Translate this page into:
Protease-activated receptor 1 mediated altered Ca+2 signaling in gliomas
⁎Corresponding author. anandprakash@mgcub.ac.in (Anand Prakash)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
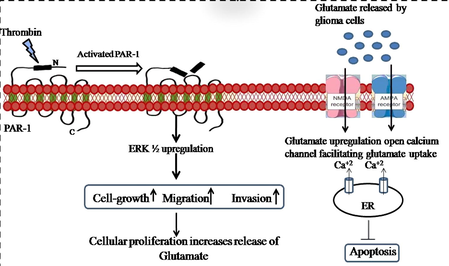
Thrombin-activated receptor-1 induces angiogenesis, cell proliferation, and invasion in tumors. The protein-activated receptor-1 (PAR1) is a G-protein coupled receptor that can be catalyzed by blood-derived serine proteases. Though Protease-activated receptor 1 plays an imperative role in coagulation and hemostasis, recent studies have found that PAR1 activation also affects the central nervous system (CNS) in addition to the vasculature. The present study focused on examining the prevalence of PAR1 in two human gliomas cell lines, D54 and U87, as well as the downstream signaling pathway. PAR1 was significantly more expressed in D54 cells than in U87 cells. An increase in PAR1 activity caused an increase in ERK1/2 activity, along with increased migration and invasion. SCH79797 (antagonist) significantly reduced the migration and invasion of the glioma cell lines. These cell lines' migration and invasion upon activator and inhibitor stimulation were related to PAR1 expression. In cells, Ca + 2 concentration ([Ca + 2]i) is altered by PAR1. Thrombin and TFLLR both induced an increase in [Ca + 2]i that was inhibited by SCH79797 (PAR1 inhibitor). The study concludes that PAR1 signaling contributes to the progression of gliomas. PAR1 and its downstream signaling may act as potential therapeutic targets for the prevention of glioma formation.
Keywords
Gliomas multiforme
Protease-activated receptor (PAR1)
G-protein coupled receptor
Thrombin
Calcium flux
1 Introduction
Glioblastomas are the most common primary tumor within cranim, accounting for 80 percent of all malignant brain tumors. Malignant high-grade gliomas are anaplastic astrocytoma, oligodendroglioma (both WHO grade III), and gliomas multiforme (GBM; WHO grade IV), the most lethal of all glial neoplasms. (WHO grade II) Lower-grade (LG) tumors are slow-growing, infiltrating lesions that inexorably progress to a more aggressive tumor, commonly and pre-terminally a GBM. In the current treatment strategies, such as surgical resection, radiation and chemotherapy, patient survival is only modestly improved, and patients suffer permanent deficits due to treatment-related toxicity (Gibson and Monje, 2012).
It has been reported that thrombin is expressed in a wide range of gliomas, including human gliomass and rat gliomas (Hua et al., 2005, 2008). Additionally, in rat gliomas, thrombin stimulates the release of VEGF from hypoxia-inducible factor-1* (HIF-1*) via mitogen-activated protein kinase (MAPK) pathways (Hua et al., 2003; Coughlin, 2000). Small molecular weight (MW 508) thrombin inhibitor argatroban treated rat F98 gliomas to reduce tumor mass, attenuate neurological deficits, and prolong survival time (Hua et al., 2003). Protease-activated receptors, PAR1, PAR-3, and PAR-4 (Coughlin, 2000), bind to thrombin and have been found on a variety of tumor cells, including rat gliomas (Coughlin, 2005). The PAR1 gene is activated during angiogenesis (Kaushal et al., 2006), tumor cell proliferation (Nierodzik et al., 1996), and invasion (Maragoudakis et al., 2000). Calcium signaling has been linked to a range of pathological conditions, including cancer. Several studies have found that calcium signals contribute to cancer cell proliferation, treatment resistance, and the spread of metastatic tumors. It has been shown that thrombin and PAR1-activating peptide (TFLLR) induces Ca2+ signals within rat brain microvascular cells. (Darmoul et al., 2004).
