Protease from Bacillus subtilis ZMS-2: Evaluation of production dynamics through Response Surface Methodology and application in leather tannery
⁎Corresponding author. maryamshafique@fuuast.edu.pk (Maryam Shafique)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Proteolytic enzymes are the most versatile and commercially viable group of enzymes comprising over 65% share in the global enzyme market amongst which alkaline proteases have extensive applications in detergent and leather industry. Current study was designed to assess the potential of an alkaline serine protease from Bacillus subtilis ZMS-2 as a bating agent in leather processing. Initially, the production parameters were investigated through Response Surface Methodology (RSM) using Plackett-Burman Design, which identified substrate, agitation speed and incubation temperature as the most significant factors. The optimal levels of these factors were determined through the Box-Behnken experimental analysis as 0.436% substrate concentration, 36.5 °C incubation temperature and 56 rpm agitation speed. The statistical optimization experiments increased the volumetric production of enzyme by 3.94 times (2246 U mL−1) than the initial titer (571 U mL−1). The enzyme was partially purified and characterized as metal ions and detergent compatible serine protease having optimum activity at pH 8 and 60 °C. During the pilot-scale application as a bating agent, the enzyme (340 U mL−1) successfully removed the hair roots and other unwanted proteins from goat skins as observed during scudding and confirmed through Scanning Electron Microscopy. The processed skins displayed enhanced porosity, thumb impression, smoothness and pliability. These findings provide a strong basis for the use of this protease as an efficient and eco-friendly alternative for bating of animal skins in leather tanneries.
Keywords
Bacillus subtilis ZMS-2
Serine protease
Response Surface Methodology
Leather bating
1 Introduction
Proteolytic enzymes are bioactive macromolecules which constitute indispensable parts of life on earth including microorganisms, animals and plants. Microorganisms are generally preferred for production of commercially important enzymes due to their ease of genetic modification, rapid growth and limited cultivation space (Shaikh et al., 2019). Proteases have extended applications in industries such as silk degumming, leather processing, peptide synthesis, feed and food production, detergent manufacturing, photography and waste management, thus having a global market share of over 65% (Barzkar et al., 2020; Gupta et al., 2002).
Alkaline proteases constitute the largest group having optimal activity at neutral to alkaline pH, and are either serine or metallo proteases (Sharma et al., 2017). Serine proteases are specialized enzyme possessing the serine residue in their catalytic active sites (Khan et al., 2021; Barzkar et al., 2020). The compatibility with metal ions, commercial detergents, denaturants and the attributes of withstanding to the change in pH and temperature makes an enzyme ideal for its commercial applications.
The production of microbial proteases in submerged fermentation is prejudiced by numerous physico-chemical factors including carbon/nitrogen sources, salts, incubation time, size and age of inoculum, pH, agitation and incubation temperature (Khan et al., 2019; Shaikh et al., 2019). The use of Response Surface Methodology for the identification of significant parameter influencing the production dynamics of hydrolytic enzymes is highly desirable and reported in multiple studies (JayaKumar et al., 2021; Bhavikatti et al., 2020; Ejaz et al., 2019).
During the chemical dehairing, lime and sulfide act like shaving blades leaving behind the intact hair roots. These intact hair roots are then removed in the succeeding leather bating step. This is an important pre-tanning step where not only hair roots and other unwanted proteins are removed from the dehaired skin, but also help to split the fiber into fibril (Kanagaraj et al., 2015). Efficient leather bating results into high porosity and thumb impression, smoothness and better pliability of the processed skin. In current study, we have used the alkaline serine protease from Bacillus subtilis ZMS-2, to check the leather bating potential of this enzyme in comparison with the commercially available enzyme (Resopan RN®). This study also reports the statistical optimization of physico-chemical parameters for the enhanced production of protease from a desert soil microbe Bacillus subtilis ZMS-2.
2 Materials and methods
2.1 Materials
Fresh goat skins for bating studies were acquired from local slaughterhouse at Gol market, Karachi, Pakistan. The commercial leather bating enzyme (Resopan RN®) was purchased from Shafi Reso Chemicals (SRC), Lahore, Pakistan. The components of growth medium, chemicals for dehairing and azocasein/assay reagents used in this study were purchased from Oxoid Ltd. and Sigma-Aldrich, respectively.
