Translate this page into:
Propionate restores disturbed gut microbiota induced by methotrexate in Rheumatoid Arthritis: From clinic to experiments
⁎Corresponding author at: State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China. bo.yang@jiangnan.edu.cn (Bo Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objective
Gut toxicity of methotrexate restricts its long-term clinical application in patients with rheumatoid arthritis (RA). However, the disturbed gut microbiota especially beneficial bacteria induced by methotrexate remain unclear, and the way to alleviate the disturbances is limited. The aim of present study was to elucidate the effects of methotrexate on gut microbiota in RA patients and collagen-induced-arthritis (CIA) rats, and to explore a way targeted for restoring gut microbiota.
Methods
The gut microbiota including lactobacilli and bifidobacterial communities were analyzed by high-throughput sequencing of samples from RA patients and healthy controls (HCs), furthermore, propionate and butyrate were administrated to CIA rats to confirm the effects on gut microbiota.
Results
The findings showed gut microbiota significantly altered, and bifidobacterial community was tolerant to methotrexate while lactobacilli community such as L. delbrueckii, L. manihotivorans and L. intestinalis species under-represented in RA samples. Propionate supplementation was significantly associated with the rebalance of gut microbiota especially lactobacilli species including L. acidophilus, L. intestinalis and L. amylovorus in CIA rats.
Conclusion
Methotrexate disturbed gut microbiota and lactobacilli community in RA patients, and propionate supplementation contributed to normalize lactobacilli community in CIA rats. These findings suggested that propionate may be a potential alleviator for gut microbiota in RA patients treated with methotrexate.
Keywords
Gut microbiota
Lactobacilli community
Bifidobacterial community
Propionate
Methotrexate
Rheumatoid arthritis
- ACR
-
American College of Rheumatology
- CIA
-
Collagen-Induced-Arthritis
- HCs
-
Healthy Controls
- LDA
-
Logarithm Linear Discriminant Analysis
- MTX-RA
-
RA Patients Treated with Methotrexate
- PERMANOVA
-
Permutational Multivariate Analysis of Variance
- RA
-
Rheumatoid Arthritis
- SCFAs
-
Short Chain Fatty Acids
Abbreviations
1 Introduction
Rheumatoid arthritis (RA) is a type of chronic autoimmune disease characterized by synovial membrane hypertrophy, inflamed joints resulting in symmetric polyarthritis as well as bone and cartilage destruction. The antirheumatic drug methotrexate induces gut toxicity in a time-dependent manner, and eliminating the gut toxicity associates with gut microbiota (Zhou et al., 2018). The human gut microbiota has been evidenced as an opportunistic trigger or improver for RA (Scher et al., 2013; Zhang et al., 2015). Porphyromonas gingivalis and Prevotella copri have been evidenced to enhance susceptibility and even aggravate arthritis (Scher et al., 2013; Sato et al., 2017). On the other hand, several independent studies have reported that oral administration of Lactobacillus or Bifidobacterium strains, typical beneficial bacteria, can mitigate arthritic inflammation in rodents and clinical studies (Elnaz et al., 2014; Fan et al., 2020). These bacteria protected against arthritis through modulating the gut microbiota, improving intestinal mucosal immunity and the gut intestinal barrier. Importantly, the inducted and protective actions were species-dependent (Marietta et al., 2016; Achi et al., 2019; Fan et al., 2020). Hence, the characterization and alteration of beneficial bacteria at species level in RA patients treated with methotrexate (MTX-RA) warrants further investigation. Although, it has been reported that drug treatment of RA will lead to changes in gut microbiota, and changes in gut microbiota will also affect the occurrence and development of RA (Bodkhe et al., 2019; Ellie et al., 2018; Maeda and Takeda, 2019). However, at present, the research on this interaction is still limited, and we still don’t fully understand how the changes of gut microbiota caused by drug therapy affect the pathogenesis of RA.
Gut microbes affect the immune system by continually producing repertoire of metabolites, such as short chain fatty acids (SCFAs), which are the major end-products from the microbial breakdown and fermentation of dietary fiber. SCFAs being important immune mediators and have shown to regulate arthritic inflammation in mice (Maslowski et al., 2009; Luu et al., 2019). Strong evidence has shown that direct supplementation of pentanoate or butyrate, and mediterranean diet which increases the production of SCFAs, can help reduce inflammation and promote bone remodeling (Sköldstam et al., 2003; McKellar et al., 2007), and yet the interaction between SCFAs and gut microbiota in RA was remained unclear (Zaragoza et al., 2020; Renuka et al., 2019; Marine et al., 2020).
In the current study, Illumina Miseq sequencing was applied to investigate the effects of methotrexate on gut microbiota. Since Bifidobacterium and Lactobacillus are two important components of probiotics, we used molecular tools to evaluate the composition of Bifidobacterium and Lactobacillus at species level in order to further evaluate the changes of probiotics in gut microbiota. Moreover, propionate and butyrate were given to collagen-induced-arthritis (CIA) rats to assess their effects on gut microbiota and SCFAs.
2 Material and methods
2.1 Subject recruitment
Thirty-three RA patients that fulfilled the inclusion criteria of the American College of Rheumatology (ACR) 2010 were recruited in the Traditional Chinese Medicine Hospital of Ili Kazak Autonomous Prefecture in Yi’ning, China. Thirty-two healthy controls (HCs) were also enrolled. Patients with long disease duration (12–48 months) and routinely received methotrexate treatment were included. Any participants having a personal history of other autoimmune illness, or taking antibiotics, probiotics and prebiotics within three months were excluded. All the collected fecal samples were for public health purposes and were the only human materials used in the present study, and fecal sample collection involved no risk of predictable harm or discomfort to the participants. Written informed consent regarding fecal sample use was obtained from all participants. All procedures performed in study involving human participants was approved by the Ethics Committee in Jiangnan University, China (SYXK 2012–0002).