The present study aims at investigating the role of par1 (PAR1) in migration and invasion of gliomas. Further, it was also analyzed whether these receptors play a role in calcium influx which is amongst one of the important signaling molecules for the propagation of cancer cells.
2 Methods
2.1 Cell culture
Glioma cells D54 (RRID: CVCL_6748) and U87 (RRID: CVCL_EP65) were purchased from Thermo Fisher Scientific or NCCS, Pune. Cells implanted in DMEM containing 10% fetal bovine serum, 2 mM glutamine, 200 penicillin/ml, and 0.02 g/ml streptomycin under controlled humidity containing 5% CO2. Cells were calculated using a hemocytometer.
2.2 RT-PCR analysis of PAR1 mRNA expression
A small Purelink RNA kit from invitrogen (Carlsbad, CA) was used to extract the total amount of RNA from D54 and U87 cells. 1 µg of RNA was loaded using the GeneAmp RT (Applied Biosystems) kit. Protease receptor primers are: forward 5′ CCTATGAGACAGCCAGAATC 3′ and reverse 5′ GCTTCTTGACCTTCATCC 3′ of protease receptor. GAPDH used as a leading controller 5′ TTCAACGGCACAGTCAAGGC 3′ The PCR cycle was maintained at 95 °C for 10 min, then at 94 °C for 30 sec, followed by anneal discharge at 51 °C (GAPDH), 55 °C (PAR1) for 90 s and 72 °C to extend for 30 s and 10 s.
2.3 Migration in D54 and U87 cells
The filters used were Transwell polycarbonate with 6 mm filters (Costar Corporation, Cambridge, MA). D54 and U87 glioma cells were starved to serum for 12 h and harvested with EDTA (1.5 mM). They were washed with PBS and returned to DMEM at 1.5 * 106 cells/ml. An aliquot of 250 cells is placed at the top of the filter. SCH79797 was mixed with DMEM and added to the upper chamber (and cells), and 630 mL of DMEM with FBS was added to the lower chamber. For 5 h, the filters were also stored at 37 °C with 95% O2 and 5% CO2. The extra cells were immersed in PFA 4% for 5 min after being rubbed cells. 1% crystal violet was used to contaminate the extra cells.
2.4 Invasion assay in D54 and U87 cells
After harvesting semi-confluent suspensions of serum-stem cells, 2 lac cells per mL were established in DMEM. Cells were loaded into each matrigel-covered chamber. They were then placed back in the incubator for five hours. Lower cells are mixed with 4% paraformaldehyde (PFA) 5 min after rubbing the extra cells. Taint was carried out with 1percent violet in 0.2 M sodium borate buffer in the lower surface cells obtained. Untreated cells were used as controls.
2.5 Calcium flux assay
In order to analyze calcium permeability in glioma cells after activation and inhibition, a calcium flux assay was performed using Abcam Calcium Detection Assay Kit (ab102505 Abcam, Berlin). The cells were seeded in 96 well plates for 24 h in serum deprived media treated with TFLLR and SCH79797. Cells were rinsed twice with 1 X sterile PBS the next day. Then 90 µL of the chromogenic reagent was added to each well containing the control and treated wells. 60 µL of Calcium Assay Buffer is added to each source. Plate was placed in the dark at room temperature for 10 min. Fraction is measured at 575 nm.
2.6 Statistical analysis
GraphPad Prism 8 used for statistical analysis. Results are presented as a standard ± SEM. Independent T-test and ANOVA were used to compare the methods. All statistical analyzes were performed with a mean value of p < 0.05.
