Translate this page into:
Prospective mechanism of action of the tubulysin synthetic derivative (TAM 1344) in HCT116 colon cancer cell line
⁎Corresponding author. alqarni-aisha@hotmail.com (Aisha Alqarni),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tubulin is still a highly valued target in cancer chemotherapy. Agents that target tubulin and microtubule dynamic are considered to be of high therapeutic potential. We conducted a study to assess the effects of TAM1344, a synthetic cytolysin that is derived from the natural tubulysins on the proliferation of cancer cell lines. Tubulysins are a group of naturally occurring cytotoxic compounds that are produced by Myxobacteria. Our results show that TAM1344 exhibit strong antiproliferative activity against different cancer cell lines at low nanomolar concentration. The measured IC50 values in HCT116, A549 and MCF7 cancer cell lines were 0.14 nM, 0.24 nM & 0.09 nM, respectively. In a direct comparison, the three cell lines were more sensitive to the drugs than the myxobacterial natural products tubulysin-A and –B. Additionally, in HCT116 cells, TAM1344 induces destabilization and depletion of the interphase microtubules as indicated by Immunofluorescence staining. Furthermore, the spindle pools of dividing cells show unusual, condensed phenotyping, a characteristic phenotype of many anti-tubulin agents. The nuclei of treated cells look fragmented in comparison to control cells, as detected with DAPI or PI staining. Furthermore, at low concentrations, TAM1344 induces an accumulation of the cells in the G2/M phase of the cell cycle, and therefore apoptotic induction, as indicated with flow cytometry analysis. In addition, it provoked an apoptotic process, marked by elevated caspase-3 activity. To conclude, the results indicate that TAM1344 is a novel, highly effective microtubule-targeting agent.
Keywords
TAM1344
Microtubules
Tubulin
Apoptosis
Caspase-3
Tubulysin
1 Introduction
While increasing the number of cancer sufferers globally, the search for novel compounds to treat cancer is urgently needed. Cancer, a group of diverse illnesses that develop across time and are characterized by uncontrolled cell division, is ranked as a major cause of mortality over the globe (Bray et al., 2021). According to World Health Organization (WHO) estimation in 2019, the most cancer incidence percentage among men is lung, prostate, and colorectal cancers, whereas the mortality percentage is lung cancer, followed by liver and colorectal cancers. Among women, the incidence of colorectal cancer is second only to that of breast cancer and the third cause of cancer-related death (Sung et al., 2021). Colorectal carcinoma is one of the highest widespread malignant in Saudi Arabia, with a prevalence rate of 50.9% (Alqahtani et al., 2020). Based on Saudi cancer incidence report, it represents the first cancer among male and the third one among females of all ages (Saudi Health Council et al., 2018). Natural products always have unique biological activity and are always found in the chemical field associated to biology (Fang et al., 2021). They remain play a principal role in the process of finding and developing new drugs for human illness, especially in the field of anti-infective and anti-cancer research (Newman and Cragg 2016). A large portion of the drugs approved between 1981 and 2014 was either based on natural products or derivatives. However, because of supply issues from biological sources and their chemical complexity, natural product pharmaceutical research has declined in comparison to that of synthetic compounds (Koehn and Carter 2005).
Microtubules are highly conserved structures in eukaryotic cells. They are mainly composed of α- and β-tubulin dimers. Each of the tubulin monomer is composed of 450 amino acids (Schummel et al., 2017) with about 40% amino acid sequence homology. This homology makes the monomers similar in 3D-dimensional structures. Tubulin-targeting agents, like toxoids and vinca alkaloids, are among the highly effective drugs in cancer chemotherapy used in the clinic (Visconti and Grieco 2017). This group of compounds inhibit cell division by either stabilizing or destabilizing the microtubules dynamic. According to their mode of action, anti-tubulin drugs are categorized into two distinct groups. The first-group members (e.g., taxoides and epothilone) bind to β-tubulin and induce stabilization of microtubules (Rogalska et al., 2013, Wang et al., 2013). In contrast, members of the second group (e.g., vinca alkaloids and colchicine); destabilize microtubules' spindle (Martino et al., 2018). The members of the two groups are known to cause an arrest in the cell cycle as well as apoptosis in the treated cells.
