Translate this page into:
Production and partial purification of extracellular xylanase from Pseudomonas nitroreducens using frugivorous bat (Pteropus giganteus) faeces as ideal substrate and its role in poultry feed digestion
⁎Corresponding author. dhivahar.biotech@gmail.com (J Dhivahar),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This investigation was aimed to optimize and purify extracellular xylanase from Pseudomonas nitroreducens strain LLD06 using frugivorous bat (Pteropus giganteus) faeces as ideal substrate. Medium components and other physical parameters affecting xylanase yield were optimized using Box-Behnken design (BBD) of response surface methodology constituting 17 experimental runs with 3 variables. The BBD showed increased xylanase activity of 2598.14 U/g using bat faeces as substrate along with 100% of moisture, 1.5% w/w of birchwood xylan, and 0.8% w/w of yeast extract. Xylanase was purified using chromatographic techniques which revealed its molecular mass of approximately 40 kDa. The partially purified enzyme exhibited stability at 60 °C and alkaline pH. Poultry feed was treated with purified xylanase which showed increment in the total reducing sugar content (88%) with respect to control. In conclusion, strain LLD06 associated xylanase exhibited stability towards high temperature and pH with potential application in poultry industries. The study represents the hyper-production of xylanase from P. nitroreducens using bat faeces as ideal substrate and its pivotal role in poultry feed digestion.
Keywords
Poultry feed
P. nitroreducens
P. giganteus faeces
Statistical optimization
Xylanase
1 Introduction
Bats belong to the second most important group of mammals (Order: Chiroptera) containing more than 1400 species (Lazzeroni et al., 2018). Flying foxes (Pteropus giganteus) of family Pteropodidae are present in varied countries of Asia (Dhivahar et al., 2019). Bats eat a wide range of flowers and fruits to meet their nutritional energy demand (Herrera et al., 2001). Frugivorous bats consume leaves in addition to fruits and floral parts due to high protein content (Courts, 1998). Leaves are considered to be the unique source of xylan. Although, bats consume leaves, most of them do not secrete digestive enzymes and depend on enzyme producing bacteria for digestion mechanism (Anand et al., 2012). Disparate xylanolytic enzymes are essential for xylan hydrolysis. Xylanases catalyze hemicelluloses and xylan into xylose (Khusro et al., 2016).
Bacteria are known to produce extracellular xylanases at higher rates. Despite several reports investigating hyper production of xylanase from disparate bacterial species, the isolation of xylanolytic bacteria from bat faeces is limited. Previously, Dhivahar et al. (2019) isolated xylanase producing Bacillus spp. from P. giganteus faeces. Considering the tremendous growing demands of xylanases in diversified industries, there is desperate essentiality to identify distinct groups of bacteria from less exploited resources. In view of this, we have undertaken further attempt in this study not only to isolate hyper-xylanase producing bacterial strain but also optimize statistically the production of xylanase and determine its role in the poultry feed digestion.
2 Materials and methods
2.1 Faeces collection
Five roosts of P. giganteus were chosen for the collection of faeces from various districts of South India as shown in Table 1 (Dhivahar and Suthakar, 2018). Samples were collected as per the methodology of Akobi et al. (2012).
Site code
Location in Southern India
Bat population
Roost tree spp.
Latitude
Longitude
TIR1
Sivagiri
740
Bassia latifolia
9°34″N
77°0.42″E
TIR2
Kadayam
420
Ficus benghalensis
8°0.82″N
77°0.37″E
TUK1
Paramankurichi
930
Ficus benghalensis Azadirachta indica Polyalthia longifolia
8°0.48″N
78°0.04″E
TUK2
Srivaikundam
8600
Terminalia arjuna
8°0.62″N
77°0.91″E
KKU1
Boothapandi
1200
Bassia latifolia Termarindus indica
8°0.26″N
77°0.44″E
2.2 Selection of xylanase producing bacteria and estimation of xylanase activity
Basal agar medium constituting 1% birchwood xylan was prepared and autoclaved. The medium was transferred to sterile petriplates aseptically and allowed to cool. Samples were serially diluted, spread on the agar medium, and incubated at 37 °C for 24 h. The potent culture was selected after congo red staining method (Akobi et al., 2012). The xylanase activity of bacterial culture was determined using the method of Khusro et al. (2016). The culture was identified using molecular characterization tools. The 16S rRNA sequence was submitted to GenBank, NCBI.
2.3 Substrate used and solid state fermentation (SSF)
P. giganteus faeces were collected from Srivaikundam (8˚0.62″N:77˚0.91″E) roost colony, Tamil Nadu, South India and dried for few days. The dried sample was ground into powder form for further experiments. The SSF of substrate was carried out as per the methods of Aarti et al. (2017) and supernatant was collected for xylanase activity using the methodology of Khusro et al. (2016) as described earlier.
2.4 Xylanase activity optimization by traditional method
Abiotic and biotic factors such as incubation period (12–96 h), pH (6.0–11.0), temperature (32–60 °C), inoculum (7.31–54.82 log CFU/mL), moisture content (60–120%), carbon sources, and nitrogen sources were optimized by one-factor-at-a-time (OFAT) technique and xylanase production was assessed following the prior method discussed.
2.5 Statistical optimization
Box Behnken design (BBD) of response surface methodology (RSM) was implied for optimizing varied factors towards enhancement of xylanase yield. The BBD contained 17 experiments of three factors (A, B, and C) at three ranges (−1, 0, +1) as shows in Table 2.
