Translate this page into:
Protective and antioxidant effects of chia oil and canola oil on testicular injury induced by lead in rats
⁎Corresponding author at: Princess Dr. Najla Bint Saud Al-Saud Center for Excellence Research in Biotechnology, Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University, P.O. Box 139109, Jeddah 21323, Saudi Arabia. alattar@kau.edu.sa (Atef M. Al-Attar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Environmental pollution is a global problem that needs more efforts to overcome the increase of environmental pollution rates and the threat of ecosystem stability. Due to the increase of human activities in the field of industry and agriculture, heavy metals are increasingly spreaded and accumulated in water, air and soil, which pose a great risk to living organisms. Medicinal plants and natural products constitute an important source for relieving and treating many diseases in a safe manner, as it is characterized by low cost and fewer side effects compared to industrial chemical drugs. The present study was aimed to evaluate the ameliorative influence of chia oil and canola oil on testicular injury induced by lead (Pb) in rats. To evaluate the role of chia oil and canola oil against Pb toxicity, sixty Wistar male rats were taken and divided into six groups. Group 1 was kept as control, group 2 received Pb solution (150 mg/kg BW), group 3 was treated with chia oil at (600 mg/kg BW) and exposed to Pb (150 mg/kg BW), group 4 was treated with canola oil (600 mg/kg BW) and exposed to Pb (150 mg/kg BW), groups 5 and 6 were treated with chia oil (600 mg/kg BW) and canola oil (600 mg/kg BW) respectively. In rats of group 2, the level of serum GSH, SOD and CAT were significantly decreased, while the level of MDA was increased. Histopathological examination of testicular tissues in rats of group 2 showed severe alterations. Treatment of rats with chai oil (group 3) and canola oil (group 4) reduced the changes of oxidative stress markers (GSH, SOD, CAT and MDA) accompanied with normal structure of testicular tissues. The results of this conclude that chia oil and canola oil have shown protection against Pb induced testicular injury due to their antioxidant roles.
Keywords
Lead
Chia oil
Canola oil
Antioxidant
Testis
Rats
1 Introduction
Environmental pollution exists in the form of air, soil and water pollution, which adversely affects the entire ecosystem including human beings (Zhang et al., 2015). Zhang and Wang (2020) in their work, concludes that environmental pollution is a cyclical process that involves all environments such as air, water, and soil, and from any perspective, living beings both emitters and receivers of pollutants. The authors further found out that the number of pollutants that humans contribute compromises the planet's environmental quality every day. The utmost harm to the environment is caused by heavy metals as they contaminate the entire food chain. Heavy metals can contaminate the soil ecosystem and the whole agricultural land which could make the crop unfit for consumption both for human beings as well as animals owing to carcinogenicity and various other serious health issues (Mao et al., 2019). Lead (Pb) is a metal that is considered as a major environmental contaminant. Various studies have suggested that Pb can trigger serious health issues and diseases (Jacobs et al., 2009).
Nearly about 200 years ago, herbal medicines dominated pharmacopoeia which laid the foundation of today’s modern medicine. Various synthetic drugs used in the present time have been originated from the vast kingdom of plants. Medical herbalism is a branch of science that deals with preparing medicines with the help of plants. It experiences a rapid decline in its popularity as soon as pharmacology firmly establishes itself as the most important branch of therapeutic medicine (Ernst, 2005). Nevertheless, a wide range of population is still dependent on medicinal herbs for their health and well-being (Sabery et al., 2019). The botanical name of Chia plant is Salvia hispanica L. which is an annual plant that belongs to the family Lamiaceae. Chia seeds are utilized in many different ways such as a whole seed, as flour, for its mucilage and to extract its oil (Zettel and Hitzmann, 2018). Various benefits are associated with Chia seeds when consumed in the form of nutritional supplements. It supports the human digestive system, promotes healthy skin, helps in making muscles and bones strong, and also have a potential to reduce the possibility of diabetes and heart ailments (Ullah et al., 2016; De Falco et al., 2018; Grancieri et al., 2019; Kulczyński et al., 2019). Canola is a variety of rapeseed which is originated from the cross breeding of Brassica rapa and Brassica napus species. Its oil has a minimum percentage (2%) of erucic acid and contains a negligible amount of anti-nutritional components (Amosovam et al., 2019). Ming et al. (2018) claim that canola oil is one of the most consumed vegetable oils globally. Moreover, the seeds contain several bioactive compounds such as antioxidant vitamins, phenolic molecules, coenzyme Q and phytosterols. These bioactive compounds have healthy metabolic, anti-inflammatory and physiologic effects (Xu et al., 2014). Therefore, the present study was carried out to determine the potential protective effects of chia oil and canola oil against Pb induced testicular injury in male rats.
