Translate this page into:
Protective effect of ethanolic extract of Actinoscirpus grossus tubers against ethanol induced liver toxicity in albino rats
⁎Corresponding author. alfarhan@ksu.edu.sa (Ahmed Alfarhan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Liver detoxifies majority of chemicals and plays an important role in the maintenance of homeostasis of the body. In the recent years due to overwhelming use of drugs and alcohol there is an increased incidence of liver toxicities. The tubers of Actinoscirpus grossus are used in liver disease as a liver tonic in folklore medicine; However, there is no experimental basis for its therapeutic use. Hence the present study was conducted to evaluate the hepatoprotective potentials of ethanolic extract of Actinoscirpus grossus tubers in ethanol induced hepatotoxicity in albino rats. Daily administration of 20% ethanol v/v p.o. at a dose of 5 g/kg for 30 consecutive days caused significant liver toxicity and there after maintained for 15 consecutive days with normal rat pellet and water ad libitum. The treatment groups were administered with group specific drugs for 15 consecutive days after 30 days of ethanol administration and control group rats were maintained for 15 consecutive days with normal rat pellet and water ad libitum. Liver toxicity was assessed on 45th day by estimating biochemical, histological and antioxidant level. Daily administration of 20% ethanol significantly increased liver enzymes and caused oxidative stress by increase in the lipid peroxidation and reduction in the catalase and glutathione peroxidase activity in liver homogenate. The histopathological examination revealed micro vesicular and macro vesicular fatty changes predominantly with mild periportal inflammation, cell infiltrate and areas of mild dilatation of sinusoids with ballooning degeneration of hepatocytes. Treatment with ethanolic extract of A. grossus tuber significantly restored the liver enzymes especially SGOT and attenuated elevated lipid peroxidation and restored catalase and glutathione peroxidase activity. The histopathological changes revealed significant reduction in the hepatocyte ballooning portal fibrosis. Thus, tubers of A. grossus plant may serve as reservoir of natural products having diverse functions and could be utilised as a potent hepatoprotective agent.
Keywords
Hepatoprotective
Lipid peroxidation
Fatty infiltration
Catalase
Glutathione peroxidase
Actinoscirpus grossus
1 Introduction
Majority of xenobiotics gets metabolised in liver and thus liver plays a important role in the maintenance of homeostasis. Despite of its great capacity to handle toxicants and high regenerative power, liver exhibit symptoms after extensive damage. Recently the liver disease related complications are increasing to a greater extent due to overwhelming use of drugs and alcohol. The acute hepatitis with or without cirrhosis, liver steatosis, cirrhosis are results of chronic alcoholism and can also leads to hepatocellular carcinoma (Liu, 2014). Clinical alcoholic patients may remain asymptomatic for years and when the symptoms developed the lesions may be quite advanced. There exist only few universal treatment options for common liver diseases such as fatty liver, cirrhosis and chronic hepatitis (Mello et al., 2008; Moghe et al., 2011).
Herbal treatments have been used to alleviate the liver disorders from centuries and are well documented in traditional system of medicines and it becomes a favourable therapy for most of the liver dysfunction worldwide (Neetu et al., 2011). There are number of plants and polyhedral formulations claimed to have hepatoprotective activities, however only a handful of drugs has been studied thoroughly for their therapeutic uses (Sabayan et al., 2007). Thus increasing use of herbal medicines shows their perceived effectiveness and prevention of liver diseases and believed to be safe because of its natural origin (Lee et al., 2004).