2.2 Animal experimental designed
Six-week-old female Wistar rats (Beijing Vital River Laboratory Animal Ltd, Beijing, China) were kept in SPF conditions. After one week adaptation, rats were randomly divided into control, model, propionate and butyrate groups (n = 8 per group). The rats in propionate or butyrate groups were respectively given 150 mM propionate or butyrate in drinking water (freshen every day), and rats in control and model groups were given sodium-matched water. The CIA induction was according to the previous described method (Brand et al., 2007). Briefly, the rats were subcutaneously injected in the base of tail with 150 μg bovine type II collagen (Chondrex, Redmond, WA, USA) emulsified in incomplete Freund adjuvant (Chondrex, Redmond, WA, USA) at day 14 and 21, respectively. The thickness of hind paws was evaluated for swelling, and each paw was scored according to the previous description (Brand et al., 2007). Arthritis assessments were performed every three days until no more severe swollen throughout the trials. The animal experiment was approved by the Ethics Committee of Jiangnan University (JN. No. 20191115 W0640201 [313]).
2.3 Fecal sample collection, DNA extraction and sequencing
Fecal samples from participants and rats were collected and stored at −80 °C until further analysis. Total DNA was extracted using the FastDNA Spin Kit for Feces (MP Biomedical, Irvine, CA, USA). The V3-V4 region of the 16S rRNA gene, Bifidobacterium and Lactobacillus groEL genes was amplified as previously described (Hu et al., 2017; Yang et al., 2018; Xie et al., 2019). Amplifications were purified and sequenced on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions.
2.4 Bioinformatic analysis
The sequence reads were processed as previously described (Hu et al., 2017). Taxonomic identification of OTUs for Lactobacillus and Bifidobacterium groEL genes was performed as previously described (Hu et al., 2017; Xie et al., 2019). To overcome the random sequencing errors, high sequencing coverage depth threshold for V3-V4, Bifidobacterium_groEL and Lactobacillus_groEL genes were 10000, 4500 and 4500 reads, respectively. The 16S rRNA gene sequencing data of humans and rats were respectively deposited in SRA database: PRJNA646930 and PRJNA647234.
2.5 Fecal SCFAs measurement
The SCFAs in dried fecal samples were extracted, and then measured using gas chromatography (GC-2010 Plus, Shimadzu Corporation, Japan) as previously described (Wang et al., 2017).
2.6 Statistical analyses
Data were presented as means ± SEM. Statistical analyses were performed using GraphPad Prism 8 (Graphpad Software Inc., San Diego, CA). Mann-Whitney test was used to compare the differences between two groups, and a one-way ANOVA with Turkey post-hoc test was performed between four groups. Permutational multivariate analysis of variance (PERMANOVA) using 999 permutations was performed to determine the significance in PCoA using R software (v 3.5.6). Spearman correlated analysis was used to evaluate the relationship between SCFAs and gut microbiota. Statistical significance was defined as *p < 0.05, **p<0.01, ***p<0.001. All data were visualized in R (v 3.5.6).
3 Results
3.1 The diversity and compositions of gut microbiota in MTX-RA patients
Based on the 16S sequencing results, there were significant differences in beta diversity when calculated unweighted UniFrac distance among HCs and MTX-RA patients (Fig. 1A-B). Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria were the main phylum (Fig. 1C). Specifically, the relative abundance of Firmicutes tended to be depleted (p = 0.06) while Actinobacteria was significantly enriched (p = 0.017) within the MTX-RA cohort. Phylum-specific Bray-Curtis distances suggest that the taxonomic structure of Actinobacteria and Proteobacteria in MTX-RA patients differed from that in the HCs group (Fig. 1D). And unclassified Ruminococcaceae, unclassified Enterobacteriaceae, unclassified Lachnospiraceae, Blautia, Bifidobacterium and Streptococcus were the major genera in the two groups (Fig. 1E). Compared with the HCs group, five genera exhibited significant differences and Haemophilus and Megamonas tended to be decreased in MTX-RA patients (p = 0.058 and p = 0.076, respectively) (Fig. 1F).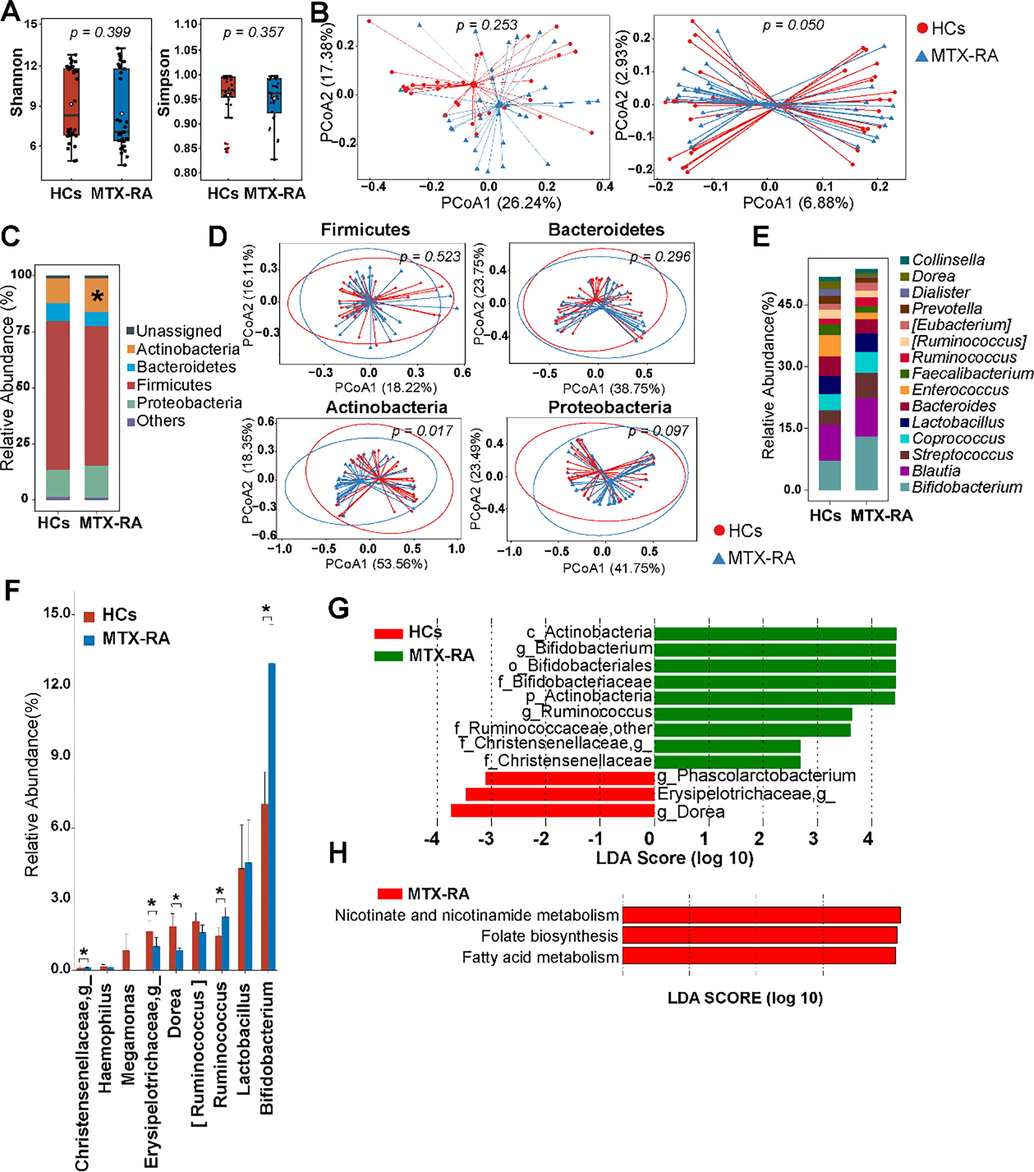