2 Material and methods
2.1 Experimental design
Sixty Wistar male rats (110–135 g) were used in the present study and randomly divided into six experimental groups. Rats were housed in cages under standard laboratory conditions (room temperature 20 ± 2 °C, humidity: 55% ±10, and 12:12 h light: dark cycle each day) with standard food and water ad libitum. For this study, rats of group 1 were untreated and kept as controls. Group 2 was orally given Pb solution (150 mg/kg body weight, BW) every day for 6 weeks. Group 3 was daily orally given chia oil (600 mg/kg BW) and treated with Pb after 4 h. Group 4 was daily treated with canola oil (600 mg/kg BW) and exposed to Pb after 4 h. Groups 5 and 6 were daily orally received chia oil (600 mg/kg BW) and canola oil (600 mg/kg BW) respectively.
2.2 Blood collection and biochemical analysis
After 6 weeks of duration all rats were fasted for 10 h and then subjected to diethyl ether anesthesia. Blood samples were collected from all groups at end of experiment for determination the levels reduced glutathione (GSH), superoxide dismutase (SOD) malondialdehyde (MDA) and catalase (CAT). Blood samples were collected from orbital plexus veins, centrifuged at 3000 rpm for 15 min and serum samples were kept at −80 °C. The levels of GSH, SOD, MDA and CAT were measured using assay kits following the manufacturer’s instructions (MyBioSource, USA).
2.3 Histopathological examinations
Samples of testicular tissues were obtained from all animals, fixed in 10% buffered formalin and histologically processed to be paraffin embedded. All sections (4–5 μm) were processed for hematoxylin and eosin (H & E) staining (Suvarna et al., 2018), and examined under light microscopy and photographed.
2.4 Statistical analysis
The obtained data in the present study were expressed as mean ± standard deviation (SD). SPSS Windows version 22.0 software was used for statistical analysis. One-way analysis of variance (ANOVA) followed by Dunnett's test were statistically applied to compare between groups with the level of significance set at P ≤ 0.05.