Actinoscirpus grossus (A. grossus, family: Cyperaceae) is a perennial plant with long rhizomes ending in small tubers and popularly known as ‘Kasheruka’ in Ayurveda. It is distributed throughout India and neighbouring Asian countries (Sharma and Sharma, 2006; Nisteshwar, 2007). In Ayurveda the decoction prepared from A. grossus tubers has been indicated for liver, digestive disorders and as anti-emetic agent (Kirtikar et al., 2007). It has been reported that the ethanolic extract of tubers possess biphasic hypotensive effect in anesthetized cats (Sharma and Sharma, 2006). The ethanolic extract of tubers of A. grossus (EAGT) has revealed the presence of flavonoids, carbohydrate, coumarins, steroids, tannins, and terpenoids (Savin et al., 2017). The acute oral toxicity test indicated that LD 50 of EAGT exceeded 2000 mg/kg in rats (Savin et al., 2018). Despite of its folklore claim as a liver tonic there is no experimental data available for its hepatoprotective activity. Thus the present study was undertaken to evaluate the hepatoprotective potentials of EAGT in ethanol induced liver toxicity in albino rats.
2 Materials and methods
2.1 Preparation of ethanol extracts of tubers of the Actinoscirpus grossus
The authenticated sample of powdered form of A. grossus tubers was purchased from Vaidya Hukam Chand Arogyadham Gharaunda, Haryana India. The sample specimen (No. 495/14101822) was deposited at SDMCA Udupi. The ethanolic extract of tubers of the Actinoscirpus grossus was prepared by soaking powdered form of tubers (480 g) in 3.5 L of ethanol for 24 h and filtered. The filtered extract was taken in a china dish and placed on water bath till free from water. It was completely dried under vacuum. The percentage of dried extract with reference to the sample taken was recorded. The concentrated extract was stored at 4 °C before the experimentation.
2.2 Animals
Wistar albino male rats (225 ± 25 g) were purchased from NITTE University, Mangalore, India. They were housed in polypropylene rat cages and maintained at a temperature of 25 ± 2 °C, humidity (50 to 55%) and natural light and dark cycles. They were fed with commercial rat pellet diet and water ad libitum. The investigation was done after the consent from animal ethics committee, NITTE University (No:115/1999/CPCSEA).
2.3 Experimental design and biochemical studies
The rats were divided into six experimental groups (n = 6). Rats in normal control group (NC) were orally treated with gum acacia (0.5%). Ethanol group rats were initially administered with 20% ethanol v/v orally (p.o.) for 30 consecutive days and there after maintained for 45 consecutive days with 0.5% gum acacia along with normal rat pellet and water ad libitum, served as ethanol group. The rats in reference standard groups were orally administered with 20% ethanol v/v for 30 consecutive days and there after treated with Liv 52 (5 ml/kg) and Silymarin (50 mg/kg) respectively for 15 consecutive days. The EAGT group rats were orally administered with 20% ethanol v/v for 30 consecutive days and there after orally administered with EAGT (200 and 400 mg/kg) for 15 consecutive days. On 45th day the body weight was recorded and blood was withdrawn from retro orbital puncture. The serum was examined for serum biochemical parameters such as SGPT, SGOT, ALP, total protein, alkaline phosphatase, HDL, cholesterol, total bilirubin, and serum glucose level (Bradly et al., 1972; Wilkinson et al., 1969). At the end all the rats were sacrificed and the liver tissues were rapidly isolated washed with ice cold saline and weighed. Antioxidant parameters such as catalase, glutathione peroxidase activities and lipid peroxidation were estimated (Sinha, 1972; Ohkawa et al., 1979; Rotruck et al., 1973).
2.4 Histopathological examination of liver tissue
The liver tissues were collected and washed with ice cold saline and trimmed into 3 mm small pieces. The sections were stained with haematoxylin and eosin stain and mounted. Histopathological studies were done according to a standard protocol (Bancroft and Gamble, 2002).
2.5 Statistical analysis
Data were statistically analysed by one way ANOVA followed by Dunnet’s multiple‘t’ test using GraphPad Prism version 6.01 (Graph Pad Software, Inc., USA). The values were in mean ± SEM and a p value < 0.05 considered as statistical significant.
3 Results
3.1 Effect of EAGT on body weight changes
Ethanol intoxication caused decrease in the body weight by 3.3% at the end of the study when compared to initial body weight. In Liv52 and Silymarin group 2.09% and 3.88% increase in the percentage increase in the body weight was observed and found to be statistically not significant. The test drug EAGT treated rats showed moderate increase in body weight as compared to ethanol group (Table 1). Data: Mean ± SEM.