2.2 Revival of culture
Bacillus subtilis strain ZMS-2 (Khan et al., 2022) previously isolated from desert soils was revived on nutrient agar for succeeding studies.
2.3 Inoculum preparation
Inoculum was prepared by adding a loopful of 24 h culture into medium containing; 1% glucose; 0.5% wheat bran; 0.1% CaCl2; 0.1% KH2PO4; 0.1% MgSO4·7H2O and incubated for 24 h at 37 °C.
2.4 Initial production medium
The 24 h old inoculum (10%) of strain B. subtilis ZMS-2 was added to production medium for containing; 0.5% starch; 1% wheat bran; 0.1% peptone; 0.1% KH2PO4; 0.1% MgSO4·7H2O and incubated for 72 h at 37 °C (Khan et al., 2019). After completion of fermentation process, the cell free filtrate was obtained by centrifugation at 8000 rpm for 15 min at 4 °C. Proteolytic units in the cell free filtrate were assessed by UV–Visible spectrophotometer using azocasein as substrate as reported by Caldas et al. (2002).
One unit of enzyme activity was defined as the amount of enzyme which yield an increase in absorbance of 0.001 optical density (OD) at 440 nm in 30 min at 37 °C.
2.5 Statistical optimization of protease production
2.5.1 Plackett-Burman Design (PBD) experiments
PBD was applied eight variables to identify the most significant variables affecting the production of protease at two levels (Table 1). The experiments were conducted by adding inoculum (5% or 10%) into media supplemented with 0.5% or 1% substrate. The pH of the medium was 6 or 8 while media contained 0.5% or 1% starch. The medium was incubated at 37 °C or 45 °C for 24 or 72 h with (150 rpm) or without agitation. Based on first order polynomial equation, 20 experiments were performed. Post incubation, cell-free filtrate was harvested and assayed for protease activity which was taken as response.
| Run Order | Inoculum (%) | pH | Substrate concentration (%) |
Substrate |
Starch concentration (%) |
Incubation time (h) |
Agitation |
Temperature (℃) | Units* (U mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 6 | 1 | WB | 0.5 | 24 | With | 37 | 181 |
| 2 | 10 | 8 | 0.5 | WB | 1 | 24 | With | 37 | 110 |
| 3 | 5 | 8 | 1 | C | 1 | 72 | With | 37 | 605 |
| 4 | 5 | 6 | 1 | WB | 0.5 | 72 | without | 37 | 338 |
| 5 | 10 | 6 | 0.5 | WB | 1 | 24 | without | 45 | 174 |
| 6 | 10 | 8 | 0.5 | C | 1 | 72 | With | 45 | 203 |
| 7 | 10 | 8 | 1 | C | 0.5 | 72 | without | 37 | 1192 |
| 8 | 10 | 8 | 1 | WB | 0.5 | 24 | without | 45 | 292.5 |
| 9 | 5 | 8 | 1 | WB | 1 | 24 | With | 45 | 72 |
| 10 | 10 | 6 | 1 | WB | 1 | 72 | With | 37 | 289.5 |
| 11 | 5 | 8 | 0.5 | WB | 1 | 72 | without | 37 | 410 |
| 12 | 10 | 6 | 1 | C | 1 | 72 | without | 45 | 421 |
| 13 | 5 | 8 | 0.5 | WB | 0.5 | 72 | without | 45 | 182 |
| 14 | 5 | 6 | 1 | C | 1 | 24 | without | 45 | 495.5 |
| 15 | 5 | 6 | 0.5 | WB | 0.5 | 72 | With | 45 | 254 |
| 16 | 5 | 6 | 0.5 | C | 1 | 24 | without | 37 | 504 |
| 17 | 10 | 6 | 0.5 | C | 0.5 | 72 | With | 45 | 364.5 |
| 18 | 10 | 8 | 0.5 | C | 0.5 | 24 | without | 37 | 445.5 |
| 19 | 5 | 8 | 1 | C | 0.5 | 24 | With | 45 | 118.5 |
| 20 | 5 | 6 | 0.5 | C | 0.5 | 24 | With | 37 | 290 |
WB = Wheat Bran, C = Casein.