3 Results
3.1 Biochemical analysis of GSH, SOD, MDA and CAT
The levels of serum GSH, SOD, MDA and CAT are shown in Fig. 1A-D. Highly significant decrease of GSH was observed in rats treated with Pb (P ≤ 0.000) and chia oil plus Pb (P ≤ 0.03) compared with control rats. The levels of serum GSH did not obviously altered in rats of groups 4, 5 and 6 (Fig. 1A). In comparison with control data, the level of serum SOD was markedly declined in rats of group 2 (P ≤ 0.000), group 3 (P ≤ 0.02) and group 4 (P ≤ 0.01). The levels of serum SOD were significantly unchanged in rats of groups 5 and 6 (Fig. 1B). Statistically, the treatment of rats with Pb (P ≤ 0.000) showed an increase on the level of serum MDA. Specifically, there were no significant differences in the levels of serum MDA in rats of groups 3, 4, 5 and 6 compared with control rats (Fig. 1C). In comparison with control rats of group 1, the administration of Pb alone significantly elevated the level of serum CAT (P ≤ 0.000). The levels of serum CAT in rats of groups 3, 4, 5 and 6 were not significantly different from those of controls (Fig. 1D).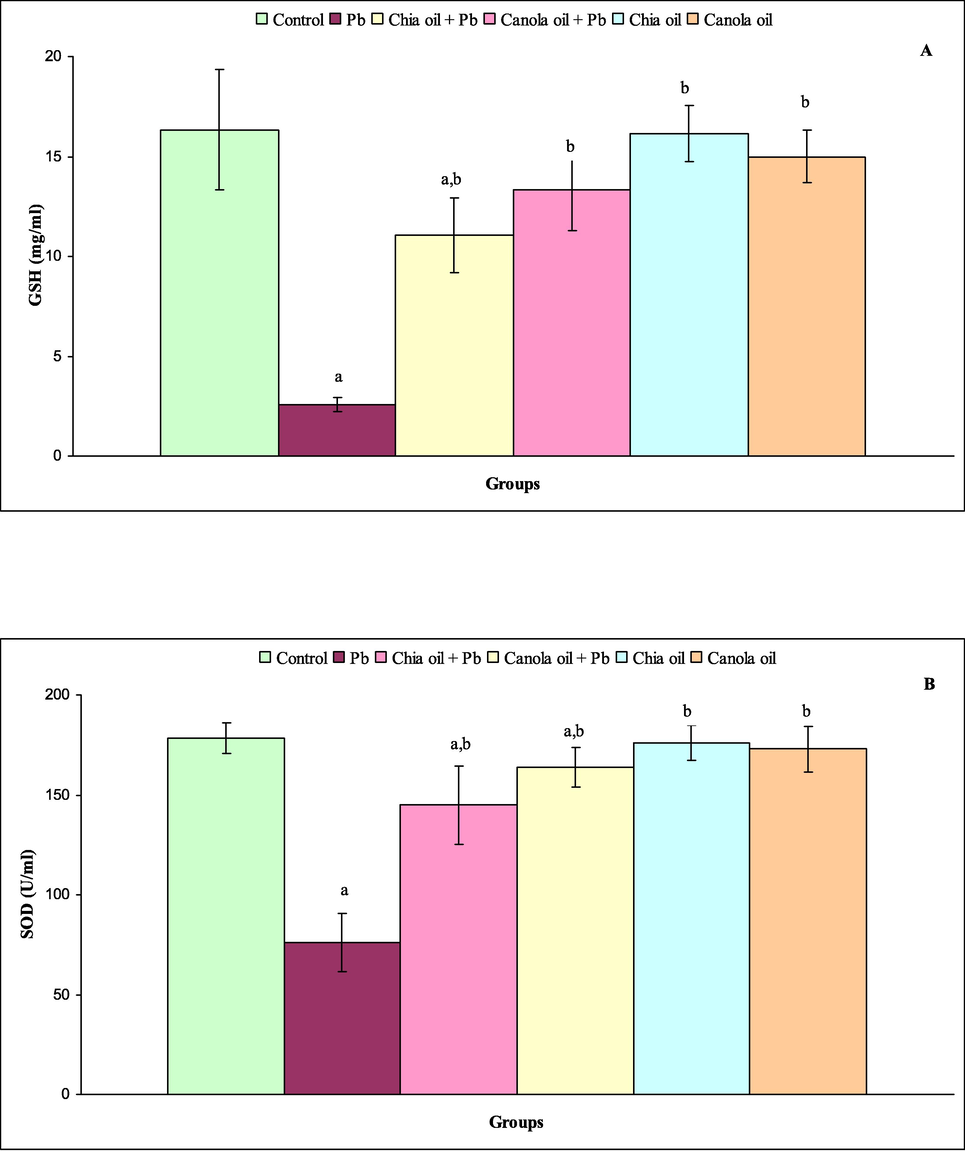
(A-D) Level of GSH(A),SOD (B), MDA (C) and CAT(D) in control (group1), Pb (group2),chia oil plus Pb (group3), canola oil plus Pb(group4),chia oil (group5)and canola oil (group6)treated rats. Significance levels shown for diffrence between control (group 1) and treated groups (2, 3,4, 5, and 6)were indicated by a. Significance levels shown for difference between Pb (group 2),and groups 3,4, 5 and 6 were indicated by b.
