Translate this page into:
Proline and other physiological changes as an indicator of abiotic stress caused by heavy metal contamination
⁎Corresponding author. hemadrireddy_s@apollouniversity.edu.in (Salla Hemadri Reddy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Heavy metal pollution due to globalization becomes the incurable hazardous problem for the agriculture productivity is due to industrialization with increased population. In this article impact of stress caused by heavy metals on shoot and root development along with phytoaccumulation was studied. The heavy metals cadmium (Cd), copper (Cu), zinc (Zn), lead (Pb) and nickel (Ni) are taken at varying concentrations ranging from 0 to 250 ppm, while cadmium concentrations ranged from 0 to 50 ppm. The findings revealed that seed germination and plant growth were impacted due to higher percentages of these metals under study. Among them copper, nickel, lead and cadmium exhibited the most pronounced effects on chlorophyll content, root and shoot growth as well as on seed germination, moreover, the physiological analysis indicated that increasing heavy metal concentrations induced greater stress, resulting in decreased chlorophyll content and elevated proline levels, underscoring the significant impact of stress caused by heavy metals on the growth of plants. Delayed germination and growth were observed across all replicates with increased concentrations of all tested heavy metals. Despite zinc being an essential nutrient for plant growth, it was noted that it exhibited a positive effect on chlorophyll content and stimulated the production of fibrous roots, although growth was delayed at higher concentrations. The phytoaccumulation of heavy metals was analysed using atomic absorption spectroscopy (AAS) to quantify the absorption of heavy metals by seedlings, thereby assessing the accumulation or evaporation of heavy metals.
Keywords
Phytoremediation
Proline content
Chlorophyll content
AAS
Heavy metals
Soil extraction
Seed germination
1 Introduction
Increased industrialization and globalization enhanced the pollution and contamination in water, air, soil etc. There is a drastic reduction in agricultural production due to soil pollution by heavy metals, thereby altering the economy of the affected areas. Phytoremediation refers to a range of technologies employing various plants for containment, absorption, adsorption, or extraction purposes. This approach can effectively detoxify sites contaminated with pesticides, metals, explosives, solvents, hydrocarbons, crude oil, and landfill leachates. Metals like cadmium, lead cobalt, chromium, nickel, mercury, and copper are essential for plant growth in small amounts, as they constitute important components of enzymes and proteins, while higher concentrations of these metals can transform them into soil pollutants. Such elevated levels can exert toxic effects on plants, leading to reduced productivity and posing significant threats to agricultural ecosystems (Hall, 2002a; 2002b). In plants heavy metals can cause oxidative stress in plants by generating reactive oxygen species and free radicals, thereby affecting various physiological aspects of the plant (Dietz et al., 1999). While there are several evidence regarding how heavy metals induce stress and how plants have adapted to overcome it, there is ample evidence available (Sethy and Ghosh, 2013). The spoilage of soil for agricultural purposes due to heavy metals has emerged as a principal ecological issue, because of its potential unexpected ecological impacts on the soil ecosystem. In a recent article it was observed that there was a noticeable color shift and fluorescence switch off when this chemo sensor was subjected to Cu2+ and CN-ions in solution. The Cu2+ and CN– detection limits were 6.36 and 3.75 nm respectively, which were significantly lower than WHO standards (Kumar et al., 2023). In a similar article it was noticed that tissues treated with active Pd (II) complexes showed normal cell architecture, in contrast, tissues treated with cisplatin caused significant harm to the mice's normal tissues (Haribabu et al., 2023). Similar research was conducted to determine the potential of the Pd II complexes towards biomolecular interactions, additional experiments involving interaction with calf thymus DNA (CT DNA) and bovine serum albumin (BSA) were conducted (Haribabu et al., 2018). Recent studies also explained that the aqueous soluble probe TQA detects cysteine based on “ON-OFF” effect with excellent absorbance and emission properties (Kavitha et al, 2023). These toxic elements are widely regarded as soil pollutants because of their predominant presence and their capacity to cause acute and chronic toxicity in plants cultivated in affected soil (Kunjam et al., 2015). Soil contamination by heavy metals, whether through natural processes or pollution, frequently exerts significant impacts on vegetation. This phenomenon often leads to the development of metallophytes and plants with a high tolerance to heavy metals. From the previous findings it is noticed that heavy metal toxicity may influence on the chlorophyll content and reduces the photosynthetic ability. Many studies have shown the substantial impact of heavy metals on chlorophyll content, consequently affecting the photosynthetic capacity of plants. This effect often results in a reduction in carbon metabolism, either by inducing deficiencies in essential elements required for the formation of chlorophyll (Van Assche and Clijsters, 1990) or through direct inhibition of enzymatic synthesis. The accumulation of amino acid proline has been identified as a marker of environmental stress and is believed to play a protective role as an osmotic regulator. When plants experience osmotic injury, they typically exhibit increased levels of free proline. Proline is thought to be used as a scavenger or as an osmolyte of reactive oxygen species, aiding in the plant's defense against stress (Reddy et al, 2015).
