Promise of latanoprost and timolol loaded combinatorial nanosheet for therapeutic applications in glaucoma
⁎Corresponding author at: Department of Ophthalmology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, XianXia Road, Shanghai 200336, China. AmiePittsvjv@yahoo.com (Li Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
-
Nanosheet for glaucomawas found to be non-irritable to the cornea of eye.
-
LATINA maybe the best option for drug delivery to the cornea.
-
LATINA may also relieve a subject from administration of ophthalmic formulation.
Abstract
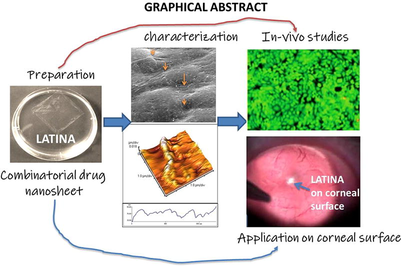
To demonstrate a combinatorial drug loaded nanosheet made of alginate and chitosan which reduces intraocular pressure in a slow and sustained manner depicted by in-vivo studies. The combinatorial drugs loaded were latanoprost along with timolol and nanosheet was termed LATINA. LATINA was fabricated using spin coating procedure and characterized using scanning electron microscopy, atomic force microscopy, adhesive peel strength, burst and cytotoxicity tests. In-vitro studies of drugs were also performed. It was clearly seen that LATINA was stable, flexible, durable, biocompatible and biodegradable drug delivery system. The main advantage provided by the nanosheet technology is the properties which could be controlled by altering the thickness of the sheet. The slow and sustained drug release from LATINA ensured that the nanosheet may be renewed once weekly instead of daily as for other ophthalmic formulations for glaucoma. This also led to reduction of intra ocular pressure (IOP) of eye. LATINA was also found to be non-irritable to the cornea of eye.
Hence, it may be safely concluded that with additional research, LATINA may prove to be the best option for drug delivery to the cornea.
Keywords
Glaucoma
Nanosheet
Latanoprost
Timolol
Cornea
Ocular
1 Introduction
Clinical biotherapeutics and diagnostics have attained new standards with the advancements in bioengineering. Nanobioengineering generates tailor-made novel sensors and drug delivery systems which cater to individual maladies (Weng et al., 2017). The novel efforts are enormous in cases of development of smart nanobiomaterials which may minimize the risk of side effects like development of new infections (Atabay et al., 2017). A class of these smart nanobiomaterials are nanosheets which are ultra-thin sheets of polymer which have remarkable nanothickness and unique properties which may be controlled in a manner that may be thickness dependent (Forrest and Dalnoki-Veress, 2001; Okamura et al., 2015; Ricotti et al., 2010). Nanosheets are also highly flexible and biodegradable, hence with any anti-glaucomic drug may prove to be an ideal drug delivery system for reduction of glaucoma (Fujie et al., 2007; Okamura et al., 2015; Kim et al., 2014). Glaucoma is an ophthalmologic condition which may impair vision irreversibly (Nair et al., 2016; Natarajan et al., 2014) and requires extreme lifelong care for the person. Until now, reduction in intraocular pressure (IOP) is only the demonstrated remedy. It has been also seen that patients with glaucoma use anti-glaucomic solutions throughout their lifetime to keep the condition in check (Centinel and Montemagno, 2016). But the flipside of this therapy is poor adherence of these anti-glaucomic solutions with decrease in adherence on increased use (Sleath et al., 2015). This topical administration may also be associated with decreased continuity (Vanelli et al., 2009; Stone et al., 2009; Bachu et al., 2018). Latanoprost is an analogue of prostaglandin F2α (PGF2α) where the last three carbon atoms on the omega chain have been replaced by a phenyl group (Alm et al., 1995; Hotehama et al., 1993; Stjernschantz, 1992; Alm et al., 1993). Latanoprost produces ocular hypotensive effect and thereby has been seen to cause IOP reduction during glaucoma (Wong et al., 2014). Timolol, on the other hand is a beta blocker which is also effective in reduction of IOP (Camras, 1996; Dubey et al., 2015). However, it has been clearly demonstrated by several studies that latanoprost is the best which lowers the IOP, is very well tolerated systemically and has a very favourable adverse-effect profile (Wentz et al., 2014; Natarajan et al., 2012). We hypothesized that loading timolol and latanoprost on the nanosheet would cause the IOP to decrease further, and would be effective for a period of time without having to renew drugs daily. Moreover, it was also hypothesized the synergistic effect of latanoprost and timolol delivered via a biodegradable nanosheet would probably be a best solution to glaucoma therapeutics. The nanosheets loaded with the drugs were prepared, characterized with the cytotoxic effects being noted and finally their performance evaluated via in-vivo studies.