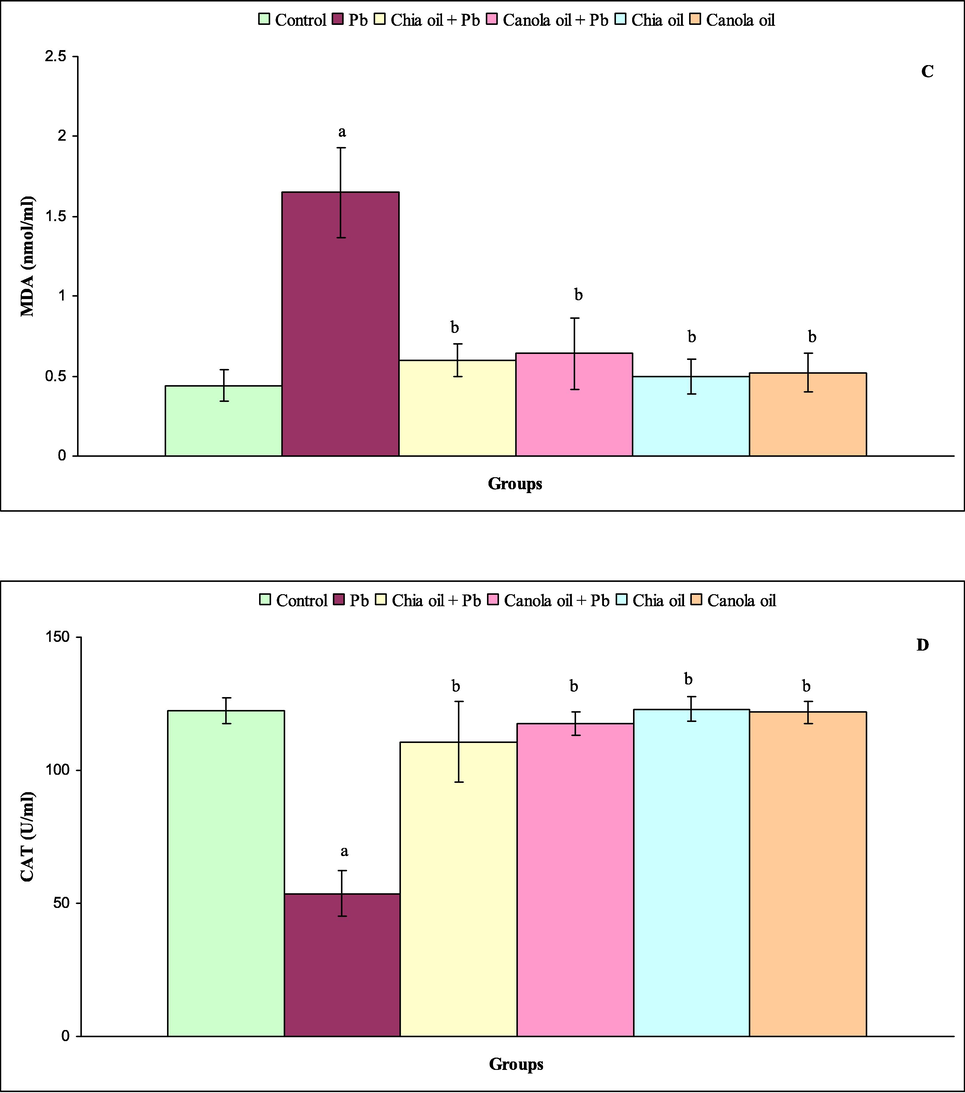
(A-D) Level of GSH(A),SOD (B), MDA (C) and CAT(D) in control (group1), Pb (group2),chia oil plus Pb (group3), canola oil plus Pb(group4),chia oil (group5)and canola oil (group6)treated rats. Significance levels shown for diffrence between control (group 1) and treated groups (2, 3,4, 5, and 6)were indicated by a. Significance levels shown for difference between Pb (group 2),and groups 3,4, 5 and 6 were indicated by b.