The differences of gut microbiota in MTX-RA and HCs. The alpha-diversity (A) and beta-diversity (B) illustrated by PCoA on weighted (left panel) and unweighted (right panel) UniFrac distances. The average relative abundances of predominant phyla (C) and the top fifteen classified genus (E). (D) PCoA based on the Bray-Curtis distance using OTUs from Firmicutes, Bacteroides, Actinobacteria and Proteobacteria. The selected significantly different genera (F) and differentially abundant taxa (LDA score > 2 and p < 0.05) based on LDA analysis (G). (H) The significantly discriminative predicted metabolic pathways on 16S rRNA gene (LDA score > 2 and p < 0.05). Statistical significance was considered at *p < 0.05.
Logarithm linear discriminant analysis (LDA) was performed and identified a total of 12 feature taxa responsible for microbial differences among individuals from HC and MTX-RA groups (LDA score > 2, p < 0.05) (Fig. 1G). The MTX-RA patients showed significantly increased feature taxa including Bifidobacteriaceae alongside its genus Bifidobacterium, Christensenellaceae alongside its unclassified genus, and Ruminococcaceae alongside its unclassified genus and Ruminococcus, while no difference was observed for genus Lactobacillus. PICRUSTs was performed to infer the functional genes of the gut microbiota. Three KEGG pathways were significantly enriched in MTX-RA patients including nicotinate and nicotinamide metabolism, folate biosynthesis and fatty acid metabolism pathways (Fig. 1H).
3.2 Diversity of bifidobacterial and lactobacilli communities in HCs and MTX-RA patients
After filtering coverage depth, 61 sequenced samples were used to evaluate the bifidobacterial community profiles in individuals. A total of 1,502,045 reads were acquired with an average of 26,429 and 21,876 reads per sample in MTX-RA patients and HCs, respectively. There were no significant differences in alpha and beta diversity (Fig. 2A,B). The bifidobacterial community was dominated by three typical species (B. longum, B. adolescentis and B. pseudocatenulatum) in both groups (Fig. 2C). No bifidobacterial species changed significantly between MTX-RA and HC groups, as well as among gender-specific groups (Fig. 2D).
The bifidobacterial and lactobacilli communities in MTX-RA and HCs. The alpha diversity and beta diversity in bifidobacterial community (A,B) and lactobacilli community (E,F). PCoA were based on weighted (left panel) and unweighted (right panel) UniFrac distances (B and E). The average relative abundances of major Bifidobacterium (C,D) and Lactobacillus (G–I) species in MTX-RA and HCs.
There were 54 qualified sequenced samples after filtering coverage depth at 4500 targeting the Lactobacillus groEL gene. From those samples, 1,020,972 high-quality sequenced reads were identified with an average of 18,702 and 19,144 reads per sample in MTX-RA patients and HCs group, respectively. MTX-RA samples tended to have higher alpha diversity (Fig. 2E). In line with the differences of PCoA on weighted and unweighted UniFrac distance, the major species were L. delbrueckii (20.54%), L. mucosae (17.00%) and L. plantarum (11.92%) in HCs, however, in MTX-RA patients it was dominated by L. mucosae (27.47%), L. salivarius (19.02%) and L. ruminis (12.24%) (Fig. 2F–G). In addition, L. delbrueckii, L. manihotivorans and L. intestinalis were under-represented in the MTX-RA group (Fig. 2H), conversely, L. mucosae and L. salivarius expanded in some MTX-RA patients (Fig. 2I).
3.3 Fecal SCFAs in HCs and MTX-RA patients
Acetate, propionate and butyrate were the major fecal SCFAs in both groups, without significant difference between the two groups (Table 1), though 16S rRNA gene-based predictions indicated that fatty acid metabolism were upregulated in MTX-RA patients (Fig. 1H). However, a significant decline of the propionate/butyrate ratio was observed in the MTX-RA group compared with HCs.