2 Materials and methods
2.1 Materials
Chitosan (molecular weight 88,000) along with sodium alginate (106,000) were acquired from Mokpo (Korea). Polyvinyl alcohol (PVA) was obtained from Sigma, China. The polymers were utilized as purchased. All the other chemicals were purchased from Sigma, China.
2.2 Fabrication of the nanosheet
The latanoprost-timolol nanosheet (LATINA) fabrication was carried out in a clean room of class 10,000 to evade contagions of any kind. By utilizing deionized water (DI), chitosan (1 mg/mL, 1% [vol/vol] acetic acid) and sodium alginate (1 mg/mL) mixtures were made as per the previous reports (Cho et al., 2001; Jiang et al., 2004).
2.3 Atomic force microscopy of the LATINA
The morphology of the LATINA made was also characterized using atomic force microscopy (AFM). The thickness of the LATINA was also calculated by atomic force microscopy. The AFM measurements were executed in air by means of the tapping mode. A silicon probe was utilized with an oscillating frequency of 200 kHz for the tapping mode. 3–10 μm2area was scanned through 48 N/m as the force constant and the tip height was 12 μm. Ip_2.1 software was utilized to analyze the images. The procedure of AFM measurements was adapted from Das et al. (2011) with proper modifications.
2.4 Adhesive strength of the LATINA
A micro-scratch test was done to estimate the macroscopic adhesive properties of nano-thin LATINA. The procedure was adapted with modifications from Komachi et al. (2017).
2.5 Burst test of the LATINA
The pressure to burst the LATINA with three to ten layers was assessed by means of a bursting test procedure established by (Fujieet al., 2009; Okamura et al., 2009; Ariga et al., 2016).
2.6 in-vitro release studies of LATINA
The release of latanoprost/timolol, latanoprost and timolol from LATINA was studied in vitro. The procedure was adapted with modifications from Kashiwagi et al. (2013).
2.7 Cytotoxicity of the LATINA
2.7.1 Cell culture
Human ocular epithelial cell line was obtained from Beijing, China. For the cell culture, prearranged conditions of 37° with 5% CO2 in a humid environment were created (Gong et al., 2017; Dou et al., 2017).
2.7.2 Cytotoxicity test
Cytotoxicity of control and LATINAs 1, 2, 3 were evaluated according to Dou et al. (2017) with applicable changes. Following the procedure mentioned above, confluent cell cultures were obtained.
2.7.3 Animals
The procedure followed was modified from Kashiwagi et al. (2013).
2.7.4 IOP reduction by LATINA
IOP was measured immediately before applying control, LATINAs 1, 2, 3, latanoprost and timolol to the surface of the cornea. Subsequently, on 1, 2, 4, 7, and 9 days after application of LATINA under general anaesthesia as mentioned, IOP was measured with a rebound tonometer (TonoLab, Finland).
2.7.4.1 Estimation of eye scratching activity after application of LATINA
For this study, only 4 adult Sprague-Dawley rats were used. The designing of the experiment was subjective to the study by Kashiwagi et al. (2013).
2.8 Statistical analysis
Means ± SD is the standard form of expression of all data. Statistical study was made with one-way ANOVA for various assessments. Student’s t-test was done for evaluation among two groups. P < 0.5 was deliberated to be statistically significant.