3.2 Histopathological examinations
Testicular histopathological examinations of Fig. 2A (group 1), 2C (group 3), 2D (group 4), 2E (group 5) and 2F (group 6) showed normal structures and spermatogenesis process. Fig. 2B showed several histopathological changes in the structure of testis in rats exposed to Pb (group 2). Degeneration of the intertubular tissues, an increase of distances between the seminiferous tubules and partial absent of spermatogenesis process were observed in testicular structures of group 2.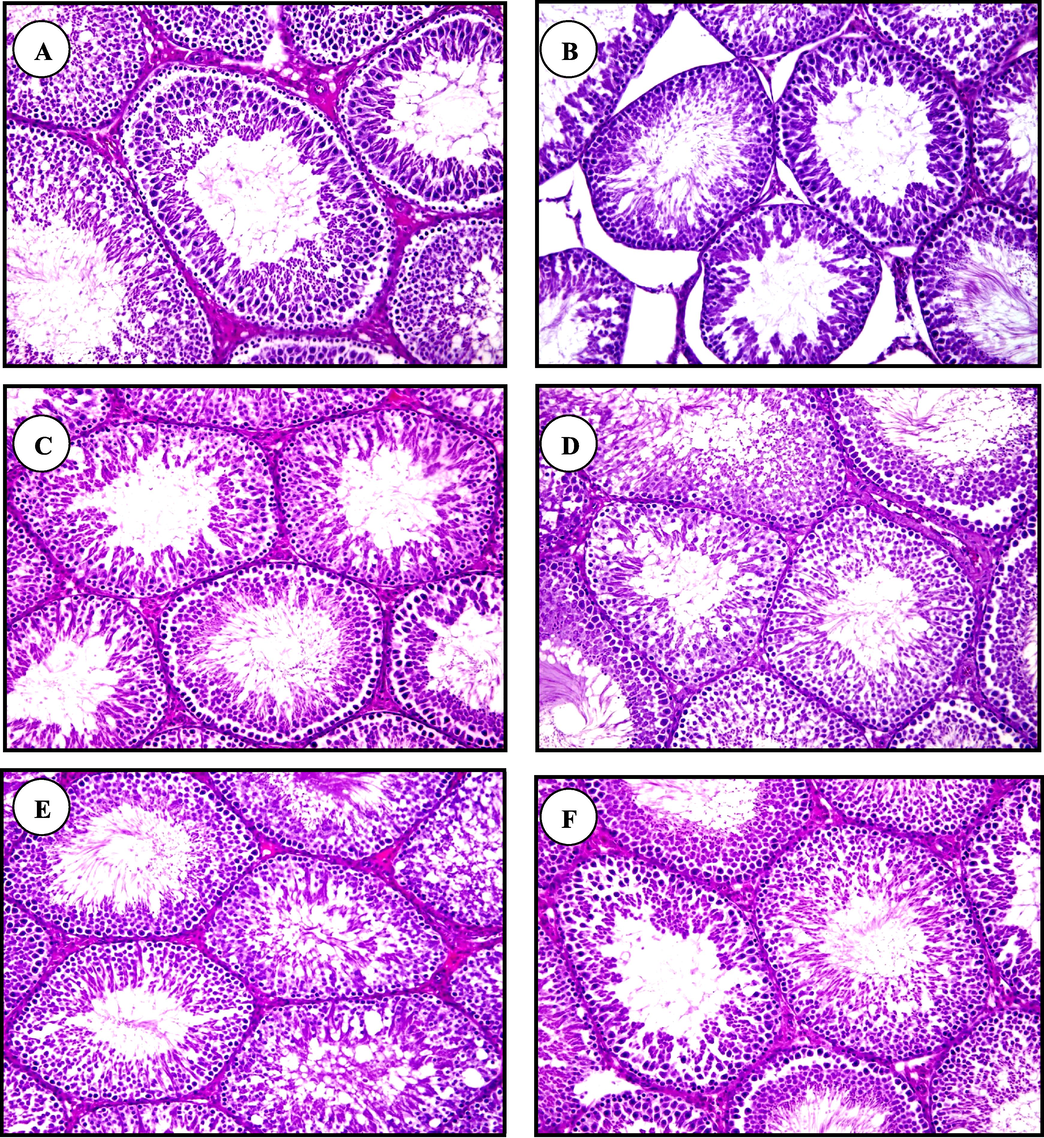
Light micrographs of testis sections of control (A), Pb(B), chia oil plus Pb(C), canola oil plus Pb(D),chia oil (E) and canola oil (F)treated rats(H & E staining, X200).
4 Discussion
Currently, the environment is affected by many pollutants that threaten the stability of ecosystem. Environmental pollutants are believed to be factors adversely affecting living organisms. The diverse deleterious health effects upon exposure to heavy metals in the environment are a matter of serious concern and a global issue. Pb is a toxic environmental pollutant and affect many of tissues in body such as kidney, lung, liver, and testis, as well as immune system and nervous system (Flora et al., 2012; Pal et al., 2015; Abdelhamid et al., 2020; Bozdağ and Eraslan, 2020; Gazeri and Aminzadeh, 2020; Abdel-Emam and Ahmed, 2021; Amin et al., 2021; Shaban et al., 2021).
Histopathologically, the present study evaluated the changes of testis structure induced by Pb exposure. Previous studies showed that the exposure to Pb caused several alterations of testis function and structure. Seminiferous tubules necrosis, decrease of sperm count, spermatogenic cells degeneration were observed in the structure of testis in rats exposed to Pb (Sudjarwo et al., 2017; Ezejiofor and Orisakwe, 2019; El-Khadragy et al., 2020).