3 Results
3.1 PAR1 receptor mRNA expression
In D54 and U87 cell lines, PAR1 expression was examined using RT-PCR. Evidently, the D54 cell line has a higher level of mRNA expression than the U87 cell line [p < 0.001; F (2:12) = 56.71] (Fig. 1a). Densitometric analysis was performed by Image Studio Lite Ver. 5.2 (Fig. 1b).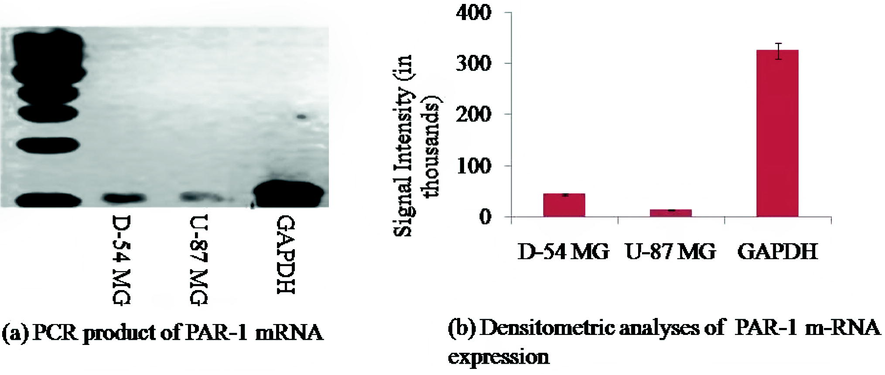
(a) Represents the RT-PCR analysis of the PAR1 mRNA receptor. PCR is performed using specific sets of protease receptor and GAPDH. (b) Densitometric analysis of protease receptor m-RNA expression in D54 and U87 cell lines. m-RNA was genetically modified for GAPDH and expressed as descriptive ± SEM.
3.2 Active agent for PAR1 activation based on p-ERK
ERK½ signaling has been reported to be linked to protease receptor signaling downstream and is associated with pathogenesis of gliomas. In order to analyze the effect of protease activation on D54 and U87 cell lines, we used 30 µM TFLLR and different western blocks to release proteins not treated cells were defined as control group.. Continuous activation of p-ERK½ was detected in the D54 cell-line [p < 0.001; F (5, 32) = 864.0] but not in U87 cell-line [p < 0.01; F (5, 32) = 1059.0] when p-ERK½ activation decreased significantly (Fig. 2a). p-ERK½ in the D54 cell line redirect after 30 min and yet in the U87 cell-line signal intensity remained steadily lower after 30 min. (Fig. 2b). ERK1/2 is activated via improved phosphorylation and activation of the Protease receptor and may play a role in glioma pathogenesis.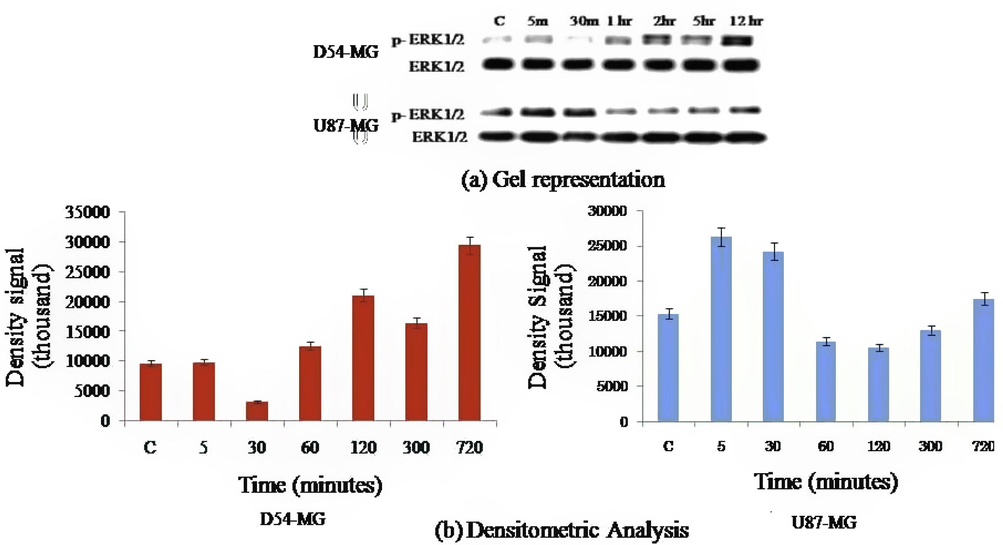
(a) Cells stimulated by TFLLR and ERK ½ phosphorylation were tested for Western blot. (b) ERK ½ time interval activated by TFLLR (30 µM). Densitometric analysis results are defined as ± S.E.M. D54-MG cells showed continuous ERK ½ activity for >12 h while in U87 ERK ½ cells appeared to appear for only ½ hour.