TAM 1344 is a synthetic derivative of the natural product tubulysins that had been previously isolated from myxobacteria (Sasse et al., 2000). Tubulysin can inhibit tubulin polymerization in vitro and cancer cell lines (Khalil et al., 2006). The synthetic derivatives of tubulysins including TAM 1344 will be published in due time.
The purpose of the current research aimed to figure out the mode of action of a novel synthetic compound named TAM 1344 on selected cancer cell lines.
2 Materials and methods
2.1 Synthetic compound and cells treatment
TAM 1344 was obtained from Tube Pharmaceuticals GmbH, Leberstrasse 20, 11,100 Vienna, Austria.
2.2 Cell culture
The human HCT116 colon carcinoma, A549 lung carcinoma and MCF7 breast adenocarcinoma cell lines were obtained from the German Collection of Microorganisms and Cell Cultures GmbH (Braunschweig - Germany). DSMZ number are ACC581, ACC107 and ACC115; respectively. The cell lines were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) in a humid environment with 5% CO2 at 37 ℃. Cell culture reagents were supplied by GIBCO (MA -USA). Plastic ware was from NEST (CA-USA).
2.3 Cell viability assay
The detection of cell viability and cell growth were performed using MTT Assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, cat#M6494, Invitrogen, MA-USA). Briefly, aliquots of 120 µl of the suspended cells (5x 104 mL−1) were given to 60 µl of a serial dilution of the TAM1344 in a 96-well plate. After 4 days of incubations, 20 µl of MTT-solution were given to each well, and the cells further cultivated for an additional two hours. The cells were washed twice with PBS, and formazan crystals were dissolved in isopropanol. The intensity of the resulting color was measured at 595 nm as described previously (Elnakady et al., 2004).
2.4 Immunofluorescence staining of microtubules
HCT116 Cells were cultivated on glass coverslips in four-well plate and treated with TAM1344 for different periods of time 2, 3 & 4 h. Cells were fixed with ice cold acetone–methanol (1 + 1) for 15 min. cells were incubated with a primary antibody anti-β-tubulin (1:1000; Sigma) at 37℃ for 1 h, then with a secondary goat anti-mouse IgG antibody conjugated with Alexa fluor 488 (1:5000; Invitrogen) at 37℃ for 1 h. The cells were washed with PBS between all incubations. The coverslips were mounted using Fluoroshield™ with PI (SIGMA-ALDRICH, MO, USA), and the images were viewed with a ZEISS LSM 800 confocal microscope (Elnakady et al., 2004).
2.5 Cell cycle analysis
HCT116 cells were cultivated at a density of 5x104 cell ML-1 into 6-well plates and treated with 1 µg/ml TAM1344 for 24 h or methanol after they reached 60–70% confluence. The cells were then spun down and fixed immediately in 80% ice-cold methanol for half an hour. After that, the cells were rinsed with PBS and with 0.1% saponin in PBS. Finally, 400 µl of 20 mg/ml propidium iodide (SIGMA-ALDRICH, MO, USA) and 100 µl of RNAse 1 mg/ml (PureLink™ RNase A) were added to the cells and left to incubate at 37℃ for 40 min. Flow cytometry (Beckman Coulter Epics XL, USA) was used to conduct the analysis of the samples, (Elnakady et al., 2004).
2.6 Annexin-V‑FITC/PI staining
HCT116 cells were treated with TAM1344 for 2 h. Annexin VFITC/PI Apoptosis Staining/Detection kit (Abcam, Cambridge, UK) was used to analyze apoptosis induction according to the manufacturer's protocol. The cells were harvested by centrifugation and washed 3 times in phosphate-buffered saline (PBS). After being resuspended in 500 µl of 1X binding buffer, the cells were stained in the dark for 5 min with 5 µl each of Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI). The percentage of apoptosis was determined by BD Accuri™ C6 Flow Cytometer (NJ, USA), (Uddin et al., 2006).