3 Results and discussion
3.1 Fabrication of the LATINA
The main advantage of fabrication of the LATINA is its biocompatibility and biodegradability which makes it an attractive option for glaucoma therapeutics. Since LATINA is prepared of biocompatible polysaccharides, alginate and chitosan, these are degraded within the eye without the trouble of removing it. Chitosan is a cationic polymer whereas alginate is an anionic polymer. This multilayered LATINA composed of anionic and cationic polymers may embed the drug(s) without the need for any modifications in chemistry or any chemical reagents. Moreover, another important benefit lies in their adhesive which may be controlled with the thickness of the nanosheet. This is to note that although nanosheets may be handled as a single nanofilm, they are actually not so. There are multiple layers within the interior spaces of the nanosheet. In our study, we had prepared a three layered LATINA along with some ten layered LATINA in order to compare the adhesiveness. The process of creating a multilayered nanosheet was difficult. We focussed on creating an alginate/chitosan multilayer over the SiO2 substrate. However, the chitosan and alginate were repelled from the hydrophobic SiO2 substrate. Hence, at the same time 10% PVA was being added drop by drop which improved the spin coating procedure. Therefore, the small amount of PVA is an important constituent in the formation of multilayered nanosheet. Another delicate mechanism is the removal of the LATINA from the substrate. There was a vulnerability that the multilayers of the LATINA may separate while removal from substrate. However, the film was easily picked by tweezers, handled in air and scooped in a petridish. This may probably due to the interaction between the layers which renders them inseparable. LATINA 2 and 3 were prepared in order to check the efficiency of single-drug loaded LATINA as compared to combinatorial drug loaded LATINA in IOP reduction.
The image of LATINA is shown in Fig. 1.
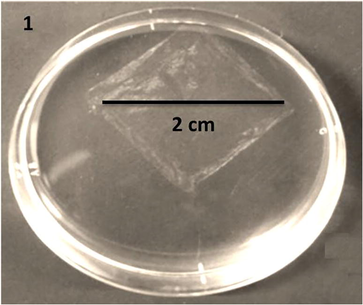
- LATINA nanosheet.
3.2 Scanning electron microscopy of LATINA
The SEM images of LATINA are depicted in Fig. 2. The images clearly show the multilayered structure of the nanosheet. Especially the control with alginate/chitosan shows an irregular and rough surface whereas the ones loaded with drug(s) are relatively smooth. LATINA 1 (10 layered) shows a thick layer of deposited drug whereas LATINA 1, 2 and 3 also show deposition of drug in the crevices of nanosheet. Although the thickness of the LATINA may improve its performance during IOP reduction, the extent of the adhesiveness remains to be seen in adhesive strength studies.
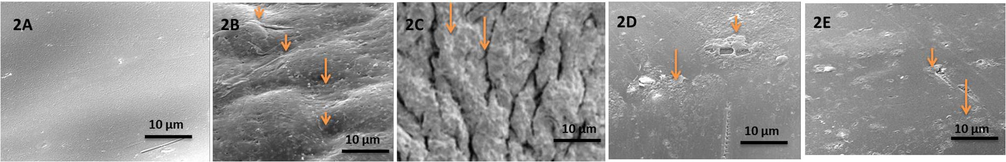
- Scanning electron Micrographs 2A. Alginate/Chitosan nanosheet (Control); 2B. LATINA 1 (3 Layered); 2C. LATINA 1(10 Layered); 2D. LATINA 2; 2E. LATINA 3. Note the multilayered coating of drugs in case of latanoprost/timolol.
3.3 Atomic force microscopy of LATINA
The atomic force microscopy images are depicted in Fig. 3. The multilayered nanosheet is clearly visible. The thickness of the nanosheet is also determined; the values are given in table 1. The values are given as mean ± SD. The thickness is determined from the height profile of the image.