3.3 PAR1 induced migration in D54 and U87 cells
In order to confirm PAR1 as a migration factor, we tested the effect of SCH79797 on TFLLR-based migration. The PAR1 inhibitor SCH79797 (3 mM) inhibited TFLLR-induced migration of D54 cell-line [F (2, 12) = 61.50, p < 0.001] (Fig. 3A), confirming role of PAR1 in the migration of D54 cell-line. U87 cells showed similar results to TFLLR-induced cells (Fig. 3B), where F (2, 12) = 8.45, p < 0.01].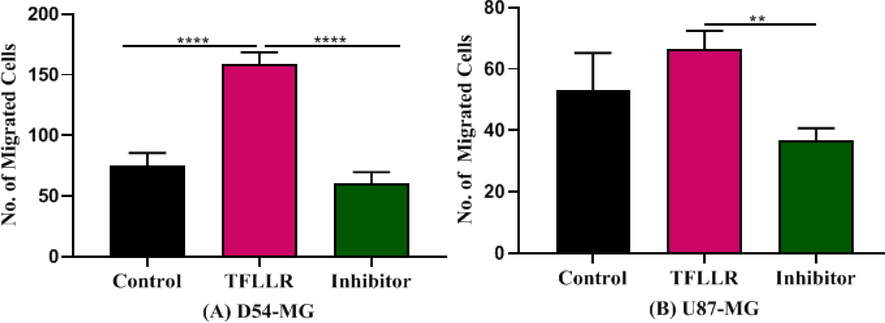
Represents the effect of agonist and antagonist of protease receptor in migration of cells. The increase in migration of D54 cells by TFLLR was inhibited by protease inhibitor SCH79797 (3 µM). Number of migrated cells were decreased a concentration increased (n:5). (A) SCH79797 in the presence of TFLLR on the migration of cells D54 (B) SCH79797 in the presence of TFLLR on the migration of cells U87.
3.4 PAR1 induced invasion in D54 and U87 cells
Thrombin, an endogenous ligand of PAR1, was used to confirm the activation and invasion of PAR1 by its agonist. D54 and U87 cell lines determined the concentration (EC50) of thrombin. The D54 cells are activated by thrombin in low concentrations (0.1U/ml), but the U87 cells are activated only in high concentrations (1U/ml) (Fig. 4).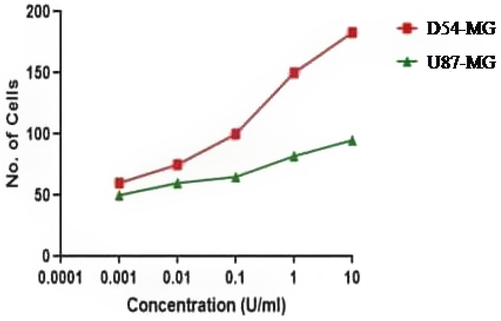
D54 cells are activated at lower concentrations of thrombin, whereas U87 cells are activated at higher concentrations.
Thrombin was then studied for its effect on invasion. D54 cell-line invasion was significantly induced by thrombin [F (2, 12) = 88.48, p < 0.0001] (Fig. 5A) compared to U87 [F (2, 12) = 39.13, p > 0.05] (Fig. 5B).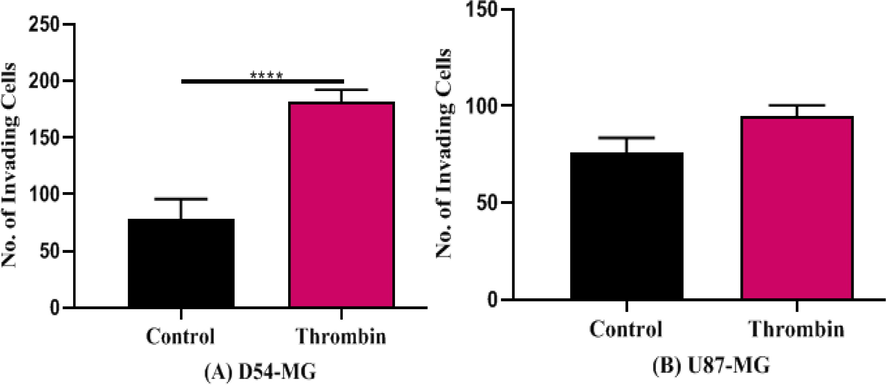
Represents the EC50 of thrombin in D54 (A) and U87 (B) cell-line. D54 cells show increase at reduced concentrations of thrombin (0.1 U/ml) but U87 shows activation only at enhanced concentrations (1 U/ml) (n:5).