2.7 Western blotting
Cells were treated with TAM1344 drug for 24 &48 h and lysed in a similar method stated by (Hussain et al., 2007). Using SDS-PAGE, 20 µg of proteins were separated and transferred to polyvinylidene difluoride (PVDF) membrane (Trans-Blot Turbo midi polyvinylidene difluoride (PVDF) trans Packs, Cat #1704157, Bio-Rad Laboratories, CA, USA. Immunoblotting was performed with a primary rabbit monoclonal procaspase-3 (1:1000; abcam) and mouse monoclonal β-actin (1:5000; santa cruz) antibodies, followed by with a secondary goat anti-rabbit IgG antibody conjugated with Alexa fluor 647 and goat anti- mouse IgG antibody conjugated with Alexa fluor 647; respectively (1:5000; Invitrogen) and visualized using ChemiDoc XRS System Imaging, Cat # 1708265, Bio-Rad Laboratories, CA, USA.
2.8 Statistical analysis
Data was presented as mean ± SD. Comparisons between groups were made with the paired Student's t-test. The limit of significance of all analysis was defined as p value of ≤ 0.05.
3 Results
3.1 TAM1344 inhibits the growth of cancer cell lines in a concentration dependent manner
We firstly tested the effect of TAM1344 (Fig. 1A) on the proliferation of three cancer cell lines representing colon, breast and lung cancers using MTT-Assay. As shown in Fig. 1B, TAM1344 inhibits the proliferation of cancer cells at low ng-level concentration. From the three cell lines tested, showed the breast cancer cell line MCF7 the highest sensitivity to the drug with IC50-Value of 0.09 nM. In contrast, the colon cancer cell line HCT116 and the lung cancer cell line A549 were relatively less sensitive to drug with IC50-Values of 0.14 and 0.24 nM, respectively. The results indicate the excellent anti-proliferative potential of TAM1344 against all cancer cell lines tested. We choose the colon cancer cell line HCT116, as a model for further investigations in this study.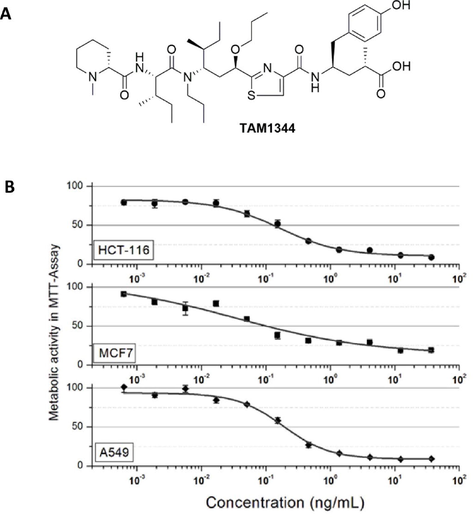
A) The chemical structure of TAM1344. B) Concentration-dependent growth inhibition of HCT116, MCF7 & A549 cell lines by TAM1344.
Using MTT-assay, we further compared the growth inhibition effect of TAM1344 with that of the natural products tubulysin-A (tub-A) and –B (tub-B) in the same cancer cell lines. As shown in Table 1, the sensitivity of the three cancer cell lines to TMA1344 was higher than that of tub-A or tub-B. The colon carcinoma cell line HCT116 was ten folds more sensitive to TAM1344 than tub-A and about 20 times more than tub-B. These results indicate that TAM1344 is a potentially anti-proliferative agent.