Group
Initial body weight (grams)
Body weight at the end (grams)
Percentage change in body weight
Normal control
216.66 ± 6.146
230.83 ± 3.516
6.53
Ethanol 5 g/kg −20% v/v
210 ± 5.164
203 ± 4.359
3.3↓
Ethanol 5 g/kg20% v/v + liv52 5 ml/kg
215 ± 8.466
219.5 ± 8.7880
2.09↑
Ethanol5g/kg 20% v/v + Silymarin 50 mg/kg
206 ± 3.33
214 ± 5.099
3.88↑
Ethanol 5 g/kg 20% v/v + EAGT 200 mg/kg
227.5 ± 2.5
232.5 ± 7.500
2.198↑
Ethanol 5 g/kg 20% v/v + EAGT 400 mg/kg
205 ± 3.416
207.5 ± 2.50
1.21↑
3.2 Effect of EAGT on percentage changes in liver weight
Ethanol administered group does not showed much change in liver weight. The test drug EAGT at 200 mg/kg (5.16%), 400 mg/kg (8.812%) and reference standards such as Liv 52 (7.412%) and Silymarin (6.865%) treated groups we could observe only an apparent increase in liver weight and volume (Table 2). Data:Mean ± SEM.
Group
Liver weight (grams)
Percentage change increase in liver weight
Normal control
8.216 ± 0.197
–
Ethanol 5 g/kg −20% v/v
8.86 ± 0.097
7.838↑
Ethanol 5 g/kg20% v/v + liv52 5 ml/kg
8.825 ± 0.2462
7.412↑
Ethanol5g/kg 20% v/v + Silymarin 50 mg/kg
8.78 ± 0.1114
6.865↑
Ethanol 5 g/kg 20% v/v + EAGT 200 mg/kg
8.64 ± 0.1568
5.161↑
Ethanol 5 g/kg 20% v/v + EAGT 400 mg/kg
8.94 ± 0.1600
8.812↑
3.3 Effect of EAGT on serum transaminases and alkaline phosphatases in ethanol intoxicated rats
Chronic administration of 20% v/v ethanol significantly increased the liver specific enzymes like SGOT, SGPT and ALP as compared with normal control (P < 0.01). Treatment with reference standards like Liv 52 and Silymarin has shown significant attenuation of elevated serum SGOT (p < 0.01), SGPT (p < 0.05) and marginal reduction in the serum ALP level in comparison to ethanol group. EAGT at both dose levels significantly reduced the elevated serum SGOT (p < 0.01) and a moderate decrease in the serum SGPT and ALP levels as compared to ethanol group (Table 3). Data: Mean ± SEM, @@p < 0.01, #P < 0.05, ##p < 0.01, #- compared with normal control, @- Compared with Ethanol group.
Group
SGOT activity
(U/L)SGPT activity
(U/L)ALP Activity
(U/L)
Normal control
179.33 ± 14.68
90 ± 5.046
346.16 ± 1.953
Ethanol 5 g/kg −20% v/v
416 ± 19.962 @@
187.4 ± 26.641@@
501.6 ± 73.56@@
Ethanol 5 g/kg20% v/v + liv52 5 ml/kg
285.3 ± 20.906##
93.5 ± 14.530#
418.16 ± 22.71
Ethanol5g/kg 20% v/v + Silymarin 50 mg/kg
296.83 ± 4.438 ##
97.5 ± 5.726#
445.33 ± 28.54
Ethanol 5 g/kg 20% v/v + EAGT 200 mg/kg
325.8 ± 25.850##
152.2 ± 14.759
445.6 ± 21.10
Ethanol 5 g/kg 20% v/v + EAGT 400 mg/kg
285.4 ± 32.1138##
120.2 ± 10.50
398.8 ± 35.07
3.4 Effect of EAGT on serum lipid profile in ethanol induced liver toxicity
Repeated administration of ethanol caused moderate increase in the serum total cholesterol and triglyceride level and a significant increase in the serum HDL level as compared to normal control rats. Silymarin administered group has shown a significant increase in the serum HDL level as compared to ethanol group. EAGT administered at 200 mg/kg significantly increased serum HDL level as compared to normal control rats (Table 4). Data: Mean ± SEM, @@p < 0.01, ##p < 0.01, #- Compared with normal control, @- Compared with ethanol group.