SCH79797 was then used to evaluate the effect of par1 inhibition on TFLLR-induced invasion of cells. D54 cells were significantly reduced in invasion with SCH79797 [F (2, 12) = 85.72, p > 0.0001] (Fig. 6A) and U87 cells were significantly decreased in invasion with SCH79797 [F (2, 12) = 8.984, p > 0.01] (Fig. 6B). The invasion of D54 cells across the matrigel was, however, highly significant as compared to U87 cells.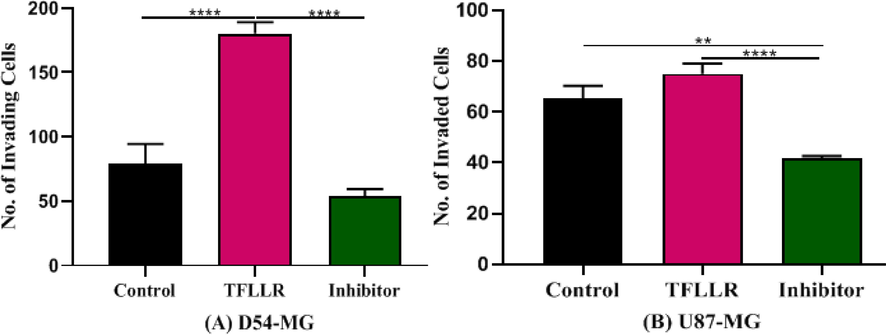
Represents the effect of activator and inhibitor of par1 in invasion of cells. (A)Effect of antagonist SCH79797 in the presence of agonist TFLLR on the invasion of D54 (B) Effect of antagonist SCH79797 in the presence of agonist TFLLR on the invasion of U87.
3.5 Calcium flux assay
Intracellular calcium concentration was analyzed using calcium flux assay. It was found that D54 cells show significant calcium influx [F (2,12) = 142.7] (Fig. 7A) when treated with TFLLR and SCH79797 while U87 show less significant calcium concentration [F (2,12) = 19.83] (Fig. 7B) when compared to the control group cells showed less concentration of intracellular calcium.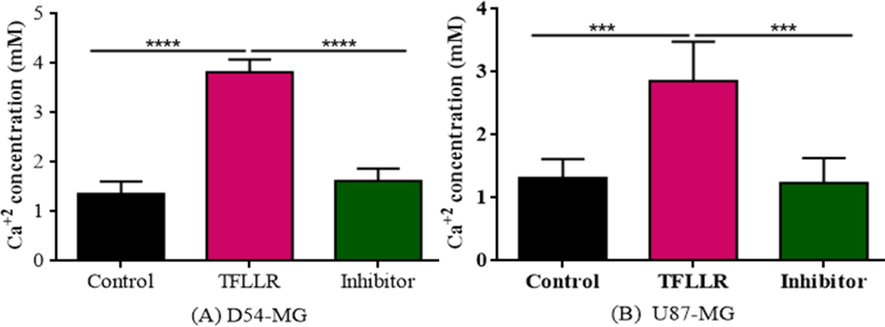
Represents the effect of agonist and antagonist of PAR1 in influx of calcium. SCH79797 decreased the calcium flux. (A)Figure shows the effect of SCH79797 with TFLLR on the calcium flux of D54 cells. (B) Figure shows the effect of SCH79797 with TFLLR on the calcium flux of U87 cells.