SCFAs (mg/g)
HCs (n = 27)
MTX-RA (n = 24)
P value
Acetate
1.308 ± 0.171 (1.075)
1.266 ± 0.173 (1.236)
0.978
Propionate
1.151 ± 0.176 (0.869)
0.991 ± 0.166 (0.817)
0.688
Butyrate
0.701 ± 0.109 (0.444)
0.756 ± 0.113 (0.626)
0.543
Iso-butyrate
0.191 ± 0.061 (0.085)
0.110 ± 0.009 (0.104)
0.531
Pentanoate
0.150 ± 0.029 (0.101)
0.137 ± 0.035 (0.0904)
0.874
Propionate/Butyrate
2.076 ± 0.291 (1.652)
1.423 ± 0.192 (1.349)
0.033
Spearman correlation analyses were performed to identify correlations between fecal SCFAs and the taxa. The results revealed that the abundances of Adlercreutzia and Turicibacter had significant positive correlations with acetate, propionate and butyrate in MTX-RA patients (Fig. 3A). Furthermore, [Ruminococcus] and Bifidobacterium, both of which promote SCFAs production, were inversely correlated with all the SCFAs. The microbe-SCFAs interaction in male MTX-RA patients was distinct from that in female patients. The interactions of some genera with SCFAs in male patients were reversed in the female group, such as Enterococcus, Coprococcus, Dorea, Megamonas and unclassified Christensenellaceae. In line with the insignificant undiminished total SCFAs, no obvious differences were found in SCFA-producing bacteria Lachnospiraceae, Lactobacillaceae, Ruminococcaceae and Bacterioidaceas (Fig. 3B).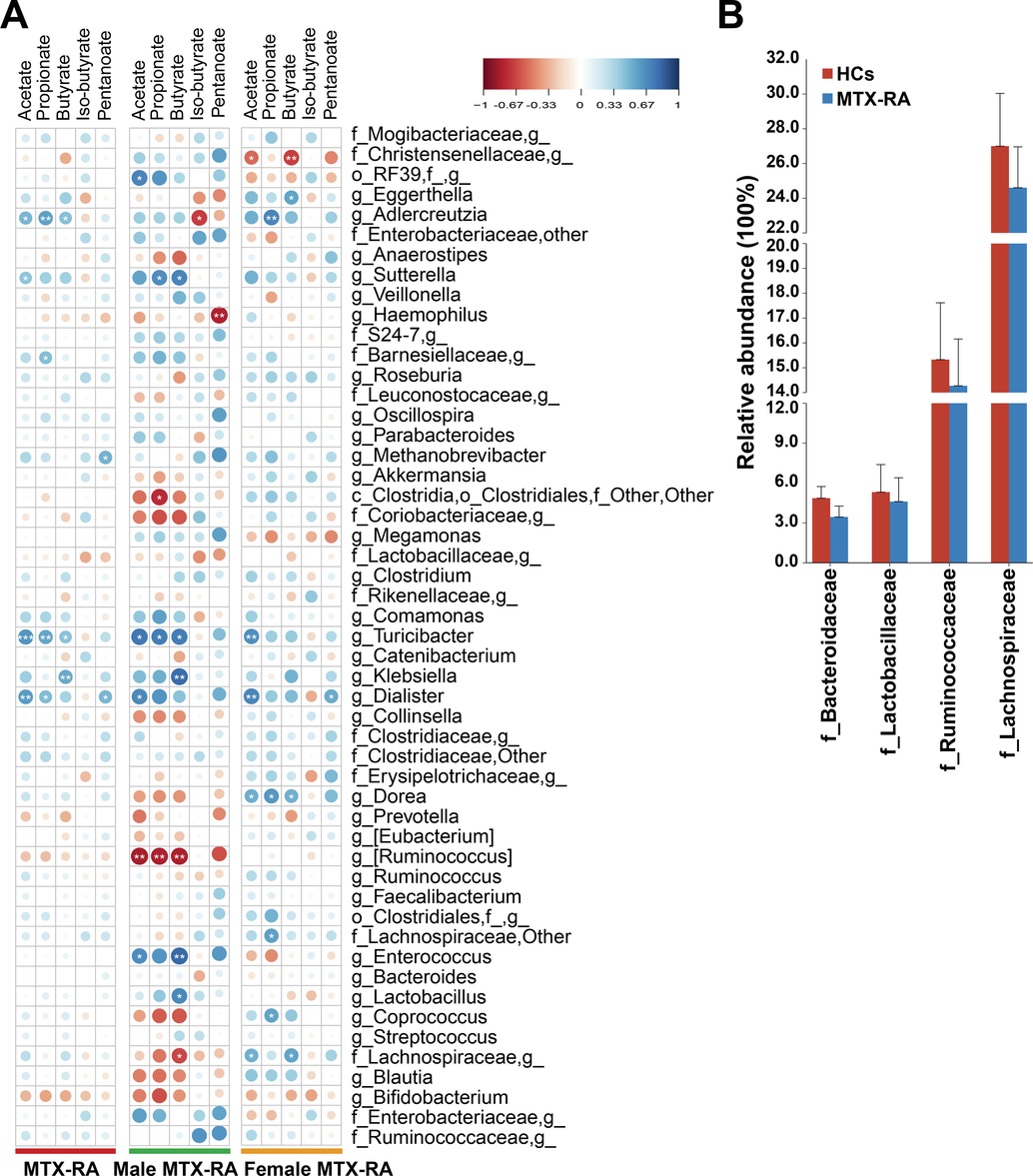
Spearman correlation between SCFAs and gut microbiota. (A) The correlation between the genus (the average abundance > 0.1%) and SCFAs. (B) The abundance of SCFA-producing bacteria at family level. Statistical significance was considered at *p < 0.05, **p < 0.01, ***p < 0.001.