2.5.2 Box-Behnken Design (BBD) experiments
In BBD, each significant variable was studied at three levels i.e., substrates concentration (0, 0.5 or 1%; agitation 0, 75 or 150 rpm and temperature 35, 37.5 or 40 °C resulted into a combination of 15 experiments. The non-significant factors including starch concentration (0.5%), pH 8, inoculum (10%) and incubation time (72 h) were kept constant. The response function representing the activity of protease was partitioned into linear, quadratic, and interactive components. After the analysis of BBD, response optimizer was run and experiment was performed in order to get optimized values of parameters.
2.5.3 Statistical analysis
The experimental responses were evaluated using two-way analysis of variance (ANOVA) and results were produced for the independent variables. The linear, quadratic and interaction regression coefficient of each entity in the model were resolved. Using the confidence level of 95% and f-value at a probability (P) of 0.05, the significance of all entities in the polynomial was statistically investigated and all coefficients were figured out using Minitab version 17.0.
2.6 Partial purification
The cell-free filtrate (1000 mL) having 2246 U mL−1 was subjected to ammonium sulfate precipitation up to 70% saturation level at 4 °C on magnetic stirrer for partial purification. Precipitates were recovered through centrifugation at 8000 rpm for 15 min at 4 °C and re-suspended in an equal volume of 50 mM Tris-HCl buffer (pH 8) followed by overnight dialysis to remove excessive salts. The proteolytic units of partially purified protease were estimated by performing protease assay as mentioned in section 2.4 and stored at −20 °C till further use.
2.7 Biochemical characterizations
The partially purified enzyme was diluted by adding 2 mL of enzyme into 10 mL 50 mM Tris-HCl buffer before the characterization. The optimum pH and temperature for proteolytic activity was identified by assaying the enzyme at varied temperatures (20–80 °C) and pH (4–11). Similarly, the effects of metal ions and inhibitors were evaluated by adding 50 µl of respective 5 mM solution to the assay reagents (Table 4). Furthermore, the effect of commercial detergents on the proteolytic activity was also evaluated using in 1% detergent solution (Mechri et al. 2019).
2.8 Pilot-scale application of protease as bating agent
After optimized production of protease a series of trials was conducted to check its application in leather processing as a bating agent at tannery section of Leather Research Centre, PCSIR, Karachi, Pakistan. Experimental rotary drums (Diameter: 45 cm, Width: 30 cm, capacity 50L) were used to carry out bating process employing dip method having a speed of 20–30 rpm. The proteolytic units of enzyme were adjusted using 50 mM Tris- HCl buffer to that of a commercial enzyme Resopan RN® (340 U mL−1) before the pilot-scale bating studies. Four goat skins were processed through conventional dehairing method using 4% Na2S and 6% Ca(OH)2. After dehairing, liming and de-liming, skins were subjected to bating by adding 2% w/w of ammonium sulfate followed by addition of 2% w/w of both Resopan RN® bat (as per manufacturer’s recommendations) and protease of the present study into separate experimental rotary drums and run for 60–90 min.
2.9 Topological analysis using scanning electron microscope (SEM)
Skin pieces from the enzymatically processed skin were dried in oven at 40–50 °C for 4–5 h, coated with 250A° thick gold using Ion Sputtering Device (JEC-1500, Jeol, Japan) and examined by Analytical SEM (JSM-6380, Jeol, Japan). The microscopy imageries were recorded at different magnifications ranging from 100 to 400x at a voltage of 10 kV.
3 Results
The optimization strategy used in this study by employing PBD revealed titers of the protease from 72 to 605 U mL−1 by varying experimental conditions (Table 1). Results of pareto chart and ANOVA showed that substrate concentration, agitation and temperature are significant factors (Fig. 1, Table 2). The Pareto chart shows the absolute values of the standardized effects from the largest effect to the smallest effect. The reference line for statistical significance depends on the significance level (denoted by α) (Fig. 1). The ANOVA analysis of the calculated enzyme units from the proposed 15 experiments runs (Table 2) revealed that the model for protease production was significant, giving p-value 0.03 and F-value 3.48 (Table 2). Three significant factors were then optimized by BBD (Table 3). Results of ANOVA showed that one-way interaction of substrate concentration and agitation were found significant as indicated by p-value < 0.05 (Table 4). Regression analysis showed the value of 86.81% and F-value, P-value were also found significant.