4 Discussion
The most malignant astrocytes are gliomas. Curing this disease is very difficult due to the pathogenic nature of the cells. Several studies have shown that the par1 gene facilitates the progression of gliomas and increases their severity (Xie et al., 2016). To investigate the association between the prevalence of protease receptor and its impact on the pathogenesis of gliomas we performed the present study. In two gliomas cell lines, the protease receptor expression was assessed. Using pharmacological interventions, we studied the role of this receptor in invasiveness and signaling. Cells from the D54 cell line express significantly more par1 m-RNA than those from the U87. These findings suggest that the expression of protease-activated receptors on the cell surface is positively correlated with the expression of protease-activated receptors in messenger RNA. The expression of these receptors has been linked to an increase in malignancy in multiple studies (Maklad et al., 2019). Early in this century, studies linked ERK 1/2 signaling to par1 downstream signaling and to the pathogenesis of gliomas (Auvergne et al., 2016).
The activation of these receptors is then investigated to see if it alters the ERK12-dependent signaling pathway in these cell lines, which is linked to cell proliferation, migration, and invasion. Par1 activation causes astrocyte growth by activating MAPKs implicated in several pathways (Covic and Kuliopulos, 2018). TFLLR (a synthetic PAR1 activator) activated PAR1 in D54 and U87 cells, resulting in a rise in p-ERK 12. TFLLR administration resulted in continuously enhanced expression of p-ERK 1/2 in the D54 cell-line, but expression in the U87 cell-line reduced after 30 min, according to the study. The greater expression of PAR1 in the D54 cell line compared to the U87 cell line supported this conclusion. Recent research shows the protease-activated receptor and their ligand thrombin in glioma progression (Wojtukiewicz et al., 2015; Liu et al., 2017). A thrombin-binding protein is an endogenous PAR1 receptor ligand. By activating the receptor, it promotes the expansion and proliferation of gliomas (Zhu and Reiser, 2014). The central nervous system tumor cells of this study expressed PAR1, suggesting that thrombin or thrombin-like proteases (membrane attached or released by the tumor) are responsible for the auto-activation of these receptors. As a result of the activation of PAR1, thrombin causes neuroglial signaling to be amplified, inflammation, differentiation, proliferation, tumor-related brain edema, and malignancy in glial cells (Cheema et al., 2005). However, U87 cells migrated in a concentration-independent manner and increased only at a high concentration.
Next, we examined whether there was a link between the level of PAR1 receptor expression and the migration. Migration and invasion of D54 cells support the concentration, but it increases only at higher concentrations for U87 cells. Compared to U87 cells, D54 cells expressed more PAR1, suggesting a link between the PAR1 signaling pathway and pathogenicity. Several gliomas cell lines are shown to hold PAR1 receptors (Fan et al., 2005), putting them in danger for the event of gliomas cancer. Further studies have also linked angiogenesis with over-expression of PAR1 in Gliomas multiforme (Morrow et al., 2012).
As compared to U87 cells, D54 cells were significantly more likely to be induced to migrate and invade by TFLLR. As a result, PAR1 receptors are over-expressed within the D54 relative to the U87. The presence of PAR1 was confirmed by using SCH79797, a selected PAR1 inhibitor. The TFLLR-induced increase in migration and invasion of D54 was reduced significantly when the precise inhibitor SCH79797 was used. We confirm that PAR1 receptors are actively involved within the progression of Gliomas multiforme in rodents and in human tissues (Hua et al., 2008). These concomitant effects suggest the association of PAR1 expression with glioma migration. Previous studies have found that activated PAR1 was not regulated in tumors (Chapman, 2013) by continued use of ERK1/2 and thus induced cellular pathogenesis.