Therefore, the aim of this manuscript is to investigate the role/impact of metals under study at varying concentrations on germination of seeds, physiology of seedling, the content of chlorophyll and growth. At the same time proline accumulation can be used as marker to determine number of proposed metals that has been absorbed by the experimental seedling.
2 Materials and method
Lead Acetate, zinc acetate, copper, nickel, cadmium, ‘AAS’ (Thermo scientific ICE 300 series A), 3 % sulfosalicylic acid, ninhydrin Solution in phosphoric acid, acetic acid glacial, toluene, L proline, microcentrifuge, spectrophotometer, 80 % acetone.
2.1 Heavy metal role on Vigna radiata germination and seedling growth
2.1.1 Preparation of pots and heavy metal stock solution
The Stock solutions were prepared for Ni, Cd, Cu, Zn, and Pb at different concentration (50 ppm, 100 ppm, 150 ppm, 200 ppm, 250 ppm) with the control. The soil cultures were prepared by mixing 70 % of Soil with 30 % of compost and filled the pots with the prepared soil until 1/3 of the pots. Each respective concentration at a volume of 100 ml was poured into three replicate of pots and each replicate was inoculated with 8 healthy seeds of Vigna radiata at equal distance. The pots were irrigated with 100 ml of normal tap water once a day for 21 days. After 21 days seedlings were collected and the root, shoot length, leaf number and morphological characteristics were measured.
2.1.2 Analysis of soil using AAS
5 g of soil from each replicate was taken before and after adding heavy metal solution, as well after 21 days of culture also the same amount was collected for heavy metal analysis through AAS. The soil samples were grounded into fine solution using mortar and pestle. The 20 ml of extracted solution (25 ml HCL was mixed with 12 ml H2SO4 and volume was made up to 500 ml) was added to each powdered soil sample and placed in shaker for 20 min at 150 RPM. After continuous agitation, the samples were filtered through Whatman no 1 filter paper and are subjected to AAS for analysis
2.1.3 Impact of heavy metal stress on chlorophyll content
0.10 g of 21-day old seedling leaves were excised into small pieces, pulverized material was grounded in mortar and pestle with 10 ml of acetone (80 %). The homogenate was collected through the filter paper and the OD of the filtrate was measured at different wavelengths 645 and 663 nm using acetone blank. All the extracts were analyzed through this method at 2 different wavelengths (Arnon, 1949)
Formula to calculate chlorophyll
To convert absorbance measurements to chlorophyll (Chl) content in milligrams per gram (mg/g) of leaf tissue Arnon's equation is used.
Chl a (mg/g) = [(12.7 × Abs 663) − (2.6 × Abs 645)] × ml acetone / mg leaf tissue
Chl b (mg/g) = [(22.9 × Abs 645) − (4.68 × Abs 663)] × ml acetone / mg leaf tissue
Total Chlorophyll = Chlorophyll a + Chlorophyll b.
2.1.4 Impact of metal stress on proline
Proline content was measured using the modified Bates et al. method (Bates et al, 1973). Fresh seedlings (0.5 g) with their hypocotyl regions crushed in 1.5 mL of a 3 % w/v sulfosalicylic acid solution. Proline levels were determined using the ninhydrin method (0.125 g of ninhydrin was dissolved in acetic acid and orthophosphoric acid (6 M) in measured quantities. The mixture was separated using toluene in ice bath following the ninhydrin reaction and the chromophore that contains proline was measured at wavelength of 520 nm against toluene as blank. Each determination was calibrated using standard proline solutions falling within the method's detection range (0–39 µg/ml). Proline concentrations were calculated using a standard curve and shown as μmol/g based on fresh weight. Proline content was calculated per unit as per the formula given below:
Proline (μMols) in g−1 FW= (mg proline / ml × ml toluene) / 115.5 mg / μMols) / (g FW / 5) (Ruscitti et al, 2011).