The present study showed that Pb induced oxidative stress which confirmed by inhibition of serum GSH, SOD and CAT levels and enhancement of MDA level. The present findings are in agreement with previous experimental investigations, which indicated that the exposure to Pb induced significant decreases of GSH, SOD and CAT and statistically increase of MDA levels (Sudjarwo et al., 2017; Hasanein et al., 2018; Abdelhamid et al., 2020; El-Khadragy et al., 2020; Fan et al., 2020). Exposure to Pb induces oxidative stress in the body, resulting in an imbalance of the antioxidants defense mechanisms (Ighodaro and Akinloye, 2018). GSH is an important oxidant and functions to relieve the body from oxidative stress. The reduction in serum GSH levels results from the inactivation of GSH by Pb (Ali et al., 2020). GSH neutralized the radicals-generated from Pb metabolism and make chelation and detoxification of it (Fan et al., 2020). An antioxidant enzyme SOD, acts on superoxide anions free radicals. Biochemically, superoxide dismutases (SODs) play an important role in the decline of reactive oxygen species (ROS) production and this process prevents body tissues from oxidative stress and severe damages. Measurement of MDA is usually used as indicator of lipid peroxidation and to evaluate the level of oxidative damage (Cherian et al., 2019; Mao et al., 2019; Shaafi et al., 2021). The increase of MDA level is associated with an enhancement of oxidative stress and induction of many diseases. CAT is one of the most important intracellular antioxidant enzymes and it protects cells against an excess formation of ROS (Begenik et al., 2013; Ighodaro and Akinloye, 2018; Nandi et al., 2019).