3.4 Propionate protected arthritis and rebalanced fecal SCFAs in CIA rats
To further address the effect of SCFAs on gut microbiota under arthritis, the CIA rats were orally administered with propionate or butyrate. The swollen symptoms in joint and paws appeared at 14 days after the first immunization, and the three most abundant SCFAs (acetate, propionate and butyrate) were lower in CIA rats. Supplementation with butyrate or propionate showed no differences in remitting swelling and slowing inflammatory progress (Fig. 4A). Administrating of propionate upregulated SCFAs concentration of which butyrate reach a significant level, whereas butyrate supplementing didn’t change any types of SCFAs (Fig. 4B). Comparing with CIA rats, the propionate/butyrate ratio was lower in propionate (p = 0.06) and butyrate (p = 0.14) treated rats.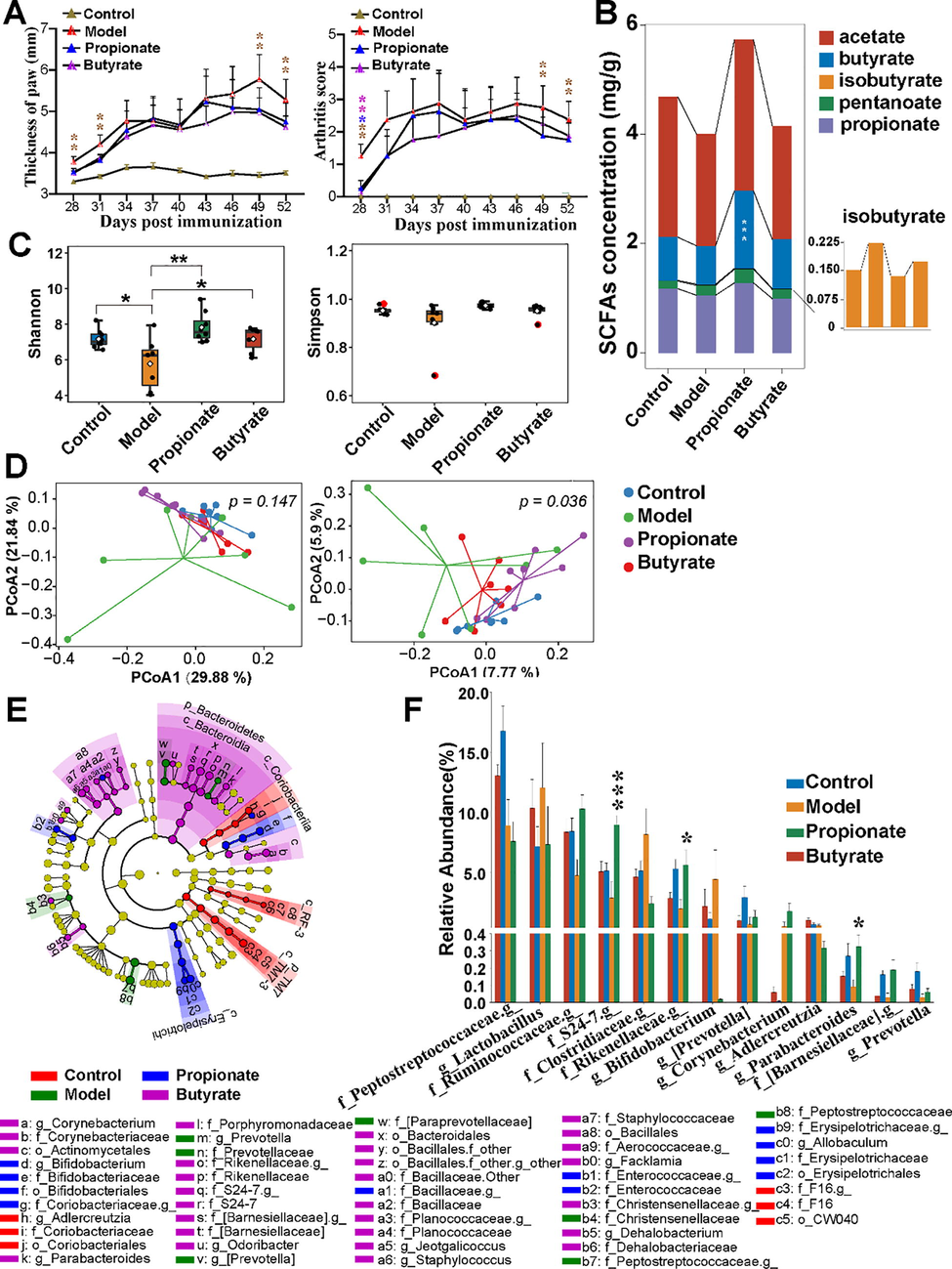
The effects of propionate on CIA rats. Thickness of paw and arthritis score (A) and the distribution of fecal SCFAs (B) in rats. Propionate changed the gut microbiota of CIA rats in alpha diversity (C) and beta diversity (D). PCoA were analysed on weighted (left panel) and unweighted (right panel) UniFrac distances (D). Differentially microbiota taxa were shown as cladogram (E) and bar charts (F). Statistical significance was considered at *p < 0.05, **p < 0.01, ***p < 0.001.
3.5 Propionate modulated gut microbiota in CIA rats
The gut microbiota were analyzed to evaluate the effects of propionate or butyrate on gut microbiota in arthritic rats. Alpha and beta diversity recovered in propionate and butyrate treated rats (Fig. 4C,D). The LEfSe analysis further accounted that Bacteroidetes, containing Prevotella, [Prevotella], Parabacteroides, unclassified [Barnesiellaceae], Rikenellaceae and S24-7, clustered differently, and Bifidobacterium, Allobaculum, unclassified Enterococcaceae and Erysipelotrichacea were enriched in feces of CIA rats compared with other groups (Fig. 4E). Propionate significantly restored the abundance of Bacteroidetes along with genus Parabacteroides and unclassified [Barnesiellaceae], Rikenellaceae and S24-7 (Fig. 4F).