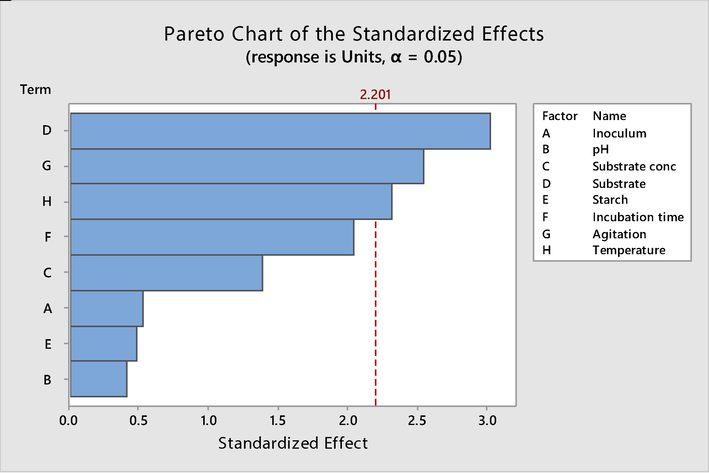
- Pareto charts showing the effect of factors on alkaline serine protease production.
| Term | T-Value | F-value | P-Value |
|---|---|---|---|
| Model | 3.48 | 0.030 | |
| Inoculum | 0.52 | 0.27 | 0.611 |
| pH | 0.41 | 0.17 | 0.687 |
| Substrate conc | 1.39 | 1.92 | 0.193 |
| Substrate | −3.03 | 9.18 | 0.011 |
| Starch | −0.49 | 0.24 | 0.637 |
| Incubation time | 2.04 | 4.18 | 0.066 |
| Agitation | 2.55 | 6.51 | 0.027 |
| Temperature | −2.32 | 5.38 | 0.041 |
| Run Order | Substrate (gm) | Agitation (rpm) | Temperature (℃) | Units* |
|---|---|---|---|---|
| 1 | 0 | 0 | 37.5 | 714.0 |
| 2 | 1 | 0 | 37.5 | 99.0 |
| 3 | 0 | 150 | 37.5 | 513.0 |
| 4 | 1 | 150 | 37.5 | 402.7 |
| 5 | 0 | 75 | 35 | 1629.3 |
| 6 | 1 | 75 | 35 | 735.3 |
| 7 | 0 | 75 | 40 | 667.0 |
| 8 | 1 | 75 | 40 | 712.3 |
| 9 | 0.5 | 0 | 35 | 1938.7 |
| 10 | 0.5 | 150 | 35 | 412.3 |
| 11 | 0.5 | 0 | 40 | 1759.0 |
| 12 | 0.5 | 150 | 40 | 713.3 |
| 13 | 0.5 | 75 | 37.5 | 2129.3 |
| 14 | 0.5 | 75 | 37.5 | 2210.0 |
| 15 | 0.5 | 75 | 37.5 | 2199.3 |
| Source | F-Value | P-Value |
|---|---|---|
| Model | 3.66 | 0.044 |
| Substrate | 1.47 | 0.279 |
| Agitation | 3.62 | 0.116 |
| Temperature | 0.44 | 0.535 |
| Square | 8.59 | 0.020 |
| Substrate*Substrate | 17.83 | 0.008 |
| Agitation*Agitation | 9.57 | 0.027 |
| Temperature*Temperature | 0.97 | 0.370 |
| 2-Way Interaction | 0.54 | 0.675 |
| Substrate*Agitation | 0.30 | 0.606 |
| Substrate*Temperature | 1.05 | 0.353 |
| Agitation*Temperature | 0.27 | 0.623 |
Correlation analysis among the factors was performed in order to determine the interaction of factors and their collective effect on response. The interaction between the factors affecting the production of protease was examined contrary to two independent variables and the optimum values were computed by contour plots (Fig. 2). Increase in temperature along with increase in substrate concentration had significant effect on alkaline serine protease production which showed the directly proportional relationship between temperature and substrate concentration (Fig. 2).