Cell lines
(Human)Cell type
TAM1344
(IC50 value nM, SD)Tubulysin A (IC50 value nM, SD)
Tubulysin B
(IC50 value nM, SD)
HCT116
Colon Carcinoma
0.14 (±0.02)
1.48 (±0.07)
2.77 (±0.09)
A549
Lung Carcinoma
0.24 (±0.01)
1.01 (±0.03)
2.69 (±0.04)
MCF7
Breast Carcinoma
0.09 (±0.04)
0.65 (±0.08)
1.33 (±0.11)
3.2 TAM1344 induces depletion of microtubules of interphase HCT116 cells and abnormal spindle of mitotic cells
Microscopic investigation using immunofluorescence technique showed that TAM1344 affect the microtubules structures in (Fig. 2). The alternation of the interphase microtubules structures could be already observed two hours after treatment the cells with 1ug/mL TAM1344 (Fig. 2B). In Fig. 2A the control sample showed the intact nuclei (red) and normal microtubule network organization (green) in which tubulin filaments are spread out in regular pattern throughout the cytoplasm of the cell (A). In contrast TAM1344 treated cells exhibit very short and at the seem time denser filaments. Such morphology seems to be characteristic for TAM1344 treatment. Additionally, the microtubules web disappeared with longer incubation time (Fig. 2C and 2D). Furthermore, the PI staining showed enlarged and fragmented nuclei of the treated cells. Moreover, the mitotic cells exhibited irregular spindles with a condensed abnormal configuration. In conclusion, ATM1344 destabilizes the microtubules of treated cells, and make them shorter and denser. It induces abnormal spindle configuration in mitotic cells as well as nuclei fragmentation in other cells as characteristic phenotypes of drug treatment.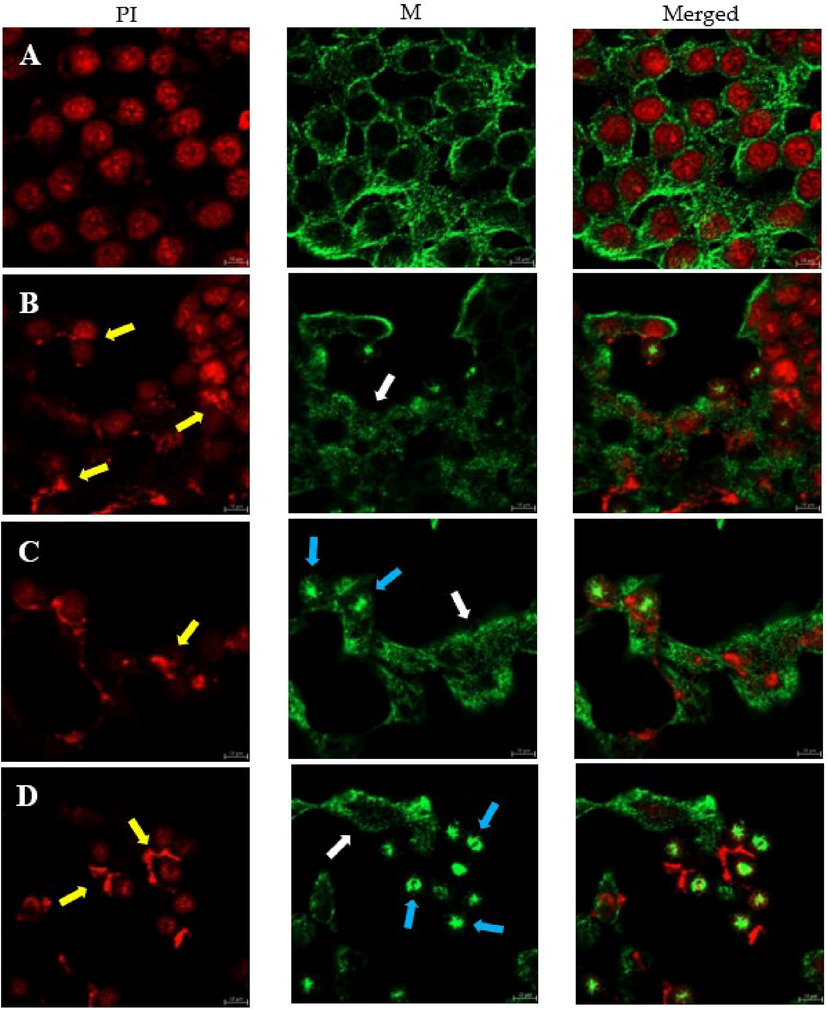
HCT116 colon cancer cells were examined by immunofluorescence confocal microscopy. Cells were treated with 1.4 nM of TAM1344 for 2, 3 & 4 h. A represents MeOH; control and B, C & D represent the effect of the drug.