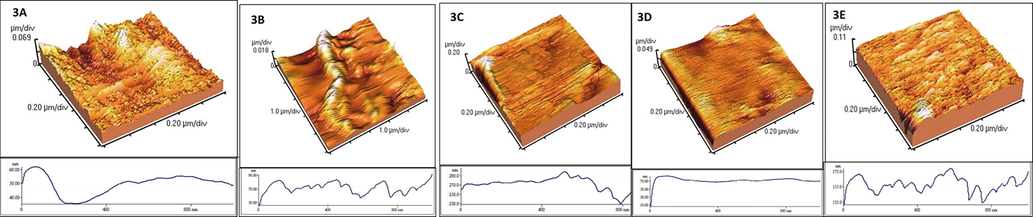
- Atomic Force microscopy Images 2A. Alginate/Chitosan nanosheet (Control); 2B. LATINA 1 (3 Layered); 2C. LATINA 1(10 Layered); 2D. LATINA 2; 2E. LATINA 3. The thickness was calculated from the height profile.
| Sl. No. | Type of Nanosheet | Mean thickness in nm |
|---|---|---|
| 1. | Control (chitosan/alginate) | 50 ± 10 |
| 2. | LATINA 1 (3 layered) | 70 ± 10 |
| 3. | LATINA 1 (10 layered) | 260 ± 30 |
| 4. | LATINA 2 | 50 ± 20 |
| 5. | LATINA 3 | 55 ± 10 |
As seen and hypothesized, the control (figure a) had the least thickness (40 nm) and was seen to be filled with pits and crevices, although layered structure. The 10 layered LATINA 1 was the thickest as expected at 260 nm. These images also show that because of the drug coating, the pits and crevices seen in the control have become smooth (figures b, d, e). Figure c also show that the surface of 10 layered LATINA 1 was still rough despite drug coating because of probably being multilayered. The structure was seen to be amazingly flexible one while handling with tweezers. This would prove to be beneficial while transferring to cornea. Along with the flexibility, from the AFM images, a micropatterning of the nanosheets was visible too (figure a). The micropatterned nanosheet may facilitate efficient drug loading and delivery in the narrow ocular space. The pits and crevices seen in the surface of the control nanosheet may have significant potential to effectively encapsulate the drug(s).
3.4 Adhesive strength of LATINA
The adhesive strength studies are depicted in Fig. 4. This shows an interesting trend. The adhesive strength was determined using a micro-scratch tester and calculated depending upon the critical load just after detachment of the LATINAs from the SiO2 substrate. It was seen that the adhesive strength was of 3 layered LATINA 1 (thickness 70 nm) was determined to be (1.22 ± 0.12) × 105 N/m whereas that of LATINA 2 (thickness 50 nm) was determined to be (1.12 ± 0.10) × 105 N/m. but surprisingly with increase in thickness of the LATINA, the adhesive strength decreased significantly. 10 layered LATINA 1 (thickness 260 nm) demonstrated adhesive strength of (0.59 ± 0.10) × 105 N/m whereas LATINA 3 (thickness 55 nm) confirmed an adhesive strength of (1.03 ± 0.10) × 105 N/m. This may be due to the fact that multilayers of the nanosheet were not detached in a step by step manner but removed together in one single step.
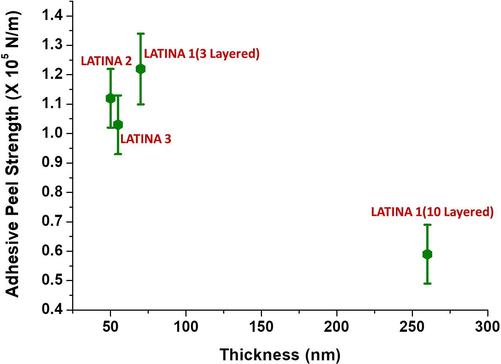
- Adhesive Peel strength of the LATINAs.
3.5 Burst studies of LATINA
The burst test studies results were shown in Fig. 5. As expected the burst pressure increased with increase in thickness of nanosheet. For bursting the control (thickness 40 nm), burst pressure required was 1.8 ± 0.10 kPa. For 3 layered LATINA 1 (thickness 70 nm), burst pressure required was 5.5 ± 0.10 kPa, 10 layered LATINA 1 (thickness 260 nm) required a burst pressure of 19.1 ± 5.10 kPa, LATINA 2 (thickness 50 nm) required burst pressure of 2.1 ± 0.12 kPa and LATINA 3 (thickness 55 nm) required a burst pressure of 2.3 ± 1.12 kPa.