Group
Total cholesterol
(mg/dl)Triglycerides
(mg/dl)HDL
(mg/dl)
Normal control
50.86 ± 1.835
84.44 ± 13.396
19.06 ± 1
Ethanol 5 g/kg −20% v/v
58.4 ± 3.370
86.8 ± 11.240
38 ± 1.225@@
Ethanol 5 g/kg20% v/v + liv52 5 ml/kg
70.5 ± 6.607
87.5 ± 16.778
38.6 ± 6.071
Ethanol5g/kg 20% v/v + Silymarin 50 mg/kg
68.33 ± 10.610
98.83 ± 20.42
45.66 ± 4.998##
Ethanol 5 g/kg 20% v/v + EAGT 200 mg/kg
67.6 ± 10.970
98.8 ± 27.683
46.2 ± 3.121##
Ethanol 5 g/kg 20% v/v + EAGT 400 mg/kg
58 ± 8.963
95.4 ± 19.99
31.8 ± 2.672
3.5 Effect of EAGT on serum biochemistry in ethanol induced liver toxicity
Ethanol intoxication significantly increased serum bilirubin (both direct and total) and albumin level (p < 0.01). The reference standards (Liv 52 and Silymarin) and EAGT at both dose levels significantly reduced the elevated serum bilirubin both direct and total in comparison to ethanol intoxicated group (p < 0.01). Silymarin administration restored the elevated serum albumin and total protein as compared to ethanol group (Table 5). Data: Mean ± SEM, @@p < 0.01, #P < 0.05, ##p < 0.01, # Compared with normal control, @ Compared with Ethanol group.
Group
Total Bilirubin
(mg/dl)Direct Bilirubin
(mg/dl)Albumin
(g/dl)Globulin
(g/dl)Total protein (g/dl)
Normal control
0.27 ± 0.02
0.13 ± 0.02
3.635 ± 0.03
3.574 ± 0.23
7.11 ± 0.26
Ethanol 5 g/kg −20% v/v
1.24 ± 0.16@@
0.54 ± 0.13@@
2.88 ± 0.30@
3.42 ± 0.32
6.5 ± 0.56
Ethanol 5 g/kg20% v/v + liv52 5 ml/kg
0.44 ± 0.12##
0.15 ± 0.02##
3.133 ± 0.12
4.06 ± 0.19
7.18 ± 0.27
Ethanol5g/kg 20% v/v + Silymarin 50 mg/kg
0.28 ± 0.03##
0.106 ± 0.02##
3.66 ± 0.12#
4.5 ± 0.62
8.183 ± 0.46#
Ethanol 5 g/kg 20% v/v + EAGT 200 mg/kg
0.34 ± 0.15##
0.12 ± 0.02##
3.28 ± 0.29
4.45 ± 0.23
7.04 ± 0.38
Ethanol 5 g/kg 20% v/v + EAGT 400 mg/kg
0.26 ± 0.05##
0.14 ± 0.024##
3.02 ± 0.22
4.06 ± 0.18
6.96 ± 0.49
3.6 Effect of EAGT on antioxidant parameters
The antioxidant activity of EAGT was evaluated in the liver homogenate of rats administered with repeated dose of ethanol. The activity of endogenous antioxidant enzymes such as catalase and glutathione peroxidase in ethanol intoxicated group significantly reduced as compared to normal control. The lipid peroxidation was significantly increased after ethanol intoxication (p < 0.01). Treatment with EAGT at both dose level significantly restored the catalase and glutathione peroxidase to normal level (p < 0.01) and significantly attenuated ethanol induced elevated lipid peroxidation (p < 0.05) (Fig. 1).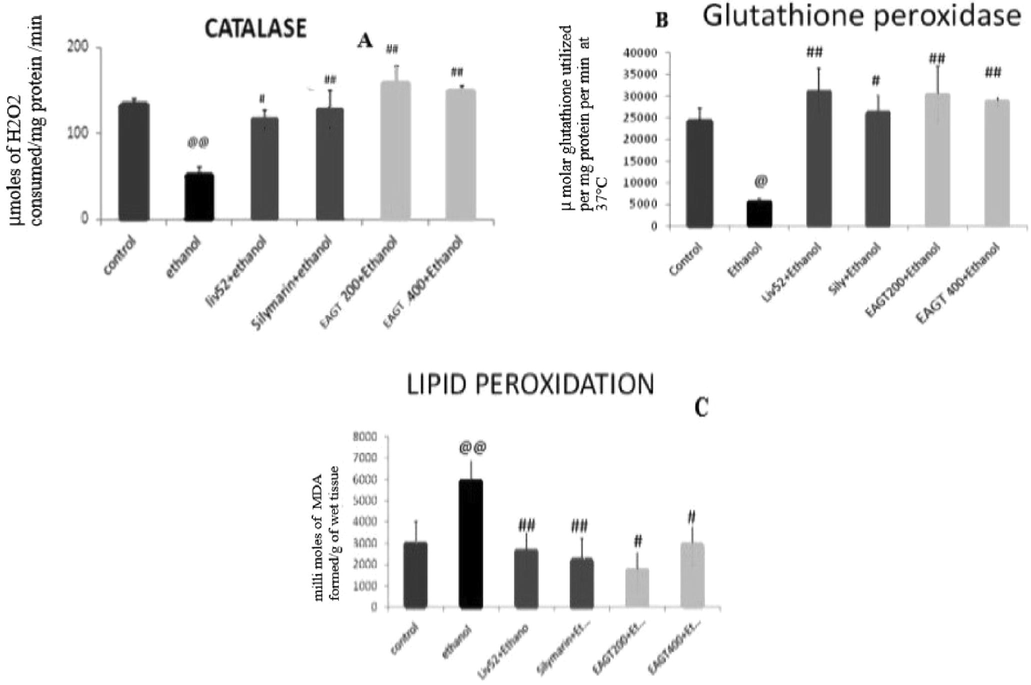