The present study showed that the administration of chia oil and canola oil in rats can prevent severe alterations of testicular structure and oxidative markers (GSH, SOD, MDA and CAT) induced by Pb toxicity. Chia and canola oils have underlying higher concentrations of antioxidants. Antioxidants protect the body from the effects of free radicals within the body cells (Jakubczyk et al., 2020). The antioxidants present in chia oil are associated with the presence of several compounds such as chlorogenic acid, caffeic acid, and kaempferol (Knez Hrnčič et al., 2020). The antioxidant composition of canola oils is associated with vanillic acid, protocatechuic acid, and quercetin (Hussain et al., 2020). The protective effects of chia and canola oils result from introducing antioxidants that seek to reduce the impact of ROS. Excessive ROS formation results in oxidative stress that results in cell damage and death (Knez Hrnčič et al., 2020). Almalki (2019) investigated the effects of canola oil against malathion toxicity in rats. Malathion induced hematological and biochemical changes with blood serum CAT decrease and an elevation of MDA. These changes were alleviated in rats treated with canola oil. The results suggested that the protective effect of canola oil against malathion toxicity is attributed to its antioxidant activity. Ahmed et al. (2021) studied the cardioprotective effect of chia oil in rats exposed to doxorubicin (DOX). The results showed that the chia oil inhibited DOX-induced the decline of GSH and the increase of MDA levels. Additionally, chia oil inhibited the alterations of electrocardiogram (ECG), creatine kinase (CK), creatine kinase-MB (CK-MB) and aspartate aminotransferase (AST) values induced by exposure to DOX. From the present study, it can be concluded that chia oil and canola oil showed protective effect and antioxidative action against Pb-induced testicular toxicity in rats. Moreover, the present findings demonstrate for the first time the ability of chia oil and canola oil as protective factors against Pb toxicity, therefore the results of the current study give a clear indication towards the importance of using natural products as effective therapeutic agents. Undoubtedly, the current results confirm the ability of chia oil and canola oil to reduce Pb toxicity due to their antioxidant properties. The present study revealed new results on the role of chia oil and canola oil in reducing Pb toxicity, which prompts more new studies in the field of medical and pharmaceutical sciences. Finally, further experimental investigations are needed to evaluate the therapeutic effect of different doses of these oils against Pb toxicity and other toxicants and pathogens.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci.. 2021;7:1426-1435.
- [Google Scholar]
- Ameliorative effect of curcumin against lead acetate-induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res. Int.. 2020;27:10950-10965.
- [Google Scholar]
- Chia seed oil ameliorates doxorubicin-induced cardiotoxicity in female wistar rats: An electrocardiographic, biochemical and histopathological approach. Cardiovasc. Toxicol.. 2021;21:533-542.
- [Google Scholar]
- Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem.. 2020;44:13145.
- [Google Scholar]
- The potential protective effect of sesame oil and canola oil on rats exposed to malathion. Currt. Sci. Inter.. 2019;8:687-698.
- [Google Scholar]
- Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J. Food Biochem.. 2021;45:e13241.
- [Google Scholar]
- Phenotypic, biochemical and genomic variability in generations of the rapeseed (Brassica napus L.) mutan, lines obtained via chemical mutagenesis. PLoS One. 2019;14:e0221699.
- [Google Scholar]
- Serum malondialdehyde levels, myeloperoxidase and catalase activities in patients with nephrotic syndrome. Redox Rep.. 2013;18:107-112.
- [Google Scholar]
- The effect of diosmin against lead exposure in rats‡. Naunyn Schmiedebergs Arch. Pharmacol.. 2020;393:639-649.
- [Google Scholar]
- Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci.. 2019;11(Suppl 2):S297-S300.
- [Google Scholar]
- Metabolomics driven analysis by UAEGC-MS and antioxidant activity of chia (Salvia hispanica L.) commercial and mutant seeds. Food Chem.. 2018;254:137-143.