3.6 The modulation of propionate on lactobacilli and bifidobacterial communities in CIA rats
Lactobacillus and Bifidobacterium were enriched in the gut microbiota in CIA rats, therefore, whether propionate or butyrate can regulate the two communities at species level were investigated. The alpha and beta diversity in Lactobacillus community changed slightly (Fig. 5A-B). Comparing with control rats, the abundances of L. taiwanensis (p = 0.03), L. johnsonii (p = 0.56), L. acidophilus (p = 0.22), L. intestinalis (p = 0.24) and L. amylovorus (p = 0.22) were lower in CIA rats (Fig. 5C). Administration of propionate could upregulate the last three species’s abundance, however, butyrate treatment inversely exacerbated the depletion. The decreased abundance of L. taiwanensis and L. johnsonii evoked by arthritis can not be recovered after neither propionate nor butyrate treatment. Simpson index of bifidobacterial community was significantly increased in propionate-treated rats (Fig. 5D). In addition, B. pseudolongum was the dominant bifidobacterial species which accounted for over 99% of bifidobacterial community in all rats (Fig. 5E).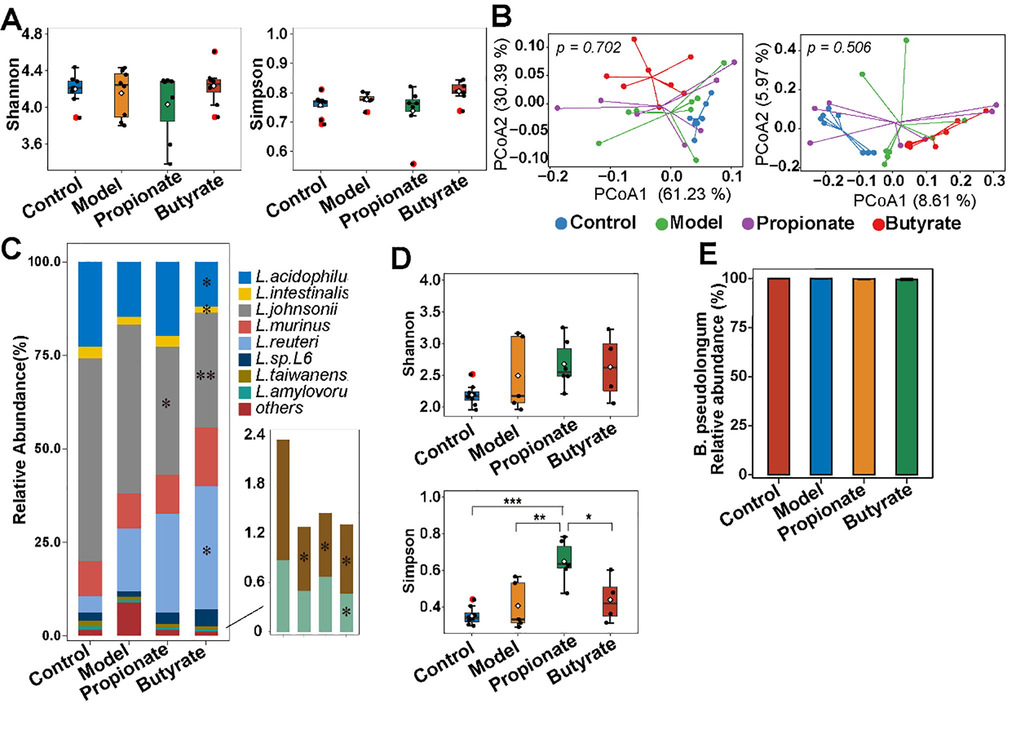
The effects of propionate on CIA rats on lactobacilli and bifidobacterial community. The indexes of alpha and beta diversity of lactobacilli (A,B) and bifidobacterial community (D) in rats. PCoA based on weighted (left panel) and unweighted (right panel) UniFrac distances (B). The relative abundances of Lactobacillus (C) and Bifidobacterium (E) species in rats. Statistical significance was considered at *p < 0.05, **p < 0.01, ***p < 0.001.
4 Discussion
It is generally accepted that RA is an autoimmune disorder and the first-line drug methotrexate is limited due to its toxicity on multi-organ including gut, cardioid, bone marrow, kidney and liver. Methotrexate impairs the intestinal epithelial cells and associated mucosal tissues, and even exacerbates to mucositis. Induction and amelioration of gut toxicity are partly related to gut microbiota, nevertheless, the effects of methotrexate on beneficial bacterial community in RA patients remain unclear. Our current study provided new evidence on the gut toxicity of methotrexate concerning the gut microbiota in RA patients, with particular interest in lactobacilli and bifidobacterial communities at species level.
Gut microbiota alpha diversity indexes were rather similar among HC individuals and MTX-RA patients, which was compatible with previous reports (Chen et al., 2016; Picchianti-Diamanti et al., 2018). Bacterial diversity significantly associated with disease duration and drug treatments, so, a consistent conclusion has not been reached. Furthermore, the significant difference of beta diversity emerged only on the unweighted UniFrac distance, revealing that low abundant taxa may be responsible for the result such as Christensenellaceae. Furthermore, the significant increase of predictive folate biosynthesis in MTX-RA might be a complementary action of gut microbiota due to methotrexate regarding as an anti-folate agent during RA clinical treatment, which means gut microbiota might cooperatively evolve via host epithelial selection to maintain the homeostasis and health.
The most abundant phyla Firmicutes and Bacteroidetes implicate in metabolic and immune activities. The relative abundance of Firmicutes decreased in the MTX-RA patients in this study, characterized by decreases of Dorea, [Ruminococcus] and unclassified Erysipelotrichaceae. The positive correlation between Ruminococcus and Dorea and serological markers in RA has been reported (Sun et al., 2019), and Ruminococcus is a genus associated with SCFAs production and mucin degradation, as found in Sun’s study, it positively correlated with acetate, propionate and butyrate (Sun et al., 2019). Additionally, the reported increases of Prevotella and Lactobacillus in new-onset RA patients has not been observed in MTX-RA patients in this study.