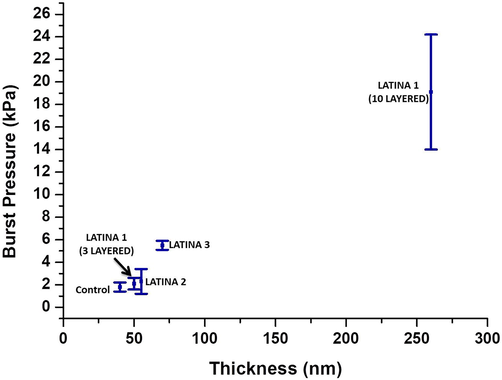
- Burst Pressure of the LATINAs and control.
3.6 In-vitro release studies
One 2.5 µg/cm2 LATINA 1(3 layered) placed in saline released only 25% of latanoprost and timolol over a period of 48 hrs whereas from LATINA 2, 82% latanoprost was released in the first 48 h. Timolol showed an even greater release of 95% from LATINA 3 in first 48 h. This is depicted in Fig. 6. It was seen that the latanoprost/timolol released from the LATINA 1(10 layered) was even slower (12%). If the LATINA 1, 2 and 3 were faced upwards instead of towards the corneal surface, the release was slower but insignificant to be of any clinically relevant use.
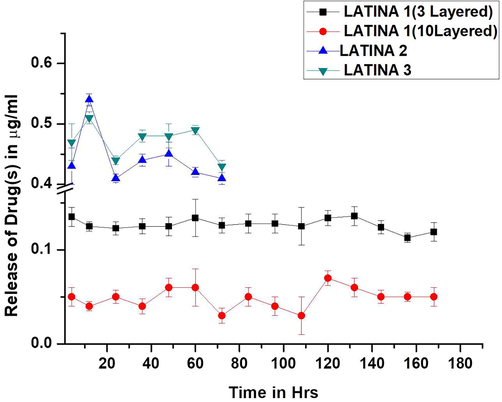
-
In vitro releases of drug(s) from the LATINA.
3.7 Cytotoxicity studies of LATINA
The cytotoxicity results of LATINA are demonstrated in Fig. 7. Since chitosan and alginate are both biocompatible polysaccharides, the nanosheets were expected to be biocompatible. Moreover, latanoprost and timolol are being regularly used for ophthalmic formulations for treatment of glaucoma for quite a long time. It has been noted in quite a few studies that no serious ocular side effects have occurred in any patient after the application of latanoprost or timolol (Camras, 1996). Hence, there was almost no chances of LATINA being non-biocompatible/cytotoxic which was reinforced by the live/dead cell assay too (Fig. 8).
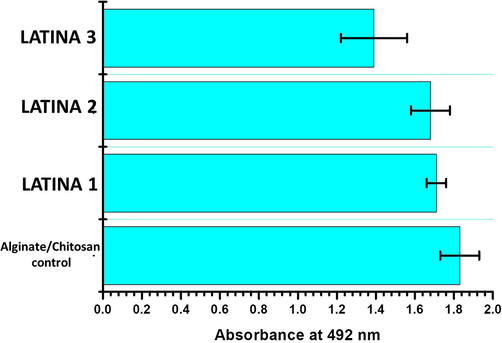
- Cytotoxicity of the control and LATINAs. Note the significance absence of difference in cell viability.
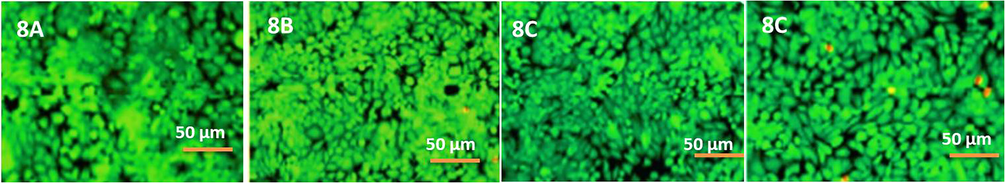
- Cytotoxicity of the LATINAs when treated with human ocular epithelial cell line. The green cells indicate live cells, whereas the red ones indicate dead cells.