3.3 Cell cycle analysis
The HCT116 cell line was treated with 1.4 nM TAM1344 or vehicle alone for 24 h. Flow cytometry was used to determine cell cycle fractions after the cells were stained. As demonstrated in Fig. 3, the percentage of G2/M population increased from 22.9% in control cells (A) to 59.7% in treated cells (B). This increase in the G2/M population was accompanied by a decline in the G1 phase population. It was difficult to determine the s-phase cell population.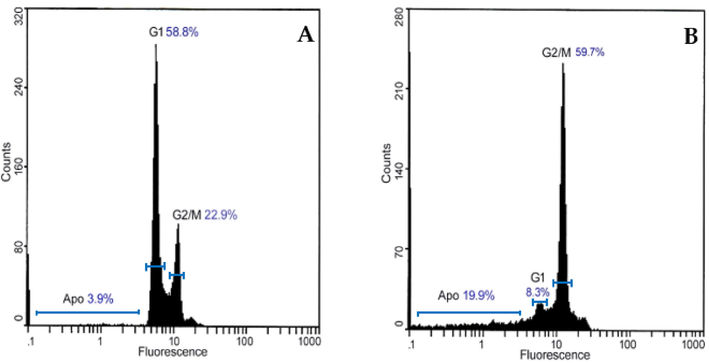
TAM1344 treatment increases G2-M populations in HCT116 cells, which were treated with 1.4 nM of the drug for 24 h. As detailed in Materials and Methods, the cells were then washed, fixed, and stained with propidium iodide before being analyzed by flow cytometry for DNA content.
3.4 Annexin V staining
Light microscopy investigation showed apoptotic morphology of the cell already two hours after drug treatment (data not shown). To further confirm the apoptosis‑inducing activity of TAM1344, HCT116 cells were treated with 1.4 nM of the drug for two hours, and cells were assayed by annexin V/PI dual staining. As shown in (Fig. 4), treatment with TAM1344 of HCT116 cells resulted in apoptosis in which the apoptotic cells accounted for 8.2% of the cells in late apoptosis (A: upper right quadrant) following a two hours post drug treatment, while that accounted for 0.1% treated with MeOH; control (B).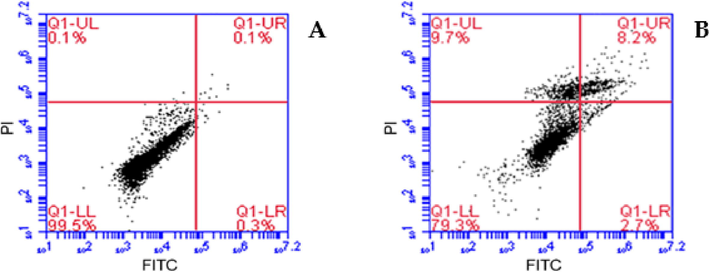
Annexin V/FITC-PI assay was used to examine the percentage of apoptosis. HCT116 colorectal cancer cells were treated with 1.4nMof TAM1344 for 2 h.
3.5 Expression of caspase-3 in HCT116 cell line
To confirm whether TAM1344 induced caspase-dependent apoptosis, we determined the effect of the drug on caspase-3, a last step hallmark of apoptosis scenario, or (the final enzyme in the apoptosis cascade). HCT116 cells were treated with TAM1344 or methanol alone for different time periods 24 & 48 h and cell lysates were separated on SDS-PAGE and probed with an anti-procaspase-3 antibody. The antibody detects the procaspase-3 inactive form of the enzyme. Fig. 5 shows that TAM1344 treatment resulted in significant decrease of the level of procaspase-3 over the time compared to the control, suggesting that the drug causes apoptosis by caspase-dependent pathway.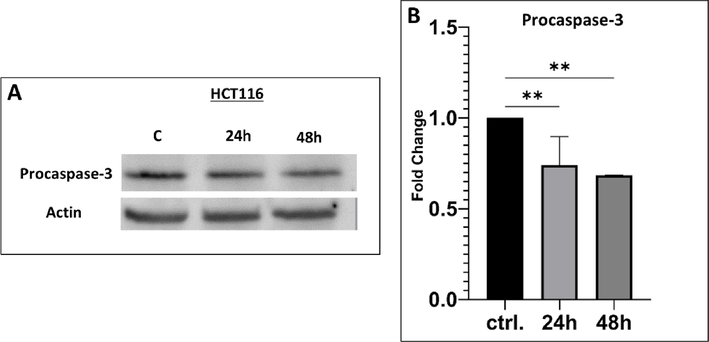
Expression of procaspase-3 by TAM1344 treatment in HCT116 cell line. The cells were treated with the 1.4 nM of the drug for 24 &48 h. Cells were lysed, and equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and probed with antibodies against procaspase-3 and β-actin (loading control). A) The visualization of procaspase-3 and β-actin protein bands was performed using Alexa Fluor Plus 647 anti-rabbit antibodies (Thermo, Cat. A-32733) and Alexa Fluor Plus 647 anti-mouse antibodies (Thermo, Cat. A-32728TR); respectively. B) The level of procaspase-3 protein expression was quantified using Image Lab 6.1 software and normalized against β-actin levels. The error bars represent the standard deviation (mean ± SD). One-way ANOVA was utilized in the statistical analysis. *: p ≤ 0.05; **: p ≤ 0.01 significant.