Lactobacillus and Bifidobacterium are members of the beneficial bacterial community and cross-talk with the host through adhering to epithelial cells, colonization in gastrointestinal mucosa, defensing pathogens and regulating immune. However, it’s a fact that various species of a same genus may exert divergent effects on arthritis. A typical example was Prevotella, in which Prevotella copri was correlated with increased arthritis susceptibility, whilst Prevotella histicola could suppress arthritis (Scher et al., 2013; Marietta et al., 2016; Pianta et al., 2017). So, identification of the distribution of the beneficial bacteria is an essential procedure to understand the intricate interaction in gut. Previously, the lactobacilli community structure in RA patients was analyzed through DGGE (Liu et al., 2013). In our study, high-throughput-sequencing offset the deficiency of DGGE on low detection and poor data reproducibility. The increased alpha diversity of lactobacilli community in MTX-RA group is consistent with previous reports (Liu et al., 2013; Zhang et al., 2015). Several Lactobacillus species, namely L. mucosae and L. salivarius, expanded in MTX-RA patients. L. salivarius is widely distributed in the oral cavity, vagina and gut. Administration of L. salivarius mitigated arthritis and modulated immune cells differentiation, while it strongly correlated with active disease in RA patients (Sheil et al., 2004; Liu et al., 2013; Zhang et al., 2015; Liu et al., 2016; Picchianti-Diamanti et al., 2018). In addition, L. salivarius overrepresents in the oral cavities of individuals with caries or periodontitis, and periodontal disease linked with an increased risk of RA (Farquharson et al., 2012; Hojo et al., 2014; Caufield et al., 2015). However, limited sample size and the lack of serological indicators in RA patients restricted the construction of linkages between gut microbiota, gut metabolites and disease activity. In the CIA model, propionate supplementation rebalanced Lactobacillus species such as L. acidophilus, L. intestinalis and L. amylovorus while butyrate seemed to have no effects. One possibility is that microbiota-derived butyrate regulate gut microbiota different from other SCFAs based on the fact that butyrate mainly serves as substrate for enterocytes and little was released into portal circulation (Henrike et al., 2008).
Intriguingly, Bifidobacterium was enriched in MTX-RA patients, which also found in patients with juvenile Spondyloarthritis or ankylosing spondylitis in previous studies (Stebbings et al., 2003; Stoll et al., 2014). To date there is no reasonable explanation for the paradoxical expansion. B. catenulatum (an adult Bifidobacterium species) and B. angulatum (an elderly Bifidobacterium species) expanded in some MTX-RA patients, rather than edible species such as B. breve, B. bifidum and B. longum (Fig. 5F). B. breve NCIM5671, B. longum NCIM5672 and B. bifidum NCIM5697 have been shown to downregulate inflammatory markers to protect against arthritis in rats (Achi et al., 2019), while B. catenulatum and B. angulatum were associated with production of proinflammatory cytokines such as TNFα and IFN-γ in vitro (Pozo-Rubio et al., 2011). Herein, the expansion of undesired Bifidobacterium species may account for the expanded of Bifidobacterium together with missing ‘probiotic’ effects in RA patients.
SCFAs positive modulate arthritis in RA patients or CIA mice (Maslowski et al., 2009; Kim et al., 2018; Lucas et al., 2018; Luu et al., 2019; Takahashi et al., 2020). Our results show that the distributions of SCFAs in MTX-RA patients were similar to that of healthy individuals, which likely to be explained by the insignificant changes of the SCFA-producing bacteria. Our results revealed that the concentration of fecal butyrate, rather than propionate, increased in propionate treatment rats, and butyrate treatment did not influence on acetate, propionate and butyrate in CIA rats. Directly propionate or butyrate supplementation does not always mean increase of correspondent SCFA in feces. Most likely, this is due to fecal SCFAs account for only 5% of total SCFAs and the rest of them present in circulation, and butyrate being quickly utilized as energy source for colonocytes. Additionally, the family S24-7, a typical butyrate-producing inhabitant, and other producers Bacteroidaceae and Ruminococcaceae were markedly increased in propionate-treated rats. In addition, we also found that Bifidobacterium decreased significantly in propionate-treated CIA rats, which may be due to the increase in the proportion of other species. From another point of view, the significant increase in gut microbiota diversity in propionate-treated CIA rats also confirmed this view from the side.
5 Conclusion
This study delves into exploring the effects of methotrexate on bifidobacterial community in RA patients. Our findings support the conclusion that the bifidobacterial community was more tolerance to methotrexate therapy than that of lactobacilli community in RA patients. Exogenous supplementation of propionate, not butyrate, could restore gut microbiota especially lactobacilli community and increase butyrate concentration in CIA rats. These results provide new insights that exogenous propionate can normalize gut microbiota when methotrexate be adopted in RA patients.
Acknowledgements
We gratefully acknowledge the assistance and guidance of Doctor Jianchun Su from Traditional Chinese Medicine Hospital of Ili Kazak Autonomous Prefecture.
Disclosure of funding
This work was supported by the National Natural Science Foundation of China (Nos. 32021005, 31820103010, 31771953), National First-Class Discipline Program of Food Science and Technology (JUFSTR20180102), the Fundamental Research Funds for the Central Universities (JUSRP52003B), 111 Project (BP0719028) and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Achi SC, Talahalli RR, Halami PM. Prophylactic effects of probiotic Bifidobacterium spp. in the resolution of inflammation in arthritic rats. Appl. Microbiol. Biotechnol., 103 (15) (2019), pp. 6287-6296. 10.1007/s00253-019-09864-2.