Graphical representation of the effect of EAGT on antioxidant parameters.
3.7 Effect of EAGT on liver histopathological examination
Repeated administration of ethanol caused extensive changes in the liver histology (Fig. 2A & B). The changes observed were micro and macro fatty changes, hepatocyte necrosis, cell infiltration, appearance of balloon cells and sinusoidal dilatation. Liver sections from reference standard drugs especially the Silymarin exhibited good protection against ethanol induced liver toxicity. We could observe mild to moderate fatty infiltration and regenerative changes and almost normal cytoarchitecture (Fig. 2C &D). The test drug EAGT administered group rats exhibited good protection against ethanol toxicity. We could observe only mild to moderate fatty changes without perisinusoidal fibrosis, Lobular infiltration and necrosis. Test drug EAGT exhibited almost normal cytoarchitecture in sections in both dose level (Fig. 2E & F).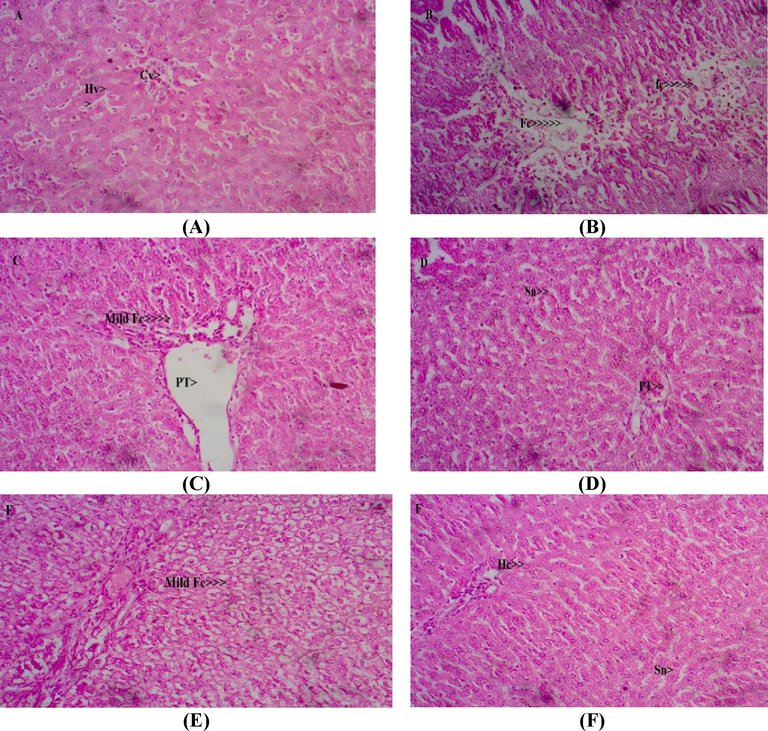
Photomicrographs of representative liver sections. Normal control shows normal Cytoarchitecture of liver (A). Liver sections from ethanol administered group shows extensive fatty changes with necrosis and appearance of balloon cells (B). Liver sections from ethanol and Liv 52 administered rats (C) and ethanol + silymarin administered rats (D). Photomicrographs of representative liver sections from ethanol + EAGT (200& 400 mg/kg) rats shows mild fatty changes and mild cell infiltration with normal cytoarchitecture (E and F). HC– Hepatic cell; KC- Kupffer cell; Sn- Sinusoid; PT- Portal triad; CI- Cell infiltration; Fc- Fatty changes; CV- Central vein.
4 Discussion
Liver is the primary site of ethanol metabolism and hence more prone to ethanol intoxication. Thus, repeated and or acute high dose of ethanol has high chances of developing alcoholic liver diseases (Baraona et al., 1977; Hasumura et al., 1975). Ethanol undergoes oxidation and produce highly toxic substances like acetaldehyde by alcohol dehydrogenase. Along with alcohol dehydrogenase the enzymes like Cytochrome P450 isoenzyme CYP2E1 and catalase also involved in the metabolism. In the ethanol metabolism molecular oxygen (O2) is recipient of the electrons from ethanol via microsomal enzyme