3.8 In-vivo studies
Latanoprost (Perry et al., 2003) and timolol as individual ophthalmic formulations have to apply daily to reduce IOP during glaucoma. It was also seen that timolol may have to be applied twice daily sometimes in more acute conditions. Therefore the aim of our study was to create a potential drug delivery system which would deliver the drug(s) without having to be replaced or renewed daily. This should ideally be able to deliver a combination of drugs to the cornea without causing any adverse ocular events. Moreover the drug delivery system should be ideally biodegradable. Therefore we had prepared the LATINA which was proven to be biocompatible, strong and flexible with a sustained release of the combination of drugs.
The insertion of LATINA in the eye of the rats was seen in Fig. 9 through slit lamp microscopy images. The LATINA was smoothly inserted in the eye with blunt tweezers under a surgical microscope and afterwards with a few blinks, it was not even visible. The LATINA is a highly transparent structure and it spread smoothly and adhered tightly to the corneal surface. Initially after administration, few wrinkles were seen in the LATINA which immediately disappeared after rapid blinking.
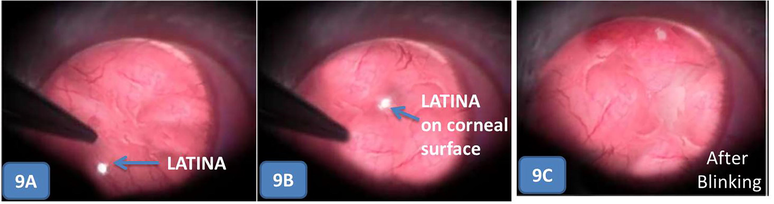
- Incorporation of LATINA on the corneal surface. Note that in 9C the LATINA is almost invisible after blinking.
3.8.1 IOP reduction profile
The IOP reduction profile by the LATINA is tabulated in Table 2. IOP reduction by LATINA was seen after 1, 2, 4, 7 and 9 days after application and the initial reading 10 days before application was considered as the baseline.
| Baseline IOP (mm of Hg) | Day 1 IOP (mm of Hg) |
Day 2 IOP (mm of Hg) |
Day 4 IOP (mm of Hg) |
Day 7 IOP (mm of Hg) |
Day 9 IOP (mm of Hg) |
|
|---|---|---|---|---|---|---|
| Control | 23.3 ± 1.1 | 23.2 ± 1.1 | 23.0 ± 1.1 | 23.3 ± 1.0 | 23.1 ± 1.2 | 23.3 ± 1.0 |
| LATINA 1 (3 layered) | 23.1 ± 1.4 | 21.1 ± 2.1 | 19.4 ± 1.3 | 12.6 ± 1.2 | 12.3 ± 1.1 | 12.2 ± 1.2 |
| LATINA 1 (10 layered) | 23.2 ± 1.1 | 21.4 ± 1.1 | 21.5 ± 1.1 | 22.1 ± 1.1 | 22.4 ± 1.1 | 23.4 ± 1.1 |
| LATINA 2 | 23.3 ± 1.4 | 17.8 ± 1.0 | 17.2 ± 1.0 | 17.8 ± 1.1 | 19.0 ± 1.8 | 19.1 ± 1.0 |
| LATINA 3 | 23.3 ± 1.2 | 17.9 ± 2.1 | 17.8 ± 1.5 | 19.1 ± 1.3 | 20.0 ± 1.1 | 21.1 ± 1.6 |
| Latanoprost | 23.2 ± 1.1 | 16.8 ± 1.4 | 22.8 ± 1.5 | 23.1 ± 1.5 | 23.3 ± 1.1 | 23.1 ± 1.0 |
| Timolol | 23.1 ± 1.0 | 17.3 ± 1.4 | 21.9 ± 1.3 | 23.0 ± 1.1 | 23.2 ± 1.2 | 23.3 ± 1.4 |
3.8.2 Estimation of eye scratching activity after application of LATINA
The scratching activities of eye of the Sprague-Dawley rats are depicted in Fig. 10. There were no noteworthy difference in scratching activity of eye with forelimbs and hind limbs between control eyes and eyes to which LATINA were applied. There was no significant difference between scratching movement of eye with forelimbs and hind limbs of control and sham-procedure. This may be due to the fact that LATINA are biocompatible and do not lead to any sort of irritation in the cornea. This particular study highlights the fact that biodegradable and biocompatible nanosheet may prove to be an ideal drug delivery system to most sensitive of organs too.