3 Results and discussion
3.1 Effect of heavy metal role on seedling growth and seed germination
From the Fig. 1 it is clearly observed that reduction in the growth of seedling at increased concentrations of heavy metals. The decline in growth that is observed in plants growing on heavy metal-polluted soil is ascribed to modifications in physiological and biochemical metabolic pathways. This decline in plant growth decreases yield, consequently contributing to food insecurity. Hence, the importance of remediation efforts for heavy metal-polluted soils cannot be overstated (Chibuike and Obiora, 2014).
Impact of varying percentages of heavy metals on seed physiology and germination.
It is well known that zinc is essential heavy metal and helps in plant growth (Singh et al., 2007, 2007, Hojiboland et al., 2006; Clemens, 2006). The values represent the increased growth of seedling was more in zinc at different concentrations evaluated in comparison with other heavy metals. The growth in the control was 100 %, but the growth was delayed slightly with the increased concentration, were 97 %, 90 %, 84 %, 82 %, and 80 % at 50 ppm, 100 ppm, 150 ppm, 200 ppm, 250 ppm, respectively. The length of Zinc treated seedling shoot growth decreases as the concentration of Zinc increases, and similarly, in roots, the length decreases with higher Zinc concentration. This finding confirms that Zn is less toxic among the other heavy metals though it delayed seed germination % due to selective cell membranes permeability (Muhammad et al., 2008). At 150 ppm and 200 ppm, it was found that the lead treatment also increased the length of the shoots and roots. Lead mostly enters the apoplast of the root and then travels radially across the cortex, gathering close to the endodermis. Lead flow between the root and the shoot is partially inhibited by the endodermis (Jones et al., 1973; Verma and Dubey, 2003). The primary enzyme in the production of chlorophyll, α-amino levulinate dehydrogenase, is severely inhibited by Pb ions (Prasad and Prasad, 1987) (See Figs. 2–4).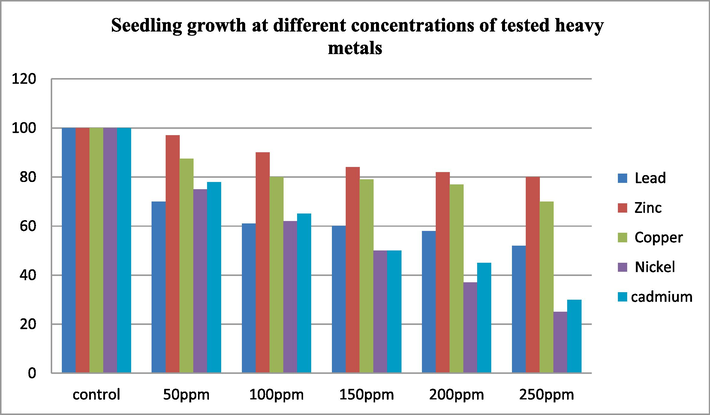
Effect of different heavy metal concentration at tested ppm on seed germination and seedling growth after 21 days of observation. (control used is only normal tap water, cadmium used at a concentration 10, 20, 30, 40 and 50 ppm).
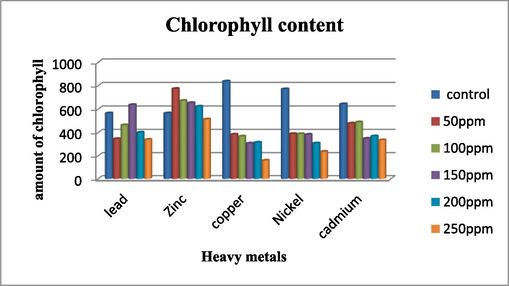
Effect of heavy metal stress on chlorophyll content. Here all the result shown in the figure is from three replicates of 21-day seedling (All the heavy metals tested at 50,100, 150,200 and 250 ppm while cadmium is tested at 10,20,30,40 and 50 ppm).