Signaling mediated by calcium is one among the foremost important regulators of cellular activity. Calcium channels and pumps are abundantly expressed on the membranes of a broad range of cell types. Therefore, glutamate-like stimulants that activate calcium-channeling channels can activate Ca2 + infiltration into many cell types. It can cause 'wave-like' calcium stimulation at all tissue levels due to the subsequent increase in intracellular Ca2 + proliferation (Itsekson-Hayosh et al., 2015). Calcium can be a signal molecule between cells that acts as a second messenger and transcripts think in many ways. Additionally, calcium channels and pumps cooperate extensively with other channels like voltage-gated proton, potassium, and chloride channels. The fate of cells is thus determined by calcium via an outsized network of signaling regulators. Enhanced metabolic changes, glial proliferation, angiogenesis, invasion, and migration, excessive calcium expression induced by the activity of the calcium channel may enhance the conversion and progression of glioma. (Pearson and Regad, 2017).
Thrombin and TFLLR activated cells are found to point out a dose-dependent rapid and transient [Ca2+] increase within the brain (Kuhn et al., 2014). This calcium influx was sensitive to the pathogenesis of gliomas. It's been reported that stimulation of PAR1 causes activation of PLC and Ca2+ release from the endoplasmic reticulum (López et al., 2019; Putney and Tomita, 2012). There has been evidence that PAR1 stimulation with TFLLR leads to a huge influx of Ca2+ into the extracellular space via SOCCs (Kim et al., 2004; Chou et al., 2021; Lin et al., 2022; Yuan, 2000). When the cells were treated with PAR1 inhibitor SCH79797 the increase in calcium flux was decreased. It had been in line with the evidence that eliminating extrinsic Ca2+ or pre-incubating with SKF-96365, an inhibitor of SOCCs, prevented PAR1-AP-induced [Ca2+] rise. According to these results, activation of PAR1 and activator peptide TFLLR results in calcium infection rather than inhibition. A secondary messenger, calcium (Ca2+), is involved in many cellular processes, including cell growth, apoptosis, differentiation, metabolism, contraction, neuronal plasticity, and transcription (Prevarskaya et al., 2014; Berridge et al., 2003; Monteith et al., 2017). Ca2+ signaling is involved in many physiological processes also as pathological processes, like cancer. Proliferation, angiogenesis, invasion, and metastasis are all Ca2+ -dependent processes critical for cancer progression (Ali et al., 2016a,b, 2015; Ali and Ali, 2014). In recent years, Ca2+ signaling has also been shown to contribute to the expansion and tumorigenesis of cancers of the brain. Despite the bulk of those studies performed thus far on gliomass, an increasing body of evidence indicates the importance of Ca2+ signaling in other sorts of brain cancer. Further, signaling of calcium in gliomas promoting growth and migration remains to be identified.
5 Conclusion
PAR1 is involved in the pathogenesis of gliomas, as discussed throughout the paper. Activator and inhibitor treatments of PAR1 demonstrate that the protein contributes to migration and invasion of gliomas. In addition, it is one of the most important mechanisms of calcium entry into cells. For the first time, the study shows that PAR1 is directly involved with glioma development through increased calcium influx.
Acknowledgment
Researchers Supporting Project number (RSP2022R414), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
None.
References
- Ali, D., Ali, H., 2015).Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells. 96:941–950. doi: 10.1080/02772248.2014.987510
- Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Devel. Ther.. 2015;9:2793-2800.
- [CrossRef] [Google Scholar]
- Ali, D., Verma, A., Pathak, A.K., et al., 2016. UVR-induced toxicity of sludge and polycyclic aromatic hydrocarbons on seed germination and seedling growth of Triticum aestivum L. 101080/0275754020161146711 32:446–459.
- Ali, H., Dixit, S., Ali, D., et al., 2016. Isolation and evaluation of biological efficacy of quercetol in human hepatic carcinoma cells. Drug Des. Devel. Ther. 10:155–162. doi: 10.2147/DDDT.S95275.
- PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene. 2016;35(29):3817-3828.
- [Google Scholar]
- Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol.. 2003;4:517-529.
- [Google Scholar]
- Coagulation in inflammatory diseases of the central nervous system. Semin. Thromb. Hemost.. 2013;39:876-880.
- [Google Scholar]
- Subnanomolar concentrations of thrombin enhance the volume-sensitive efflux of taurine from human 1321N1 astrocytoma cells. J. Pharmacol. Exp. Ther.. 2005;315(2):755-763.