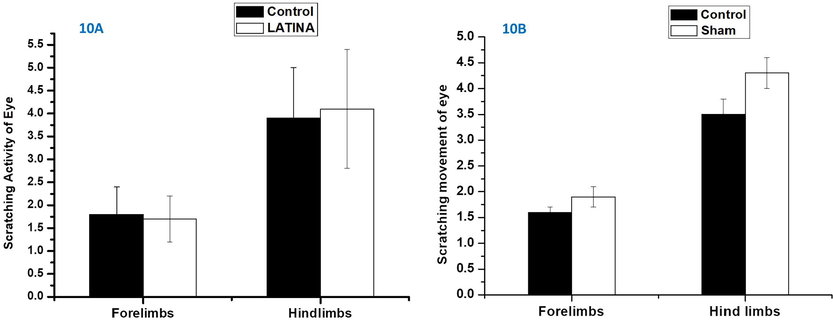
- Scratching movements of eye by fore and hind limbs after treatment with control/LATINA and control/sham. Students t test followed, P < 0.5.
4 Conclusion
A devastating malady, glaucoma affects thousands of persons worldwide and severely hampers their lifestyle. As researchers uncover more information regarding glaucoma and its pathogenesis, more methods of novel treatments may be elucidated. In this study, a novel combinatorial drug loaded nanosheet therapy to reduce IOP during glaucoma has been detailed. This nanosheet is easy to prepare, does not cause any side-effects as other ophthalmic formulation and is biocompatible. This therapy may provide novel addition to the currently available IOP-lowering techniques. This novel LATINA may also relieve a subject from daily routine of administration of ophthalmic formulation. However, abundant caution must be exercised while moving away from the established treatments and undertaking novel ones. While it may take some time to get approval for a new therapy to gain acceptance, more in-vivo research can definitely be taken up on nanosheet which acts a perfect drug delivery system which may benefit glaucoma-affected persons in future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Intraocular pressure-reducing effect of PhXA41 in patients with increased eye pressure: a one-month study. Ophthalmology. 1993;100:1312-1317.
- [Google Scholar]
- Latanoprost administered once daily caused a maintained reduction of intraocular pressure in glaucoma patients treated concomitantly with timolol. Br. J. Ophthalmol.. 1995;79:12-16.
- [Google Scholar]
- Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater.. 2016;28:1251-1286.
- [Google Scholar]
- Adsorption and immobilisation of human insulin on graphene monoxide, silicon carbide and boron nitride nanosheets investigated by molecular dynamics simulation. Mol. Simulat.. 2017;43:298-311.
- [Google Scholar]
- Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10:28-59.
- [Google Scholar]
- Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month, masked, multicenter trial in the United States. Ophthalmology. 1996;103:138-147.
- [Google Scholar]
- Fabrication of highly ordered multilayer films using a spin self-assembly method. Adv. Mater.. 2001;13:1076-1078.
- [Google Scholar]
- A Novel two-step procedure for plasma surface modification of low-density polyethylene for improved drug adhesion in intra uterine devices (IUDs) J. Adhes. Sci. Technol.. 2011;25:151-167.