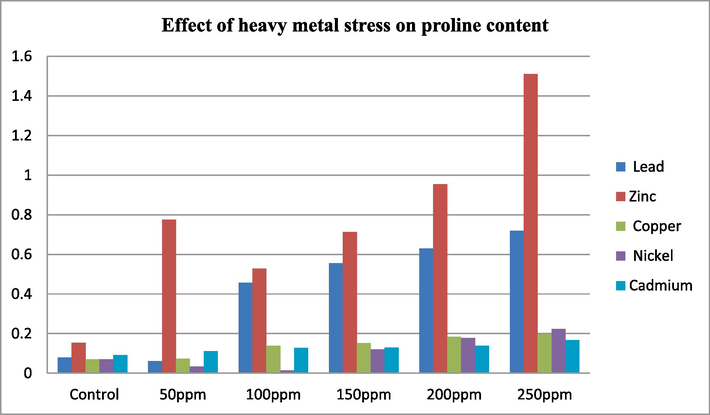
Effect of Heavy metals stress on proline content.
As the concentration of nickel decreases, the length of both shoots and root increases in the nickel treated seedling. In control the growth was 100 % but at 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm was 70 %, 61 %, 60 %, 58 %, and 52 % respectively. It has been demonstrated that in a range of plant species, elevated nickel concentrations hinder seed germination and seedling growth. (Espen et al., 1997, Farooqi et al., 2009). At exceptionally low concentrations, Nickel can enhance numerous enzymatic activities and is found as part of the active metallocentre in the enzyme urease which is hexametric (Gerendás et al., 1999).
With copper treated seedling it was observed that, there is a very minute difference in the length of root and shoot, the growth being 100 % in the control but at 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm it was 87.5 %, 80 %, 74 %, 77 %, and 70 % respectively. Copper is abundant in the environment and present everywhere, and organisms can tolerate its presence. One of the most common heavy metals in agricultural soils is copper. It plays an active part in cellular activities due to its in numerous proteins (Hall, 2002a; 2002b).
When the concentration of cadmium is high, it delays the growth of seedlings and inhibits germination percentage, taken more time for germination and growth of seedling. Seedling growth was only observed up to maximum at 50 ppm cadmium. Using concentrations ranging from 10 ppm to 50 ppm from the table above, it is evident that shoot and root length decrease with increasing cadmium concentration. Some studies suggest that increased presence of cadmium in plants leads to varied phytotoxicity symptoms, resulting in the inhibition of development and growth in plants (Di Cagno et al., 1999; Milone et al., 2003). Many research studies have demonstrated that an excess amount of cadmium in plants can lead to slow down of plant growth, leaf rolling, chlorosis, and necrosis (Xue et al., 2013). Plants have physiological mechanisms that enable them to withstand increased concentrations of heavy metals in their environment. Resistance or tolerance mechanisms may be responsible for this resistance. Through physiological systems, plants can defend themselves externally against metals that they can tolerate at high tissue concentrations.
3.2 Phyto absorption
Based on the results presented in Table 3.1, the concentration of heavy metal before germination reduced after germination. These findings reveal that during seedling germination, heavy metals are absorbed from the soil and may accumulate in plant materials or evaporated through phytoremediation role of the seedling evaluated into the atmosphere. Several studies have shown that many plant species are capable of effectively absorbing contaminants such as chromium, arsenic, lead, cadmium, and various radionuclides from soil. Phytoextraction, the type of phytoremediation, involves the use of certain plant species to remove heavy metals from soil by the phyto adsorption of metals essential for plant growth (such as Cu, Mg, Mo, Fe, Mn, Zn, and Ni). Metals like Cd, Cr, Pb, Co, Ag, Se, and Hg that have no known biological purpose can also accumulate. By processes like precipitation inside the root zone, adsorption onto roots, and absorption and accumulation by roots, certain plant species have been utilized to immobilize pollutants in soil and groundwater. This method works well for metal and organic pollutants found in sludge, sediments, and soil (Tangahu et al., 2011). Values expressed are the mean of triplicates (n = 3). Here pre: Before treatment and post: After treatment.