- Bodkhe R, Balakrishnan B, Taneja V. The role of microbiome in rheumatoid arthritis treatment. Ther Adv Musculoskelet Dis., 11:1759720X19844632, (2019), pp. 1-16, 10.1177/1759720X19844632.
- Caufield PW, Schön CN, Saraithong P. et al. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res., 94 (9 Suppl) (2015), pp. 110S-8S. 10.1177/0022034515576052.
- An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med.. 2016;8(1):43.
- [CrossRef] [Google Scholar]
- Drug-microbiota interactions and treatment response: Relevance to rheumatoid arthritis. AIMS Microbiol.. 2018;4(4):642-654.
- [CrossRef] [Google Scholar]
- Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30(4):430-435.
- [CrossRef] [Google Scholar]
- The prophylactic effects of different Lactobacilli on collagen-induced arthritis in rats. Food Funct.. 2020;11(4):3681-3694.
- [CrossRef] [Google Scholar]
- Periodontitis, Porphyromonas, and the pathogenesis of rheumatoid arthritis. Mucosal Immunol.. 2012;5(2):112-120.
- [CrossRef] [Google Scholar]
- Henrike M. H., Jonkers D, Venema K. et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol. Ther., 27 (2) (2008), pp.104-119. 10.1111/j.1365-2036.2007.03562.x.
- Distribution of salivary lactobacillus and bifidobacterium species in periodontal health and disease. Biosci. Biotechnol. Biochem.. 2014;71(1):152-157.
- [CrossRef] [Google Scholar]
- Assessment of bifidobacterium species using groEL Gene on the Basis of Illumina MiSeq High-Throughput Sequencing. Genes (Basel). 2017;8(11):336.
- [CrossRef] [Google Scholar]
- Attenuation of rheumatoid inflammation by sodium butyrate through reciprocal targeting of HDAC2 in osteoclasts and HDAC8 in T cells. Front. Immunol.. 2018;9:1525.
- [CrossRef] [Google Scholar]
- Lactobacillus salivarius Isolated from patients with rheumatoid arthritis suppresses collagen-induced arthritis and increases treg frequency in mice. J. Interferon Cytokine Res.. 2016;36(12):706-712.
- [CrossRef] [Google Scholar]
- Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr. Microbiol.. 2013;67(2):170-176.
- [CrossRef] [Google Scholar]
- Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun.. 2018;9(1):55.
- [CrossRef] [Google Scholar]
- The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun.. 2019;10(1):760.
- [CrossRef] [Google Scholar]
- Host–microbiota interactions in rheumatoid arthritis. Exp. Mol. Med.. 2019;51:1-6.
- [CrossRef] [Google Scholar]
- Suppression of inflammatory arthritis by human gut-derived prevotella histicola in humanized mice. Arthritis Rheumatol.. 2016;68(12):2878-2888.
- [CrossRef] [Google Scholar]
- Marine PM, Nyasha M, Kate W et al. A Two-Way Interaction between Methotrexate and the Gut Microbiota of Male Sprague–Dawley Rats. J Proteme Res., 19 (8), (2020), pp. 3326-3339. acs.jproteome.0c00230.
- Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282-1286.
- [CrossRef] [Google Scholar]
- A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis.. 2007;66(9):1239-1243.
- [CrossRef] [Google Scholar]
- Evidence of the immune relevance of Prevotella copri, a Gut Microbe. Patients With Rheumatoid Arthritis. Arthritis Rheumatol.. 2017;69(5):964-975.
- [CrossRef] [Google Scholar]
- Analysis of gut microbiota in rheumatoid arthritis patients: disease-related dysbiosis and modifications induced by etanercept. Int. J. Mol. Sci.. 2018;19(10):2938.
- [CrossRef] [Google Scholar]
- Immunostimulatory effect of faecal Bifidobacterium species of breast-fed and formula-fed infants in a peripheral blood mononuclear cell/Caco-2 co-culture system. Br. J. Nutr.. 2011;106(8):1216-1223.
- [CrossRef] [Google Scholar]
- Perturbation of the human gut microbiome by a non-antibiotic drug contributes to the resolution of autoimmune disease. bioRxiv.. 2019;4(5):1-16.
- [CrossRef] [Google Scholar]
- Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep.. 2017;7(1):6955.
- [CrossRef] [Google Scholar]
- Scher JU, Sczesnak A, Longman RS et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife, 2 (2013), pp. e01202. 10.7554/eLife.01202.
- Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53(5):694-700.
- [CrossRef] [Google Scholar]
- An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis.. 2003;62(3):208-214.
- [CrossRef] [Google Scholar]
- Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology. 2003;41(12):1395-1401.
- [CrossRef] [Google Scholar]
- Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthrit. Res. Ther.. 2014;16(6):486.
- [CrossRef] [Google Scholar]
- Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front. Cell. Infect. Mi.. 2019;9:369.
- [CrossRef] [Google Scholar]
- Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine. 2020;58:102913
- [CrossRef] [Google Scholar]
- Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct.. 2017;8(10):3587-3600.
- [CrossRef] [Google Scholar]
- groEL gene-based phylogenetic analysis of lactobacillus species by high-throughput sequencing. Genes (Basel). 2019;10(7):530.
- [CrossRef] [Google Scholar]
- Bifidobacterium breve CCFM683 could ameliorate DSS-induced colitis in mice primarily via conjugated linoleic acid production and gut microbiota modulation. J. Funct. Foods. 2018;49:61-72.
- [CrossRef] [Google Scholar]
- DMARDs–Gut Microbiota Feedback: Implications in the Response to Therapy. Biomolecules.. 2020;10(11):1479-1483.
- [CrossRef] [Google Scholar]
- The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med.. 2015;21(8):895-905.
- [CrossRef] [Google Scholar]
- Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBio Med.. 2018;33:122-133.
- [CrossRef] [Google Scholar]