system. At last the catalase reduces hydrogen peroxide into water and produce acetaldehyde from ethanol. Acetaldehyde dehydrogenate quickly oxidises acetaldehyde into less toxic acetate. Thus, rapid removal of ethanol by the liver is clearly offering significant protection from its toxic metabolites (Baraona et al., 1977). During the oxidative process there will be release of reactive oxygen species, which inter causes severe oxidative stress followed by release of inflammatory cytokines, which intern causes inflammatory process and leads to liver cirrhosis (Gallagher, 1962).
Normally hepatocytes have both enzymatic and non-enzymatic defensive antioxidant molecules to combat the reactive oxygen species. Among non-enzymatic antioxidant molecules glutathione, thioredoxine and thiol containing molecules are well recognized antioxidant molecules. The endogenous enzymes like superoxide dismutase, catalase and glutathione peroxidase are enzymatic antioxidants. Superoxide dismutase (SOD) enzyme is the first enzyme which defends the reactive oxygen species; superoxide’s and converts superoxide anions to hydrogen peroxide and oxygen. Thus generated hydrogen peroxide is neutralized by catalase (CAT) and glutathione peroxidase (GSH-Px) enzymes. There by it cause protective action in hepatocytes against free hydroxyl radicals (Comporti et al., 2010). The catalase and glutathione peroxidase activity were measured to evaluate the antioxidant effect of EAGT in ethanol induced oxidative stress in liver tissue. Repeated administration of ethanol caused significant decrease in the CAT and GSH-Px as compared to the control rats. EAGT at 200 mg/kg and 400 mg/kg significantly attenuated the ethanol induced decrease in the CAT and GSH-Px level in a dose dependant manner. Depletion of defensive antioxidant molecules in liver cells results in mitochondrial dysfunctions and increased lipid peroxidation, which leads to the liver necrosis. The level of MDA in the ethanol group were increased significantly (p < 0.01). However the MDA level was reduced significantly with the treatment of EAGT. Thus the degenerative hepatic cells releases enzymes such as SGOT and SGPT into the circulation and it can be measured as a marker for liver abnormalities. The measurement of SGPT is considered as essential marker for mitochondrial distortion in the centrilobular zone in the liver tissues.