Sample
Concentration of Heavy Metal (before and after germination)
Pb
Cu
Zn
Ni
Cd
pre
post
pre
post
pre
post
pre
post
pre
post
C
0.001
0.001
0.01
14
0.001
0.1
135
66.6
160
3.8
50
0.5
0.005
120.1
16
0.07
0.07
135.7
70.6
164.8
9.1
100
0.52
0.005
123
15.9
0.157
0.12
137
72.2
164.6
12
150
0.60
0.006
140
15.9
0.295
0.20
137
72.4
164.8
14.6
200
0.62
0.006
170
16.1
0.721
0.60
141.1
77.7
168.2
17.17
250
0.70
0.007
178
16.1
0.768
0.66
141.4
79.5
168.9
21.8
3.3 Heavy metal stress on chlorophyll content
The findings presented in Table 3.2 elucidate the levels of chlorophyll in seedlings cultivated under varying heavy metal concentrations for a period of 21 days. Notably, the control group exhibits higher chlorophyll content across lead, copper, cadmium, and nickel, apart from zinc, recognized as an essential element for plant development. In the case of lead (Pb), the highest chlorophyll concentration was detected in the treatment with 150 ppm, while the lowest was observed in the 250 ppm treatment. It is worth noting that lead ions notably inhibit the activity of α-amino levulinate dehydrogenase, a crucial enzyme in chlorophyll biosynthesis. In the case of seedlings treated with cadmium, the chlorophyll content at 20 ppm surpasses that at 10 ppm. Nevertheless, in general, an increase in concentration leads to a decrease in chlorophyll content. Elevated levels of cadmium can inhibit photosynthesis and the efficiency of chlorophyll fluorescence (Azevedo et al., 2005). For copper (Cu), it was observed across all examined concentrations that an increase in copper treatment resulted in a decrease in chlorophyll content. The highest chlorophyll concentration was recorded at 50 ppm, measuring 383.5, while the lowest was noted at 250 ppm, measuring 160.4. The decline in chlorophyll content following exposure to copper is a commonly reported phenomenon in plants. (Devi and Prasad, 1998 & Singh et al., 2007, 2007). In the case of nickel, there are minimal discrepancies in chlorophyll content across all concentrations, with a consistent decrease observed with increasing concentration. For instance, in seedlings treated with 50 ppm, the chlorophyll content is 339, whereas at 250 ppm, it decreases to 234.8. These findings align with previous research indicating that chlorophyll content tends to diminish with higher concentrations of heavy metals, due to oxidative stress (Dubey and Pandey, 2011). Note: Values presented in the table are mean value of three replicates taken from 21 days old leaves of seedling and measured at 663 nm and 645 nm.
Concentration
Copper
Nickel
Zinc
Lead
Cadmium
Cadmium conc (ppm).
Control
837.9
770.4
565.1
565.1
643.9
Control
50 ppm
383.5
399
773.6
345
479.5
10
100 ppm
366.6
388
673
465
488.1
20
150 ppm
306
382.5
654
637
347.6
30
200 ppm
313.7
306.5
623
401
367.7
40
250 ppm
160.4
234.8
513
339
334.8
50
In contrast, seedlings grown in zinc (Zn) exhibited higher chlorophyll levels across all tested concentrations compared to other heavy metals, even at elevated levels. Zinc's crucial role as an essential micronutrient for optimal crop growth is well-documented, with its deficiency leading to various adverse effects on crop growth and yield. Zinc plays a vital role in plants by participating in chlorophyll and carbohydrate metabolism. Additionally, it is an essential element in several enzymes such as dehydrogenases, proteinases, and peptidases. Zinc also contributes to the biosynthesis of cytochrome, a pigment, and helps maintain the integrity of the plasma membrane and the synthesis of the leaf cuticle (Kumar et al., 2023).
3.4 Heavy metal stress by measurement of proline content
All the value presented in the table are mean value of three replicates taken from21 days of old seedling root.
