver section of rat treated with TAA and subjected to gallic acid (low dose) (another filed from that of A) showing dilated congested portal vein (DCPV) and bile duct hyperplasia (red arrow), deposition of thin collagen fibers mixed with a few inflammatory cells in the portal area. Red blood vessels in dilated blood sinusoids could be observed (star). C: The liver section of a rat treated with TAA along with gallic acid at a dose level of 200 mg/kg (high dose), showed some hepatic cells that more or less appeared normal (black arrow), but mild dilated and congested portal vein (DCPV), a thickened portal vein vascular wall (yellow arrow) and hyperplasia of the bile duct (red arrow). D: A congested central vein (CCV) in a rat treated with TAA and gallic acid at a high dose (another field from that of C), some pyknotic hepatocytes (black arrow), and red blood cells in the blood sinusoidal space (star) (E&H).
3.6.2 Kidney
Normal kidney sections showed standard histological structure (Fig. 8 A). While the kidneys of the group treated with TAA only revealed extensive tubular dilatation with epithelial flattening, cloudy swelling of some renal tubules with renal casts in the lumen and desquamation of some tubular epithelial cells. Vacuoles in the renal tubular epithelium characterize signs of lining epithelial cell degeneration such as pyknosis, necrosis, and cytoplasmic vacuolation. Glomerular degeneration with intraglomerular hemorrhage increased a wide space of Bowman’s capsule and hemorrhage was detected in the interstitial tissue (Fig. 8B, C and D).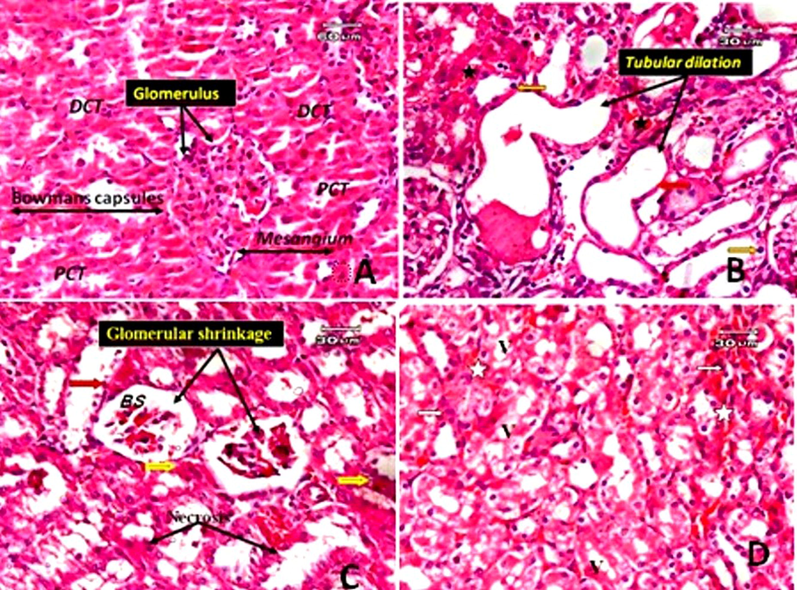
Photomicrographs of the rat kidney. A: Normal control shows proximal convoluted tubules (PCT), distal convoluted tubules (DCT), the renal corpuscle consists of Bowman's capsule and glomerular capillaries, mesangium. B: The kidney section of a rat treated with TAA only showing extensive tubular dilatation, epithelial flattening (red arrow), hemorrhage in interstitial tissue (star) and pyknotic cells (orange arrow). C: kidney section of rat treated with TAA only (another filed) showing glomerular shrinkage with wide urinary space of Bowman's capsule (BS), cloudy swelling of some tubular epithelium (yellow arrow), signs of degeneration in the form of necrosis (black arrow) and desquamation of some cells (red arrow). D: a kidney section from a rat treated only with TAA (another file from that of C) demonstrating vacuoles in renal tubular epithelial cells (v) and hemorrhage in interstitial tissue (star) (E&H).