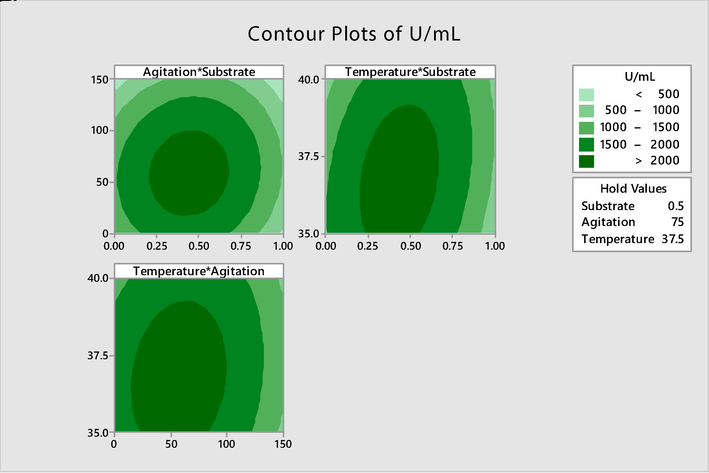
- Contour plots showing interaction between a) agitation and temperature b) temperature and substrate c) temperature and agitation.
After analyzing the data set and comparable responses, Minitab software proposed an experimental design comprised of a single run based on the previous results of BBD. A maximum of protease titer of 2254.06 units response was predicted under optimum conditions i.e., substrate concentration 0.426 %, agitation 56 rpm and temperature 36.5 °C (Fig. 3). The results revealed the experimental values 2246 U mL−1 that were comparable with the predicted values confirming the accuracy of RSM to predict the optimum levels of factors.
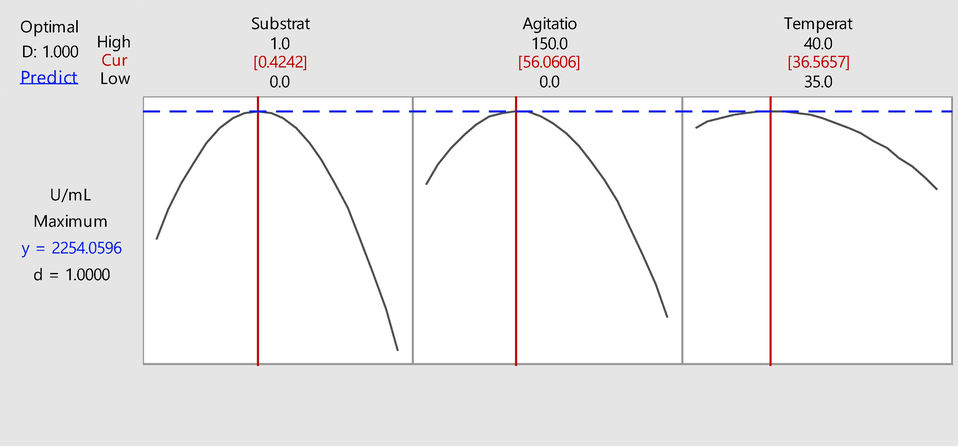
- Multiple response prediction showing the maximum production of alkaline serine protease as 2254.06 U mL−1.
During partial purification, the enzyme was completely salted out at 70% ammonium sulfate saturation with 1.87-fold increase in proteolytic activity (4200 U mL−1). The enzyme has showed maximum activity at 60 °C and pH 8 (Fig. 4a, 4b), completely lost its proteolytic activity when assayed in the presence of 5 mM PMSF and displayed compatibility with metal ions and detergents (Table 5).