According to the findings presented in Table 3.3, it's evident that in seedlings treated with lead, the concentration of proline increased as the concentration of lead increased. Proline serves as a biomarker for the non-specific defense mechanisms against lead toxicity. It mitigates metal toxicity by functioning as a metal chelator and stabilizing proteins (Sharma and Dubey, 2005). The results from cadmium treated seedlings indicate that as the concentration of cadmium increases, there is a corresponding increase in proline content. Proline accumulation in plants under heavy metal stress, such as cadmium, is triggered by a reduction in the plant's water potential caused by cadmium exposure. This proline accumulation's functional importance comes from its role in preserving the plant's water balance (Costa and Morel, 1994). Absolutely, proline plays a crucial role in enhancing stress tolerance in plants through various mechanisms. It aids in osmoregulation, helping to regulate water balance and maintain cell turgor pressure under stressful conditions. Additionally, proline acts as a protective agent for enzymes, shielding them from denaturation caused by environmental stressors. Moreover, proline contributes to the stabilization of protein synthesis, ensuring the continued production of essential proteins necessary for plant survival and adaptation to stress (Kuznetsov and Shevyakova, 1997). All the value presented in the table are mean value of three replicates taken from 21 days of old seedling root.
Concentration
The concentration of proline (mg/ml)
Lead
Zinc
Nickel
Copper
Concentration of cadmium(ppm)
Cadmium
Control
0.080
0.154
0.071
0.071
Control
0.092
50 ppm
0.061
0.775
0.034
0.073
10
0.111
100 ppm
0.457
0.529
0.014
0.138
20
0.128
150 ppm
0.550
0.713
0.121
0.153
30
0.129
200 ppm
0.630
0.954
0.178
0.184
40
0.138
250 ppm
0.724
1.510
0.224
0.196
50
0.167
In the case of nickel, there is a notable increase in proline content with rising concentrations. For instance, the proline content is 0.034 mg/ml at 50 ppm, it rises to 0.224 mg/ml at 250 ppm, Proline, being an amino acid, has the potential to play a therapeutic role in plants (Singh et al., 2012). However, numerous metabolites have been extensively studied for their role in enhancing metal tolerance in plants. Proline, sugars, glycine, betaine, and other organic solutes are among them. These compounds are believed to improve metal tolerance by contributing to osmoregulation, helping to maintain water balance within plant cells under stressful conditions. Additionally, they play a crucial role in preserving enzyme activity in the presence of toxic metal ions, thereby supporting essential cellular functions even in metal-contaminated environments.
4 Conclusion
According to the results of the present findings all the heavy metals possess toxicity at higher concentrations on seed germination as well seedling physiological growth. Zn is found to be less toxic than other heavy metals studied though it was found to be essential element in the growth. Also, it was concluded that leaf that grown in Zn polluted soil found very greenish and found highest amount of chlorophyll. The Ni also affected the growth and delayed the germination at increased concentration. Among the tested heavy metals, Cadmium affected severely the seed germination and delayed the growth at high concentration. The plant-based biomarker proline concentration was found and concluded that at increased concentration of tested heavy metals increased the proline dramatically as indication of heavy metal stress.
Acknowledgement
To perform the current work, the authors are highly thankful to college of Applied science and Pharmacy, University of Technology and Applied Science (UTAS, Muscat), for providing facilities and supporting the research. Authors are also thankful to laboratory technicians of Applied Biology and Applied Chemistry, College of Applied Science and Pharmacy, UTAS, Muscat. The authors extend their sincere appreciation to the researchers supporting project number (RSP2024R266), King Saud University, Riyadh, Saudi Arabia for the support.
References
- copper enzymes in isolated chloroplasts, polyphenol oxidase in beta vulgaris. Plant Physiol.. 1949;24:1-15.
- [Google Scholar]
- Cadmium effects on sunflower growth and photosynthesis. J. Plant Nutr.. 2005;28:2211-2220.
- [Google Scholar]
- Chibuike G.U., Obiora S.C., 2014. Heavy metal polluted soils: effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014 (Article ID 752708), 12 pages.
- Toxic metal accumulation: responses to exposure and mechanisms of tolerance in plants. Biochemistry. 2006;88:1707-1719.
- [Google Scholar]
- Water relations, gas exchange and amino acid content in cadmium-treated lettuce. Plant Physiol. Biochem.. 1994;32:561-570.
- [Google Scholar]
- Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci.. 1998;138:157-165.
- [Google Scholar]
- Effects of cadmium on growth of Helianthus Annu seedlings: physiological aspects. New Phytologist. 1999;14(1):65-71.
- [Google Scholar]
- Dietz, K.-.J, Krämer, U., Baier, M., 1999. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants: from molecules to ecosystems, 73–97. Springer-Verlag, Berlin.