- [Google Scholar]
- Investigating the therapeutic effects of N-acetylcysteine decorated poly (L-lactic acid) nanoparticles on transfusion induced acute lung injury. J. Biomater. Tissue Eng.. 2017;7:69-76.
- [Google Scholar]
- Develoment and investigation of timolol maleate and latanoprost combination liposomes for the treatment of glaucoma. Int. Res. J. Pharm.. 2015;6:256-264.
- [Google Scholar]
- The glass transition in thin polymer films. Adv. Colloid Interface Sci.. 2001;94:167-195.
- [Google Scholar]
- Adhesive, flexible, and robust polysaccharide nanosheets integrated for tissue-defect repair. Adv. Funct. Mater.. 2009;19:2560-2568.
- [Google Scholar]
- Ubiquitous transference of a free-standing polysaccharide nanosheet with the development of a nano-adhesive plaster. Adv. Mater.. 2007;19:3549-3553.
- [Google Scholar]
- Biodegradable combinatorial drug loaded pH-sensitive liposomes for enhanced osteosarcoma therapeutics. J. Biomater. Tissue. Eng.. 2017;7:952-961.
- [Google Scholar]
- Ocular hypotensive effect of PhXA41 in patients with ocular hypertension or primary open-angle glaucoma. Jpn. J. Ophthalmol.. 1993;37:270-274.
- [Google Scholar]
- Compliant, robust, and truly nanoscale free-standing multilayer films fabricated using spin-assisted layer-by-layer assembly. Adv. Mater.. 2004;16:157-161.
- [Google Scholar]
- Development of latanoprost-loaded biodegradable nanosheet as a new drug delivery system for glaucoma. Invest. Ophthalmol. Vis. Sci.. 2013;54(8):5629-5637.
- [Google Scholar]
- Nanotechnology and glaucoma: a review of the potential implications of glaucoma nanomedicine. Br. J. Opthalmol.. 2014;98:427-431.
- [Google Scholar]
- Adhesive and robust multilayered poly (lactic acid) nanosheets for hemostatic dressing in liver injury model. J. Biomed. Mater. Res. B. Appl. Biomater.. 2017;105:1747-1757.
- [Google Scholar]
- YBR/EiJ mice: a new model of glaucoma caused by genes on chromosomes 4 and 17. Dis. Model. Mech.. 2016;9:863-871.
- [Google Scholar]
- Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int. J. Nanomed.. 2012;7:123.
- [Google Scholar]
- Sustained drug release in nanomedicine: a long-acting nanocarrier-based formulation for glaucoma. ACS Nano. 2014;8:419-429.
- [Google Scholar]
- Free-standing biodegradable poly (lactic acid) nanosheet for sealing operations in surgery. Adv. Mater.. 2009;21:4388-4392.
- [Google Scholar]
- Patchwork coating of fragmented ultra-thin films and their biomedical applications in burn therapy and antithrombotic coating. Materials. 2015;8:7604-7614.
- [Google Scholar]
- Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003;20:597-630.
- [Google Scholar]
- Adhesion and proliferation of skeletal muscle cells on single layer poly (lactic acid) ultra-thin films. Biomed. Microdevices. 2010;12:809-819.
- [Google Scholar]
- Ophthalmologist-patient communication, self-efficacy, and glaucoma medication adherence. Ophthalmology. 2015;122:748-754.
- [Google Scholar]
- Phenyl-substituted prostaglandin analogs for glaucoma treatment. Drugs Future. 1992;17:691-704.
- [Google Scholar]
- An objective evaluation of eyedrop instillation in patients with glaucoma. Arch. Ophthalmol.. 2009;127:732-736.
- [Google Scholar]
- The role of patient inexperience in medication discontinuation: a retrospective analysis of medication nonpersistence in seven chronic illnesses. Clin. Ther.. 2009;31:2628-2652.
- [Google Scholar]
- Nanotechnology-based strategies for treatment of ocular diseases. Acta. Pharm. Sin. B. 2017;7:281-291.
- [Google Scholar]
- Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug. Deliv. Transl. Res.. 2014;4:303-309.
- [Google Scholar]