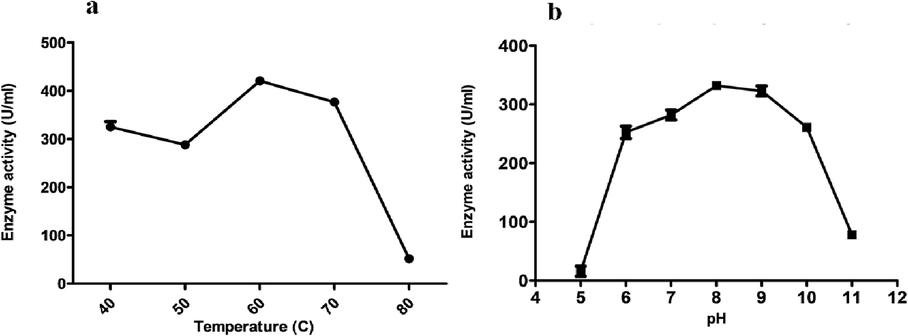
- Effect of temperature (a) temperature and (b) pH on the activity of alkaline serine protease.
| Inhibitors/metal ions/detergents | Concentration | Residual activity (%) |
|---|---|---|
| Control | – | 100 ± 4.32 |
| PMSF | 5 mM | 0 ± 2.41 |
| EDTA | 5 mM | 68 ± 3.52 |
| K+ | 5 mM | 95 ± 1.65 |
| Ca+ | 5 mM | 74 ± 2.78 |
| Na+ | 5 mM | 85 ± 3.13 |
| Fe2+ | 5 mM | 76 ± 3.61 |
| Mg2+ | 5 mM | 90 ± 4.26 |
| Surf Excel | 1% | 65 ± 2.72 |
| Ariel | 1% | 61 ± 3.37 |
| Bonus Tri-Star | 1% | 72 ± 1.81 |
| Lemon Max | 1% | 76 ± 2.52 |
| Brite | 1% | 74 ± 2.93 |
| Tween 80 | 1% | 124 ± 2.18 |
The enzyme exhibited a comparable bating potential to that of commercial bat as analyzed by the scudding process effectively removing the hair roots and other unwanted non-structural protein from the skin (Fig. 5b). Furthermore, observation of porosity, thumb impression, smoothness, and pliability of the processed skin also confirmed the effective bating property of protease. The SEM analysis of the processed skin displayed smooth morphology, better structure and degraded epidermis as well as degraded hair bulb (Fig. 5c-d).
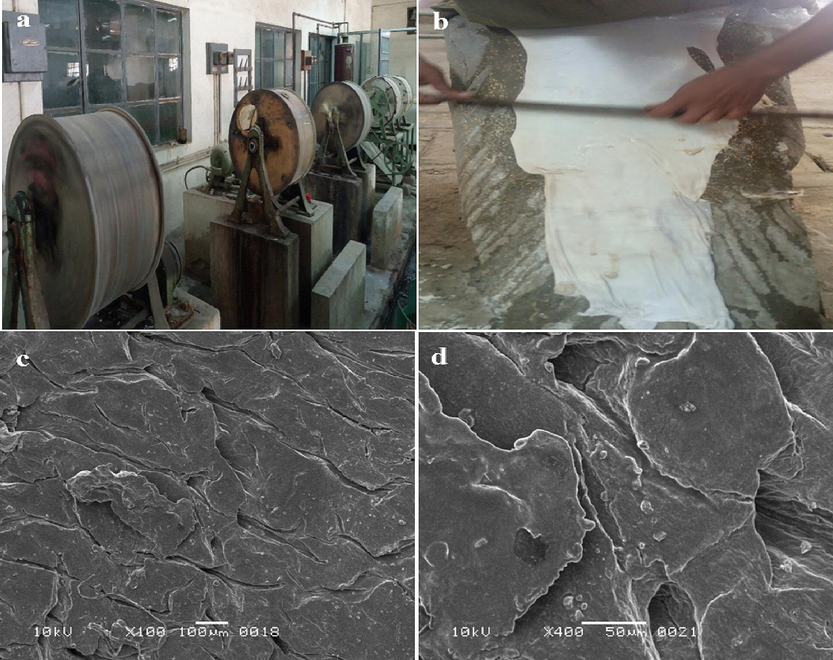
- (a)Tannery facility at Leather Research Center, PCSIR, Karachi, (b) Scudding of skin after bating showing removal of hair roots and unwanted proteins, SEM images of processed skin at (c) 100x and (d) 400x.
4 Discussion
One of major hindrances in the commercial application of enzyme is its cost, therefore, enzyme yield is improved either through genetic modification of the strain or through process optimization (Zafar et al. 2021).