On light microscopic examination, the liver of rats treated with TAA and subjected to gallic acid at a dose level of (low dose) revealed no improvement in pathological changes. The liver tissues still suffer from the deleterious effects of TAA in the form of hyaline casts present in the modularly of some tubules, desquamation in tubular epithelial cells, and a few inflammatory infiltrates and hemorrhage in the interstitial tissue. Glomerular lobulation, interglomerular hemorrhage, and some tubular epithelial cells (Fig. 9A and B). Histological examination in the kidney tissues of rats treated with TAA and gallic acid at a high dose level (200 mg/kg) exhibited improved pathological changes compared with the group treated with TAA only in the form of no hyaline cast in the lumen of tubules, no tubular dilatation. However, glomerular shrinkage, cell debris in the lumen of some tubules and cloudy swelling of the tubules were still present; some tubules appeared normal (Fig. 9C and D).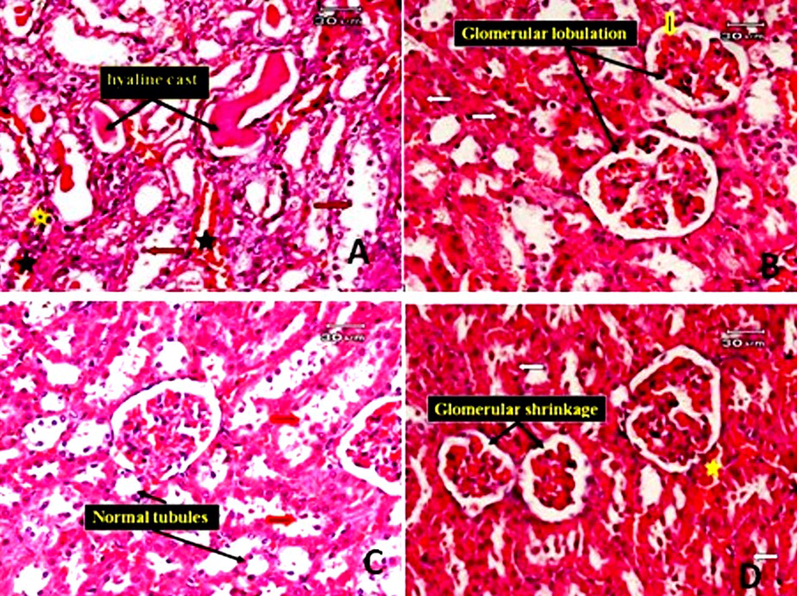
Photomicrographs of the rat kidney. A: The kidney tissue of rats treated with TAA and subjected to gallic acid at a dose level of 100 mg/kg (low dose), showed no improvement in pathological changes. The kidney tissue showed hyaline casts (black arrow) present in the modularly of some tubules, desquamation in tubular epithelial cells (red arrow), some degenerated tubules, a few inflammatory infiltrates (yellow star) and hemorrhage in interstitial tissue (black star). B: the kidney section of a rat treated with TAA and subjected to gallic acid at a low dose (another field) showed glomerular lobulation (black arrow), interglomerular hemorrhage (yellow arrow) and cloudy swelling of some renal tubules (white arrow). C: kidney section of rat treated with TAA and subjected to gallic acid at high dose showing some improvement in pathological changes in the form of no hylain cast with no dilatation of tubules, although cell debris in lumen of some tubules (red arrow), some normal tubule could be observed. D: kidney section of rat treated with TAA and subjected to gallic acid at high dose (another filed) showing glomerular shrinkage, cloudy swelling of some tubules (red arrow) and hemorrhage in interstitial tissue (star) (E&H).
4 Discussion
To combat oxidative stress, animals have evolved a complicated antioxidant system. Excess reactive species, on the other hand, cause oxidative damage. The overproduction and buildup of free radicals causes oxidative stress, which is the major cause of numerous degenerative diseases. Oxidative stress is thought to be a shared pathogenic process that contributes to the onset and development of liver and kidney damage (Li et al., 2015; Tomsa et al., 2019). Thioacetamide is a familiar hepatotoxic compound producing free radicals and oxidative stress through its S-oxide metabolite (thioacetamide-S dioxide), an unstable and reactive metabolite (Yogalakshmi et al., 2010). The present study clarified the decrease of oxidative stress results from TAA by gallic acid as evidenced by a reduction in hepatic or renal MDA content and enhancement of GSH levels. MDA is an indicator for lipid peroxidation that represents another route for propagating reactive species and their deleterious effects. It is an uncontrolled reaction that elevates lipid hydroperoxides in cellular and subcellular membranes. These highly reactive cytotoxic species can disrupt several cellular processes (Siquet et al., 2006). GA protects lipids from peroxidation by scavenging free radicals and inhibiting lipid peroxidation. A research study reported that GA scavenges the DPPH radical by a hydrogen-donating mechanism that is more efficient than Vitamin E itself (Gressner et al., 2007).