- [Google Scholar]
- Bifunctional mechanisms of autophagy and apoptosis regulations in melanoma from Bacillus subtilis natto fermentation extract. Food Chem. Toxicol.. 2021;150:112020.
- [Google Scholar]
- Thrombin signalling and protease-activated receptors. [Review] [64 refs] Nature. 2000;407(6801):258-264.
- [Google Scholar]
- Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost.. 2005;3(8):1800-1814.
- [Google Scholar]
- Protease-activated receptor 1 as therapeutic target in breast, lung, and ovarian cancer: Pepducin approach. Int. J. Mol. Sci.. 2018;19(8):2237.
- [Google Scholar]
- Activation of proteinase-activated receptor 1 promotes human colon cancer cell proliferation through epidermal growth factor receptor transactivation. Mol. Cancer Res.. 2004;2:514-522.
- [Google Scholar]
- Thrombin and PAR1-AP increase proinflammatory cytokine expression in C6 cells. J. Surg. Res.. 2005;129:196-201.
- [Google Scholar]
- Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr. Opin. Oncol.. 2012;24:672-678.
- [Google Scholar]
- A thrombin inhibitor reduces brain edema, glioma mass and neurological deficits in a rat glioma model. Acta Neurochir. Suppl.. 2003;86:503-506.
- [Google Scholar]
- Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82(4):834-848.
- [Google Scholar]
- Thrombin activity and thrombin receptor in rat gliomas model: possible markers and targets for intervention? J MolNeurosci. 2015;56:644-651.
- [Google Scholar]
- Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66(3):273-282.
- [Google Scholar]
- Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am. J. Physiol. Cell Physiol.. 2004;286(1):C31-42.
- [Google Scholar]
- Overexpression of protease-activated receptor type 1 (PAR1) in gliomas multiforme WHO IV cells and blood vessels revealed by NCAM-assisted gliomas border labeling. Neurol. Res.. 2014;36:709-721.
- [Google Scholar]
- Cell-derived artificial nanovesicle as a drug delivery system for malignant melanoma treatment. Biomed. Pharmacother.. 2022;147:112586.
- [Google Scholar]
- Protease-activated receptor-1 (PAR1): A promising molecular target for cancer. Oncotarget. 2017;8(63):107334-107345.
- [Google Scholar]
- Thrombin induces Ca2+-dependent glutamate release from RPE cells mediated by PLC/PKC and reverse Na+/Ca2+ exchange. Mol. Vis.. 2019;25:546.
- [Google Scholar]
- Calcium signaling in brain cancers: roles and therapeutic targeting. Cancers. 2019;11(2):145.
- [Google Scholar]
- Effects of thrombin/thrombosis in angiogenesis and tumour progression. [Review] [24 refs] Matrix Biol.. 2000;19(4):345-351.
- [Google Scholar]
- Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med.. 2012;366(15):1404-1413.
- [Google Scholar]
- Presence of the seven transmembrane thrombin receptor on human tumour cells: effect of activation on tumour adhesion to platelets and tumor tyrosine phosphorylation. Br. J. Haematol.. 1996;92:452-457.
- [Google Scholar]
- Targeting cellular pathways in gliomas multiforme. Signal Transduct Target Ther. 2017
- [Google Scholar]
- Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. B. 2014;369(1638):20130097.
- [Google Scholar]
- Phospholipase C signaling and calcium influx. Adv. Biol. Regul.. 2012;52(1):152-164.
- [Google Scholar]
- Protease-activated receptors (PARs)—biology and role in cancer invasion and metastasis. Cancer Metast. Rev.. 2015;34(4):775-796.
- [Google Scholar]
- Role of protease-activated receptor-1 in glioma Growth. Brain Edema XVI 2016:355-360.
- [Google Scholar]
- Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395-403.
- [Google Scholar]
- Signaling mechanism of par1 1-induced proliferation of astrocytes: Stabilization of hypoxia inducible factor-1α triggers glucose metabolism and accumulation of cyclin D1. Neurochem. Int.. 2014;79:20-32.
- [Google Scholar]