- Effect of Ni2+ during the early phases of radish (Raphanus sativus) Seed Germinat. Environ. Exp. Bot.. 1997;38(2):187-197.
- [Google Scholar]
- Toxic effects of lead and cadmium on germination and seedling growth of Albizia lebbeck (L.) Benth. Pak. J. Bot.. 2009;41(1):27.
- [Google Scholar]
- Gerendás, J., Polacco, J., Freyermuth, S.K., Sattelmacher, B., 1999. Significance of nickel for plant growth and metabolism. Z Pflanzenernaehr Bodenkd 162: 241–256.
- Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot.. 2002;53:1-11.
- [Google Scholar]
- Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot.. 2002;53(366):1-11.
- [Google Scholar]
- Synthesis, structures and mechanistic pathways of anticancer activity of palladium (II) complexes with indole-3-carbaldehyde thiosemicarbazones. New J. Chem.. 2018;42(13):10818-10832.
- [Google Scholar]
- Michael addition-driven synthesis of cytotoxic palladium (ii) complexes from chromone thiosemicarbazones: investigation of anticancer activity through in vitro and in vivo studies. New J. Chem.. 2023;47(33):15748-15759.
- [Google Scholar]
- Uptake, transport and chelation of Cu and Zn at toxic levels in tolerant and sensitive species from Northwest of Iran. J. Sci.. 2006;17:203-214.
- [Google Scholar]
- Lead uptake from solution by perennial ryegrass and its transport from roots to shoots. Plant and Soil. 1973;38:403-414.
- [Google Scholar]
- An aqueous mediated ultrasensitive facile probe incorporated with acrylate moiety to monitor cysteine in food samples and live cells. Spectrochemical Acta Part A: Mol. Biomol. Spectroscopy. 2023;293:122447
- [Google Scholar]
- Imidazole-based dual functional chemosensor for the recognition of Cu2+ and CN: applications in real water samples and colorimetric test strips. Opt. Mater.. 2023;144:114382
- [Google Scholar]
- Studies on selected heavy metals on seed germination and plant growth in pea plant (Pisum sativum) grown in solid medium. J. Pharmacog. Phytochem.. 2015;3:85-87.
- [Google Scholar]
- Stress responses of tobacco cells to high temperature and salinity. Proline accumulation and phosphorylation of polypeptides. Physiologic Plantarum. 1997;100:320-326.
- [Google Scholar]
- Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ. Exp. Bot.. 2003;50:265-276.
- [Google Scholar]
- Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. J. Appl. Sci. Environ. Manage.. 2008;12:61-66.
- [Google Scholar]
- Altered α-amino luvelinic acid metabolism by Pb and Hg in germinating seedling of Bajra (Pennisetum typhoidenum) J. Plant Physiol.. 1987;127:241-249.
- [Google Scholar]
- Reddy, S.H., Aysha Al-Weheibi, Halima Al Shekaili, Rehab Al-Naabi, 2015. Reversal of oxidative stress by selective medicinal plant extracts induced by UV radiation and their influence on seedling physiology. Adv. Biol. Res. 9 (5) (2015) 324-329.
- Inoculation with mycorrhizal fungi modifies proline metabolism and increases chromium tolerance in pepper plants (Capsicum annuum L.) Braz. J. Plant Physiol.. 2011;23(1):15-25.
- [Google Scholar]
- Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med.. 2013;4(2):272-275.
- [CrossRef] [Google Scholar]
- Response of wheat seed germination and seedling growth under copper stress. J. Environ. Biol.. 2007;28(2):409-414.
- [Google Scholar]
- Response of wheat seed germination and seedling growth under copper stress. J. Environ. Biol.. 2007;28:409-414.
- [Google Scholar]
- Tangahu, B.V., Sheikh Abdullah, S.R., Basri, H., Idris, M., Anuar, N., Mukhlisin, M., 2011. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. [939161].10.1155/2011/939161.
- Effect of metals on enzyme activity in plants. Plant Cell Environ.. 1990;13:195-206.
- [Google Scholar]
- Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci.. 2003;164:645-655.
- [Google Scholar]
- Effects of cadmium on growth, photosynthetic rate, and chlorophyll content in leaves of soybean seedlings. Biol. Plant.. 2013;57:585-590.
- [Google Scholar]