Ethanol 5 g/kg 20%v/v dose administration for 30 consecutive days caused decrease in the body weight of rats, however the treatment with EAGT mild to moderate increase in the body weight has been observed as compared to ethanol intoxicated group rats. Ethanol intoxication significantly increased serum SGOT, SGPT, ALP, total bilirubin, HDL, total protein and albumin as compared to the normal control. The histopathological results supports the evidence of reduced number of viable cells with massive necrotic cells around the centrilobular zone extending to the parenchymal zone, which is characterized by increased glycogen depletion, condensation of nuclei, pyknotic, karyolitic, lymphatic infiltrations and endothelial disruptions.
Interestingly, treatment with EAGT showed capability to increase the body weight and simultaneously caused significant reduction in the liver enzymes such as SGOT, SGPT and ALP levels in blood dose dependently. It is further supported by significant attenuation of ethanol intoxicated decrease in the CAT and GSH-Px antioxidant enzymes and decreased lipid peroxidation level in the liver homogenate. The biochemical parameters such as total bilirubin, cholesterol, total protein and albumin were significantly attenuated as compared to the ethanol intoxicated rats. Further the histopathological investigation shows the normal cytoarchitecture and presence of normal healthy hepatocytes. This liver regenerative process was almost comparable with that of reference standards like Silymarin and Liv 52 treated groups. Whereas increased viable hepatocytes, decreased hepatic enzymes and increased endogenous anti-oxidant enzymes such as CAT and GSH-Px and decreased lipid peroxidation could be observed.
Ethanol induced hepatotoxicity is mainly attributed to the ethanol active metabolite acetaldehyde induced free radical generation subsequent oxidative stress and inflammatory process in liver tissues. Thus, it is clear that the extract possesses free radical scavenging activity and prevented the process of initiation and progression of hepatocellular damage. Interestingly our present findings did demonstrate the EAGT ability of scavenging free radicals and exerting antioxidant activity (Pari and Prasath, 2008).
The plant derived phytochemicals such as flavonoids, polyphenols, unsaturated fatty acids, glycosides, tannins, saponins and alkaloids having wide range of biological activities and found useful in the treatment of liver disorders (Mundugaru et al., 2014; Xu et al., 2007). It is a valuable source for future research in the therapeutics. Some of the potent flavonoids like Silymarin, which is derived from seeds of the milk thistle, Silybum marianum is used in the treatment of hepatitis, liver cirrhosis and toxins induced liver dysfunctions (Sy-Cordero et al., 2010). Many chemicals like hydroxy citric acid, curcumin, garcinol and ginkjo alkaloids were derived from plant sources were found to be effective in liver diseases (Abdel-Wahab, 2013; Liu et al., 2013; Yang et al., 2011). The phytochemical analysis demonstrated that EAGT are rich in flavonoids, tannins, terpenoids and steroids. Flavonoids are important phenolic contents of the plants and serves as secondary metabolites having potent antioxidant and anti-inflammatory activities. Thus we could infer that the presence of flavonoids, tannins, terpenoids and steroids might have possessed free radical, anti-oxidant and anti-inflammatory and hepatoprotective activities.