Here, the significant factors as revealed by the PBD found to have been reported earlier for carbohydrolases (Rashid et al., 2020). For instance, the balance between C/N sources and substrate in production medium affect the growth rate as well as the production of enzymes (Ejaz et al., 2019; Jayakumar et al., 2021). Similarly, some studies have reported the concentration of tryptone and agitation as significant variable for the production of hydrolytic enzymes (Bhavikatti et al., 2020; Rashid et al., 2020). Moreover, majority of the microbes produce maximum titers of enzymes at their optimal growth temperature as studied by Sohail et al. (2009) and Ejaz et al. (2019).
The BBD potentially consider the quadratic effects hence providing a precise value of each significant factor (Rashid et al., 2020). The analysis of calculated units of each experiment using ANOVA resulted that the model for the enzyme production was significant (Table 4) (Sood et al., 2019). Contour plot was used to explore the potential relationship between three variables (two factors and response). The analysis of optimal values by contour plots revealed that the enzyme production was highly influenced by an increase in temperature as well as substrate concentration (Fig. 2).
The proposed single experimental run predicted 2254.06 U mL−1 by the software at specified condition; the results revealed the experimental values 2246 U mL−1 being comparable with the predicted values. This further confirms the accuracy of RSM to describe the optimum levels of factors. Furthermore, the statistical model not only resulted in a 3.94-fold increase in the production titer but also minimized the use of substrate for production further ensuring its cost-effectiveness which is essentially important for the scale up at industrial level (JayaKumar et al., 2021).
The enzyme displayed optimum activity at 60 °C and pH 8–9, and retained its catalytic potential at broad range of pH and temperature. During the interaction with PMSF the enzyme lost its activity which categorizes it as serine protease (Mechri et al., 2019). These characteristics of tempeature, pH, metal ions and detergents compatibility make this enzyme ideal for industrial applications. Further studies on structural elucidation are required to understand the underlying mechanism of this tolerance to metal ions and temperature-pH.
The enzyme (2% w/w) efficiently removed the hair roots and unwanted non-structural proteins (globulin, albumin, elastin, etc.) from the goat skin without affecting the main structural protein collagen; the data is in coherence with a previous study (Al Mamun et al., 2015). Furthermore, not all proteolytic enzymes are applicable for dehairing/bating purpose due to their collageno-lytic activity (Huang et al., 2003). Therefore, those enzymes having efficient keratinolytic and minimal collageno-lytic potentials are ideal for tanneries (Zambare et al., 2007). During the process of dehairing/bating, proteases selectively degrade the soft keratin tissues inside the hair follicle, thereby pulling out the intact hairs without disturbing the tensile strength of the processed leather (Thanikaivelan et al., 2004). This removal of hair at the level of roots is very important step during the leather processing which provide the ideal smoothness and pliability to the processed skin as displayed by this enzyme.
The skins processed using this protease as bating agent showed smooth texture, degraded epidermis and degraded hair bulb which is essentially important for pulling out the hair along with root (Fig. 5c). The surface topology of skins when observed at high resolution (400x) also indicated that the enzyme was highly specific towards the keratin and had no adverse effect on the collagen structure of the skin (Fig. 5d) (Zambare et al., 2007). These findings provide a strong basis for the use of alkaline serine protease from B. subtilis ZMS-2 as an efficient, ecofriendly and cost-effective alternative for the bating of animal skins on industrial scale.
5 Conclusion
Current study reports the optimization of physico-chemical parameters for the enhanced production of a thermophilic alkaline serine protease from B. subtilis ZMS-2. The initial production yield (571 U mL−1) was improved using statistical tools to 2246 U mL−1 with a 3.94-fold increase in activity. The result of ANOVA and second-order model revealed that the effects of casein, incubation temperature and agitation at various levels were significant for protease production. The enzyme was identified as metal ions and detergent compatible serine protease with an optimal activity at 60 °C and pH 8. The enzyme showed successful pilot-scale application as a bating agent with promising effects on leather pelt. Furthermore, scale-up production is highly desirable for its use as bating agent in leather industry.