4 Discussion
Over the past decades, several natural compounds have been isolated from myxobacteria that have anticancer, antibacterial, antifungal, antiparasitic and antiviral bioactivity (Bhat et al., 2021). Some of them interfere with either microfilaments or microtubules of the cytoskeleton (Elnakady et al., 2004, Herrmann et al., 2017). Because of the known challenges and high cost of natural product research, in addition to the development of drug resistance, the pharma industry focused mainly, during past decades, on libraries of synthetic compounds as an alternative and promising source of the drug discovery. In comparison to natural products, synthetic compounds are easer in production and resupplying. Additionally, They are compatible with established high-throughput screening (HTS) platforms. (Atanasov et al., 2015).
In this study, we demonstrated that TAM1344 is a novel antimitotic compound. It is a chemical derivative of the natural product tubulysin that had been previously isolated from myxobacteria (Sasse et al., 2000). TAM1344, like tubulysin (Sasse et al., 2000, Khalil et al., 2006) and disorazol A1(Elnakady et al., 2004), interfere with and destabilize tubulin polymerization in cancer cell lines. The study demonstrated that TAM1344, an antimitotic agent, inhibits the growth of HCT116, A549 & MCF7 cell lines in a concentration-dependent manner. The growth inhibition data of various mammalian cell lines that have been published agree with our findings. Loss of cell viability due to tubulysin treatment has been previously reported in five different mammalian cell lines with IC50 values ranging from 1 ng/ml to 20 pg/ml (Sasse et al., 2000). In addition, low picomolar concentrations (3 pM) of disorazol A1 inhibited the proliferation of numerous cancer cell lines, including a multidrug-resistant KB line (Elnakady et al., 2004). The TAM1344 IC50 values were 0.14 nM, 0.24 nM & 0.09 nM for HCT116, A549 & MCF7 cell lines; respectively. A direct comparison between TAM1344 and the natural products tub-A and -B in MTT-assay indicated a higher sensitivity of the cancer cell lines tested to TAM1344 than to the two natural products. Summing up, TAM 1344 possesses the strong anticancer property that inhibits the proliferation of all cancer cell lines tested.
Microtubule-targeting agents are widely used in the treatment of cancer due to their ability to inhibit essential cellular processes, such as mitosis, cell migration, and cell signalling (Čermák et al., 2020). The effectiveness of microtubule-targeting drugs has been demonstrated by the use of a number of Vinca alkaloids and taxanes in the treatment of a wide variety of human malignancies (Karahalil et al., 2019). Microtubule-targeting agents (MTAs) are divided into two categories according to their mechanism of action. The first group are microtubule-destabilizing agents, such as the Vinca alkaloids, disorazol (Elnakady et al., 2004) and tubulysin (Khalil et al., 2006), which inhibit the polymerization of tubulin in vitro and destabilizing microtubules in treated cells. In contrast, the second group including microtubule-stabilizing agents, such as taxanes, paclitaxel & Epothilones. In vitro, these compounds promote tubulin polymerization, and in cells that have been treated, they stabilize microtubules (Devi Tangutur et al., 2017). More importantly, these anti-tubulin agents were considered the most effective drugs in many cancer chemotherapies (Morris and Fornier 2009, Edelman and Shvartsbeyn 2012, Naghshineh et al., 2015, Yeung et al., 2018). More recently, research efforts have been concentrated on the development of a novel compounds that are both more active and safe that can target microtubule organization (Mukhtar et al., 2014, Raja et al., 2014, Cong et al., 2018).