Despite their strong prevention of lipid peroxidation, they do not scavenge ROS. A flavonol molecule was shown to have a comparable anti-lipid-peroxidative action but no antiradical potential (Gressner et al., 2007). Reduced glutathione (GSH) serves as an antioxidant and a scavenger of reactive oxygen species (ROS), including hydroxyl radicals and singlet oxygen (Özen et al., 2004). However, the restoration of GSH levels in pre-treated rats implies that gallic acid has both antioxidant and hepatoprotective characteristics.
TAA is an active hepatorenal toxic agent, manifested by histopathological changes and biochemical analysis in the present work. Our results demonstrated a significant increase in plasma liver enzymes (GGT, ALT, AST and ALP) due to TAA treatment. The rise in plasma enzymatic activity is linked to liver damage and membrane characteristics that result in the leakage of enzymes. Therefore, its level is elevated in the plasma. The increase in liver enzymes may also indicate hepatocyte necrosis (Gressner et al., 2007). Also, plasma creatinine, urea, and uric acid were elevated significantly in the TAA group, indicating renal insufficiency and tubular injury (Özen et al., 2004). The pathological alterations observed in the liver and kidney of the TAA group can be attributed to an increase in tissue MDA, an indicator of lipid peroxidation that alters the physiological functions of cell membranes and plays a vital role in cellular membrane damage through a free radical chain reaction mechanism (Wong-Ekkabut et al., 2007). It was reported that gallic acid treatment alleviates the hepatic and renal toxicities induced by tramadol by enhancing reduced glutathione and antioxidant enzymes (Sheweita et al., 2018). The reduction in degenerative abnormalities in the GA + TAA groups is most likely owing to reduced lipid peroxidation and maintenance of tissue GSH. Also, GA administration before cisplatin administration reduced histological renal damage and suppressed ROS generation, lipid peroxidation, and oxidative stress in kidney tissues (Akomolafe et al., 2014, 2014.). The histological study showed that GA reduces hepatic fibrosis due to TAA treatment, as noted in the histological analysis. It was found that GA counteracted the progression of hepatic fibrosis through a reduction of hepatic stellate cell proliferation/activation (El-Lakkany et al., 2019). Furthermore, GA reduced plasma triglycerides, cholesterol, and hepatic steatosis in liver sections. Patients with fatty liver have high plasma triglycerides and low levels of high-density lipoprotein (Cali et al., 2009). It has been observed that GA reduces fat buildup by increasing -oxidation and ketogenesis (Chao et al., 2020). The current study found that rats treated with TAA alone had significantly decreased plasma protein levels. It has been proposed that increased lipid peroxidation has an effect on protein production (Ayala and Muñoz, 2014, 2014.). GA may prevent protein decline in TAA-treated rats by lowering hepatic MDA or liver pathological alterations, as protein loss is associated with increased cellular damage (Woodman, 1996).
Furthermore, GA reduces the TAA-induced increase in IL-6 and TNF-α. TNF-α is essential in the pathophysiology of various inflammatory liver diseases (Dong et al., 2016; Hassan et al., 2021). Interleukin-6 was intimately associated to fatty liver disease, and it was mostly found in non-alcoholic steatohepatitis diagnosis(Tarantino et al., 2009). We believe that GA may protect against TAA-induced hepatic or renal damage by lowering IL-6 levels and lipid peroxidation.
5 Conclusion
The current study demonstrates that GA prevents hepatic or renal damage caused by TAA by decreasing oxidative stress and inflammatory indicators while also enhancing the antioxidant state of hepatic and renal tissues.
Ethics statement
This experiment was conducted out in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, updated 1996) and the National Research Centre in Egypt's Animal Care and Use guidelines, with ethical approval No. 18576.
Consent for publication
All authors have consented to publication.
Funding
The research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, through project no. IFKSURG-2-278.