Acknowledgment
The authors acknowledge the Higher Education Commission, Pakistan for the provision of funds for this study through TDF Grant No. 02-078 (awarded to corresponding author) and for Indigenous Ph.D. fellowship (awarded to first author). We are also thankful to the Director and technical staff of Leather Research Center, PCSIR, Karachi for the provision of tannery facility to carry out pilot-scale studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of an alkaline protease with high quality bating potential in leather processing from Bacillus licheniformis MZK05M9 mutant. Int. J. Biol. Res.. 2015;3(1):36-41.
- [Google Scholar]
- A critical review on marine serine protease and its inhibitors: a new wave of drugs? Int. J. Biol. Macromol.. 2020;170:674-687.
- [Google Scholar]
- Statistical optimisation of protease production using a freshwater bacterium Chryseobacterium cucumeris SARJS-2 for multiple industrial applications. 3 Biotech. 2020;10:279.
- [Google Scholar]
- Purification and characterization of an extracellular protease from Xenorhabdus nematophila involved in insect immunosuppression. Appl. Environ. Microbiol.. 2002;68(3):1297-1304.
- [Google Scholar]
- Methyltrioctylammonium chloride mediated removal of lignin from sugarcane bagasse for themostable cellulase production. Int. J. Biol. Macromol.. 2019;140:1064-1072.
- [Google Scholar]
- Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol.. 2002;59:15-32.
- [Google Scholar]
- Purification and characterization of extracellular alkaline serine protease with dehairing function from Bacillus pumilis. Curr. Microbiol.. 2003;43:169-173.
- [Google Scholar]
- Statistical optimization of thermostable alkaline protease from Bacillus cereus KM 05 using response surface methodology. Biotechnol Lett. 2021;43:2053-2065.
- [Google Scholar]
- Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. J. Clean. Prod.. 2015;89:1-17.
- [Google Scholar]
- Bacillus tequilensis ZMS-2: A novel source of alkaline protease with anti-microbial, anti-coagulant, fibrinolytic and dehairing potentials. Pak. J. Pharm. Sci.. 2019;32:1913-1918.
- [Google Scholar]
- Exploring the catalytic significant residues of serine protease using substrate-enriched residues and a peptidase inhibitor. Microbiol. Biotechnol. Lett.. 2021;49:65-74.
- [Google Scholar]
- Characterization of the genome and serine protease of a novel Bacillus subtilis isolate. Antonie van Leeuwenhoek.. 2022;115:281-295.
- [Google Scholar]
- Purification and biochemical characterization of a novel thermostable and halotolerant subtilisin SAPN, a serine protease from Melghiribacillus thermohalophilus Nari2A for chitin extraction from crab and shrimp shell by-products. Extremophiles.. 2019;23:529-547.
- [Google Scholar]
- Combined pretreatment of sugarcane bagasse using alkali and ionic liquid to increase hemicellulose content and xylanase production. BMC Biotechnol.. 2020;20:1-15.
- [Google Scholar]
- Streptomyces sp. MM-3 from rhizosphere of Psidium Guajava: A potential candidate for protease with dehairing properties. Pak. J. Bot.. 2019;51:735-742.
- [Google Scholar]
- Microbial alkaline proteases: optimization of production parameters and their properties. J. Genet. Eng. Biotechnol.. 2017;15:115-126.
- [Google Scholar]
- Distribution of hydrolytic enzymes among native fungi: Aspergillus the pre-dominant genus of hydrolase producer. Pakistan J. Bot.. 2009;41:2567-2582.
- [Google Scholar]
- Scientific validation of the antimicrobial and antiproliferative potential of Berberis aristata DC root bark, its phytoconstituents and their biosafety. AMB Expr. 2019;9:143.
- [Google Scholar]
- Progress and recent trends in biotechnological methods for leather processing. Trends Biotechnol.. 2004;22(4):181-188.
- [Google Scholar]
- Production of multienzyme by Bacillus aestuarii UE25 using ionic liquid pretreated sugarcane bagasse. J. Basic Microbiol.. 2021;61:1016-1028.
- [Google Scholar]
- Production of an alkaline protease by Bacillus cereus MCM B-326 and its application as a dehairing agent. World J. Microbiol. Biotechnol.. 2007;23:1569-1574.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102643.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