To test the ability of TAM1344 affect the microtubules stability, we carried out immunofluorescence study using HCT116 cell line, since it sensitive to the drug and according to the Saudi Cancer Registry, the colon carcinoma represents the first cancer among men and the third one among the women (2018). Cells that were only treated with the vehicle (methanol) showed a normal structure of the microtubule network. This normal organization is characterized by tubulin filaments being distributed in a regular pattern throughout the cytoplasm of the HCT116 cells (Fig. 2, panel A, MT). In contrast, cells exposed to TAM1344 exhibited microtubule disorganization (Fig. 2, panels B, C & D, MT). Indeed, tubulin filaments become irregular suggesting that the drug affects the microtubules structure by depleting them (see the white arrows). Also, many nuclei of the treated cells were fragmented (see the yellow arrows), in addition to appearing of centrosomes (see the blue arrows). These results indicate that, similar to tubulysin (Khalil et al., 2006), TAM1344 could act as a tubulin-polymerization inhibitor.
In mitosis, chromosomes are separated by a dynamic molecular mechanism called the mitotic spindle, which is made up primarily of tubulin. The depletion of microtubules suggests that the drug inhibits cell proliferation by blocking mitosis. Cell cycle investigations on the treated cells support this assumption. After 24hr of incubation with TAM1344, 59.7% of HCT116 cells had accumulated in the G2/M−phase (Fig. 3, B). It has been reported that many of MTAs; such as tubulysin and disorazol; arrest microtubules at G2/M−phase (Elnakady et al., 2004, Khalil et al., 2006).
Treatment of cancer cells with microtubule-disrupting agents like taxanes and vinca alkaloids causes the cells to undergo apoptosis, evidenced by their morphological changes and DNA fragmentation patterns (Raja et al., 2014). Dual annexin V-FITC and PI labeling of HCT116 cells exposed to TAM1344 for 2 h enabled detection of cell populations undergoing early and late apoptosis. As shown in (Fig. 4, B), TAM1344 induces apoptosis in HCT116 cell line. Additionally, using western blotting analysis we detected an involving of caspase-3 in TAM1344 apoptotic scenario, however this scenario has to be studied in details in a future study. This result is consistent with several other previously published findings that were examining various microtubule-destabilizing agents (Tu et al., 2013, Raja et al., 2014).
In conclusion, TAM1344 potently inhibits growth of different human carcinoma cell lines. Additionally, it induces at low nano-molar concentration, a depletion of interphase microtubules, a profound G2/M cell cycle arrest and apoptosis in Colon cancer cell line HCT116. TAM1344 shows great promise as potential antimitotic agents. However, further characterization of the mechanism of drug action in vitro as well as in an animal model still needed to fully explore the value of the drug in which, extend the current findings by examining in detail the effect of TAM 1433 on apoptosis-dependent pathway and the action of the drug on tubulin polymerization in vitro and in vivo.
Funding
This research was funded by the Researchers Supporting Project (RSP-2023/214), King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Aisha Alqarni: Methodology, Formal analysis. Yasser A. Elnakady: Conceptualization, Formal analysis. Lamya Alsadhan: Methodology. Muhammad Abbas: Methodology. Wolfgang Richter: Methodology. Badr A. Aldahmash: Supervision. Mansour I. Almansour: Resources. Layali M. Almutairi: Methodology. Ahmed Rady: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Epidemiology of cancer in Saudi Arabia thru 2010–2019: a systematic review with constrained meta-analysis. AIMS Public Health.. 2020;7(3):679.
- [Google Scholar]
- Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv.. 2015;33(8):1582-1614.