Authors' contributions
SAEB conceived the research idea and performed the experiments. HE drafted the manuscript and analyzed the results. FAM described the histology results. JHT prepared the figures and performed the statistical analysis of the generated experimental data. IMA arranged the funds. IH and JHT finalized the manuscript in the communicable format including type setting, language, and plagiarism etc. All of the authors have approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project no. IFKSURG-2-278.
References
- Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int. Scholarly Res. Notices 2014, 2014.
- [Google Scholar]
- Guide for the Care and Use of Laboratory Animals (8th edn.). London, England: SAGE Publications Sage UK; 2012.
- Argüelles S: Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014.
- [Google Scholar]
- Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896-1903.
- [Google Scholar]
- Gallic Acid Ameliorated Impaired Lipid Homeostasis in a Mouse Model of High-Fat Diet-and Streptozotocin-Induced NAFLD and Diabetes through Improvement of β-oxidation and Ketogenesis. Front. Pharmacol.. 2020;11:2469.
- [Google Scholar]
- Probing gallic acid for its broad spectrum applications. Mini Rev. Med. Chem.. 2018;18:1283-1293.
- [Google Scholar]
- Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol. Pathol.. 2011;39:949-957.
- [Google Scholar]
- Protective effect of gallic acid and gallic acid-loaded Eudragit-RS 100 nanoparticles on cisplatin-induced mitochondrial dysfunction and inflammation in rat kidney. Biochim. Biophys. Acta (BBA)-Mol. Basis Disease. 2020;1866:165911.
- [Google Scholar]
- The protective or damaging effect of tumor necrosis factor-α in acute liver injury is concentration-dependent. Cell Biosci.. 2016;6:1-10.
- [Google Scholar]
- Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J. Tradit. Complement. Med.. 2019;9:45-53.
- [Google Scholar]
- Gallic acid ameliorates sodium arsenite-induced renal and hepatic toxicity in rats. Drug Chem. Toxicol.. 2021;44:341-352.
- [Google Scholar]
- Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta. 2007;381:107-113.
- [Google Scholar]
- Selenium nanoparticles mitigate diabetic nephropathy and pancreatopathy in rat offspring via inhibition of oxidative stress. J. King Saud Univ. Sci.. 2021;33(1):101265
- [Google Scholar]
- Gallic acid and ferulic acid protect the liver from thioacetamide-induced fibrosis in rats via differential expression of miR-21, miR-30 and miR-200 and impact on TGF-β1/Smad3 signaling. Chem. Biol. Interact.. 2020;324:109098
- [Google Scholar]
- Therapeutic effect of gallic acid against paraquat-induced lung injury in rat. Jundishapur J. Natural Pharmaceut. Prod.. 2018;13:13.
- [Google Scholar]
- The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci.. 2015;16:26087-26124.
- [Google Scholar]
- Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int.. 2006;48:263-274.
- [Google Scholar]
- In vivo protective effects of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat erythrocytes. Arh. Hig. Rada Toksikol.. 2013;64:553-558.
- [Google Scholar]
- Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med. Sci.. 2015;3:78-92.
- [Google Scholar]
- Gallic acid and omega-3 fatty acids decrease inflammatory and oxidative stress in manganese-treated rats. Exp. Biol. Med.. 2020;245:835-844.
- [Google Scholar]
- Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J. Appl. Toxicol.: Int. J.. 2004;24:27-35.
- [Google Scholar]
- Tramadol-induced hepato-and nephrotoxicity in rats: Role of Curcumin and Gallic acid as antioxidants. PLoS One. 2018;13:e0202110.
- [Google Scholar]
- Antioxidant profile of dihydroxy-and trihydroxyphenolic acids-A structure–activity relationship study. Free Radic. Res.. 2006;40:433-442.
- [Google Scholar]
- Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur. J. Gastroenterol. Hepatol.. 2009;21:504-511.
- [Google Scholar]
- Oxidative stress as a potential target in acute kidney injury. PeerJ. 2019;7:e8046.
- [Google Scholar]
- Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys. J .. 2007;93:4225-4236.
- [Google Scholar]
- Woodman, D, 1996. Assessment of hepatotoxicity. Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers. 66.
- Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology. 2010;268:204-212.
- [Google Scholar]