5 Conclusion
Herbs are the natural products considered to be the rich source of structurally diverse molecules and exhibit multiple therapeutic functions. It is very important in the treatment of chronic degenerative disorders like liver diseases. We have reported for the first time that the ethanolic extract of tubers of Actinoscirpus grossus showed protective effects in ethanol induced hepatotoxicity. Thus, the plant tuber can be used as potent source of phytoconstituents from which we could further develop efficient hepatoprotective agents.
Acknowledgements
Authors are grateful to KSHEMA, NITTE University Derlakatte, Mangalore, Karnataka for the opportunity and for the constant support. The authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this work through Researchers Supporting Project number (RSP-2020/11).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J. Basic Appl. Zool.. 2013;66:263-270.
- [Google Scholar]
- Theory and Practice of Histological Techniques (5th ed.). London: Churchill Livingstone; 2002.
- Pathogenesis of alcohol-induced accumulation of protein in the liver. J. Clin. Invest.. 1977;60(3):546-554.
- [Google Scholar]
- Transaminase activities in serum of long-term hemodialysis patients. Clin Chem.. 1972;18:1442.
- [Google Scholar]
- The effect of antioxidants on poisoning by carbon tetrachloride. Aust. J. Exp. Biol. Med. Sci.. 1962;40(3):241-253.
- [Google Scholar]
- Acetaldehyde oxidation by hepatic mitochondria: decrease after chronic ethanol consumption. Science. 1975;189(4204):727-729.
- [Google Scholar]
- Indian Medicinal plants. Dehra DunIndia: International Book Distributors; 2007. p. :2644-2645.
- Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci.. 2004;74:1051-1064.
- [Google Scholar]
- Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol.. 2014;20(40):14672.
- [Google Scholar]
- Use of the combination index to determine interactions between plant-derived phenolic acids on hepatotoxicity endpoints in human and rat hepatoma cells. Phytomedicine. 2013;20:461-468.
- [Google Scholar]
- Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol. Aspects Med.. 2008;29:17-21.
- [Google Scholar]
- Histone modifications and alcohol-induced liver disease: are altered nutrients the missing link. World J. Gastroenterol.. 2011;17(20):2465.
- [Google Scholar]
- Hepatoprotective activity of fruits extract of Garcnia pedanculata. Bangladesh J. Pharmacol.. 2014;9:483-487.
- [Google Scholar]
- Textbook of Dravyagunavijnana. Varanasi, India: Chaukhamba Subharati Prakashan; 2007. p. :581.
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem.. 1979;95:351-358.
- [Google Scholar]
- Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem.-Biol. Interact.. 2008;173(2):77-83.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- A postulated role of garlic organo sulfur compounds in prevention of Valproic acid hepatotoxicity. Med. Hypotheses. 2007;68:512-514.
- [Google Scholar]
- Acute oral toxicity study of ethanolic extract of Actinoscirpus grossus (L.F) Goetgh and D.A.Simpson. Asian J. Pharm.Clin. Res.. 2018;11(7):321-323.
- [Google Scholar]
- Microscopical evaluation, Phytochemical analysis and HPTLC fingerprinting of Tuber of Actioscripus grossus Goetgh and D.A. Simpson. Pharmacogn. J.. 2017;9(5):657-662.
- [Google Scholar]
- Oshadhi varga. Kaiyadeva nigantu. Drlhi, India: Chaukambha publication; 2006. p. :644-645.
- Large-scale isolation of flavonolignans from Silybum marianum extract affords new minor constituents and preliminary structure-activity relationships. Planta Med.. 2010;76:644-647.
- [Google Scholar]
- Evaluation of a new system for kinetic measurement of serum alkaline phosphatase. Clin. Chem.. 1969;1969(15):487-495.
- [Google Scholar]
- Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Environ. Toxicol. Pharmacol.. 2007;24:140-148.
- [Google Scholar]
- Hepatoprotective effects of polyprenols from Ginkgo biloba L. leaves on CCl4-induced hepatotoxicity in rats. Fitoterapia. 2011;82:834-840.
- [Google Scholar]