- [Google Scholar]
- Myxobacteria as a source of new bioactive compounds: A perspective study. Pharmaceutics.. 2021;13(8):1265.
- [Google Scholar]
- The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029-3030.
- [Google Scholar]
- Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol.. 2020;99(4):151075.
- [Google Scholar]
- An indole–chalcone inhibits multidrug-resistant cancer cell growth by targeting microtubules. Mol. Pharm.. 2018;15(9):3892-3900.
- [Google Scholar]
- Microtubule targeting agents as cancer chemotherapeutics: an overview of molecular hybrids as stabilizing and destabilizing agents. Curr. Top. Med. Chem.. 2017;17(22):2523-2537.
- [Google Scholar]
- Epothilones in development for non–small-cell lung cancer: novel anti-tubulin agents with the potential to overcome taxane resistance. Clin. Lung Cancer. 2012;13(3):171-180.
- [Google Scholar]
- Disorazol A1, a highly effective antimitotic agent acting on tubulin polymerization and inducing apoptosis in mammalian cells. Biochem. Pharmacol.. 2004;67(5):927-935.
- [Google Scholar]
- Natural products as LSD1 inhibitors for cancer therapy. Acta Pharmaceutica Sinica B.. 2021;11(3):621-631.
- [Google Scholar]
- Natural products from myxobacteria: novel metabolites and bioactivities. Nat. Prod. Rep.. 2017;34(2):135-160.
- [Google Scholar]
- Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res.. 2007;67(8):3888-3897.
- [Google Scholar]
- An overview of microtubule targeting agents for cancer therapy. Arhiv za higijenu rada i toksikologiju.. 2019;70(3):160-172.
- [Google Scholar]
- Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. Chembiochem.. 2006;7(4):678-683.
- [Google Scholar]
- The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov.. 2005;4(3):206-220.
- [Google Scholar]
- Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett.. 2018;28(17):2816-2826.
- [Google Scholar]
- Novel anti-tubulin cytotoxic agents for breast cancer. Expert Rev. Anticancer Ther.. 2009;9(2):175-185.
- [Google Scholar]
- Targeting microtubules by natural agents for cancer therapymicrotubule-targeting agents for cancer chemotherapy. Mol. Cancer Ther.. 2014;13(2):275-284.
- [Google Scholar]
- Safranal as a novel anti-tubulin binding agent with potential use in cancer therapy: An in vitro study. Chemico-biological interactions.. 2015;238:151-160.
- [Google Scholar]
- Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.. 2016;79(3):629-661.
- [Google Scholar]
- Novel antitumour indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Invest. New Drugs. 2014;32(5):838-850.
- [Google Scholar]
- Activation of apoptotic pathway in normal, cancer ovarian cells by epothilone B. Environ. Toxicol. Pharmacol.. 2013;36(2):600-610.
- [Google Scholar]
- Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli production, isolation, physico-chemical and biological properties. J. Antibiot.. 2000;53(9):879-885.
- [Google Scholar]
- Saudi Health Council, N. C. Center and S. C. Registry, 2018. Cancer Incidence Report In Kingdom of Saudi Arabia. 104.
- Modulation of the polymerization kinetics of α/β-Tubulin by osmolytes and macromolecular crowding. ChemPhysChem.. 2017;18(2):189-197.
- [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2021;71(3):209-249.
- [Google Scholar]
- Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Med.. 2013;31(1):113-119.
- [Google Scholar]
- Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108(13):4178-4186.
- [Google Scholar]
- Fighting tubulin-targeting anticancer drug toxicity and resistance. Endocr. Relat. Cancer. 2017;24(9):T107-T117.
- [Google Scholar]
- Cell-cycle synchronization reverses Taxol resistance of human ovarian cancer cell lines. Cancer Cell Int.. 2013;13(1)
- [Google Scholar]
- Identification of Cdk1–LATS–Pin1 as a novel signaling axis in anti-tubulin drug response of cancer CellsCdk1–LATS–Pin1 signaling mediates anti-tubulin drug response. Mol. Cancer Res.. 2018;16(6):1035-1045.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102824.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