- [Google Scholar]
- Impact of coenzyme Q10 administration on lead acetate-induced testicular damage in rats. Oxid. Med. Cell. Longev.. 2020;2020:4981386.
- [Google Scholar]
- The efficacy of herbal medicine–an overview. Fundam. Clin. Pharmacol.. 2005;19:405-409.
- [Google Scholar]
- The protective effect of Costus afer Ker Gawl aqueous leaf extract on lead-induced reproductive changes in male albino Wistar rats. JBRA Assist. Reprod.. 2019;23:215-224.
- [Google Scholar]
- Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elem. Med Biol.. 2020;58:126443.
- [Google Scholar]
- Toxicity of lead: A review with recent updates. Interdiscip. Toxicol.. 2012;5:47-58.
- [Google Scholar]
- Protective effects of deferoxamine on lead-induced cardiotoxicity in rats. Toxicol. Ind. Health. 2020;36:800-806.
- [Google Scholar]
- Chia seed (Salvia Hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Compr. Rev. Food Sci. Food Saf.. 2019;18:480-499.
- [Google Scholar]
- Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia. 2018;50(1)
- [Google Scholar]
- Phenolic compounds with antioxidant properties from canola meal extracts inhibit adipogenesis. Int. J. Mol. Sci.. 2020;21:1.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54:287-293.
- [Google Scholar]
- The relationship of housing and population health: a 30-year retrospective analysis. Environ. Health Perspect.. 2009;117:597-604.
- [Google Scholar]
- Reactive oxygen species-sources, functions, oxidative damage. Pol. Med. J.. 2020;48:124-127.
- [Google Scholar]
- Chia Seeds (Salvia hispanica L.): An overview—phytochemical profile, isolation methods, and application. Molecules. 2020;25:11.
- [Google Scholar]
- The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients. 2019;11:1242.
- [Google Scholar]
- Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: a community-based cohort study. BMC Geriatr.. 2019;19:104.
- [Google Scholar]
- Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena. 2019;175:339-348.
- [Google Scholar]
- Combustion characterization of waste cooking oil and canola oil-based biodiesels under simulated engine conditions. Fuel. 2018;224:167-177.
- [Google Scholar]
- Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev.. 2019;2019:9613090.
- [Google Scholar]
- Lead exposure in different organs of mammals and prevention by curcumin-nanocurcumin: a review. Biol. Trace Elem. Res.. 2015;168:380-391.
- [Google Scholar]
- Satisfaction with and factors related to medicinal herb consumption in older Iranian adults. Eur. J. Integr. Med.. 2019;25:100-105.
- [Google Scholar]
- The significance of the oxidative stress markers in the one-year prognosis of patients with acute ischemic stroke: a case-control study. BMC Neurol.. 2021;21:258.
- [Google Scholar]
- Synergistic protective effect of Beta vulgaris with meso-2,3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Sci. Rep.. 2021;11:252.
- [Google Scholar]
- Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci.. 2017;12:381-390.
- [Google Scholar]
- Bancroft's Theory and Practice of Histological Techniques E-Book. Elsevier Health Sciences; 2018. p. :654.
- Nutritional and therapeutic perspectives of Chia (Salvia Hispanica L.): a review. J. Food Sci. Technol.. 2016;53:1750-1758.
- [Google Scholar]
- Optimized rapeseed oils rich in endogenous micronutrients ameliorate risk factors of atherosclerosis in high fat diet fed rats. Lipids Health Dis.. 2014;13:166.
- [Google Scholar]
- Applications of chia (Salvia Hispanica L.) in food products. Trends Food Sci. Technol.. 2018;80:43-50.
- [Google Scholar]
- Natural and human factors affect the distribution of soil heavy metal pollution: a review. Water Air Soil Pollut.. 2020;231:1-13.
- [Google Scholar]
- Impact of soil heavy metal pollution on food safety in China. PLoS One. 2015;10:135-182.
- [Google Scholar]