Variables
Code
Range and levels
−1
Levels
+1
Moisture (%)
A
80%
100%
120%
Birchwood xylan (% w/w)
B
0.5%
1%
1.5%
Yeast extract (% w/w)
C
0.3%
0.5%
0.8%
The average xylanase activity (Y) was determined using equation as mentioned below:
2.6 Partial purification of xylanase
Ammonium sulphate (70% saturation) was added into the collected supernatant of bacterial culture while stirring. After 2 h, the precipitated sample was centrifuged at 8,000 rpm for 20 min at 4 °C, pellet was collected, dissolved in phosphate buffer (pH 7.4), and dialyzed overnight. Sample was purified using ion-exchange chromatographic technique. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine molecular mass of the purified enzyme. Total protein content was determined using Bradford assay (Bradford, 1976).
2.7 Characterization of xylanase
Xylanase stability towards various pH, temperature, metal ions, and solvents was carried out as per the methods of Khusro et al. (2016) and Aarti et al. (2017, 2018).
2.8 Pre-digestion of poultry feed using purified xylanase
Poultry feed was obtained from poultry house, washed thoroughly, dried, and stored in clean bottles. About 15 g of poultry feed were measured in a conical flask and autoclaved using 25–30 mL distilled water. Poultry feed was separately treated with xylanase (5 mL) for 24 h incubation at 40 °C under shaking condition. After incubation, poultry feed was centrifuged at 10,000 rpm for 10 min. Supernatants were incubated with DNS and boiled for 10 min. After water bath treatment, total reducing sugar production was estimated by recording optical density at 540 nm using UV–Vis spectrophotometer (Nagar et al., 2012). Untreated poultry feed was used as control.
2.9 Statistical analysis and software used
Design Expert Version 11.0.0 (Stat-Ease Inc., Minneapolis, MN, USA) statistical software was utilized for optimization. Analysis of variation (ANOVA) was used for validation of various factors.
3 Results
3.1 Xylanolytic strain identification
Off 18 isolates, 1 bacterial isolate exhibited good xylanase activity and identified as P. nitroreducens strain LLD06 after 16S rRNA sequencing (Accession No: MK530169).
3.2 Solid state fermentation
Strain LLD06 utilized P. giganteus faeces for production of xylanase with maximum activity of 1044.05 ± 303 U/g (Figure not shown).
3.3 OFAT method based optimization
The enzyme activity was optimum at 48 h (1044.05 ± 32.3 U/g) and reduced afterwards (Figure not shown). The xylanase activity was optimum at pH 8 (1144.12 ± 32.3 U/g) and decreased at higher alkaline conditions (Figure not shown). The optimum temperature for the xylanase yield was maximum at 37 °C (1044.05 ± 24.2 U/g) and reduced at high temperature (Figure not shown). The inoculums level showed no significant alteration in xylanase production, being maximum at 18.27 log CFU/mL (Figure not shown). The xylanase productivity was optimum (1323.56 ± 31.3 U/g) with 100% moisture and reduced with lower and higher moisture contents (Figure not shown). The xylanase activity was maximum (1423.14 ± 28.3 U/g) in the presence of birchwood xylan. Similarly, optimum xylanase production (1496.12 ± 31.3 U/g) was estimated in the presence of yeast extract (Figure not shown).
3.4 RSM based optimization
Experimental strategy of parameters (moisture, birchwood xylan, and yeast extract) with 17 runs is demonstrated in Table 3. Xylanase activity (Y) was predicted using model equation as given below:
Run Order
A:A
B:B
C:C
Xylanase activity (U/g)
Experimental
Predicted
1
0
0
0
1956.23
1550.76
2
−1
0
1
2013.34
2012.58
3
0
−1
−1
1145.32
1144.04
4
1
0
1
1532.16
1612.47
5
0
0
0
1486.12
1550.76
6
−1
0
−1
1145.12
1064.81
7
1
1
0
1886.13
1804.53
8
−1
−1
0
1112.14
1193.74
9
0
1
−1
2314.12
2394.96
10
0
1
1
2598.14
2599.42
11
1
0
−1
1167.23
1167.99
12
0
−1
1
2412.67
2331.83
13
−1
1
0
2234.11
2233.59
14
0
0
0
1437.23
1550.76
15
0
0
0
1433.12
1550.76
16
0
0
0
1441.12
1550.76
17
1
−1
0
1325.34
1325.86
Y (U/g) = 1150.76 − 74.23A + 379.63B + 348.07C − 140.29AB − 125.82AC − 245.83BC − 282.22A2 + 370.88 B2 + 195.92C2
Table 4 represents ANOVA for xylanase production. Modelling codes such as BB, CC, BC, A2, and B2 were significant. The multiple correlation coefficient (R2) was close to 1, indicating an accurate modelling. Parity plot showed the predicted and observed values closeness towards diagonal line (Fig. 1). The interaction effects between parameters are shown in Fig. 2(a–c). The 3D graphs (Fig. 2a–c) represented interaction between two factors. R2-0.9357, Adj R2-0.8531, Predicted R2-0.7518, CV-11.14%, Adeq precision-10.659, Df-degree of freedom, Significant- P < 0.05, Non-significant- P > 0.05.
Source
Sum of Squares
df
Mean Square
F-value
p-value
Model
3.591E+06
9
3.990E+05
11.33
0.0021
Significant
AA
44082.23
1
44082.23
1.25
0.3002
BB
1.153E+06
1
1.153E+06
32.72
0.0007
CC
9.692E+05
1
9.692E+05
27.51
0.0012
AB
78730.75
1
78730.75
2.23
0.1786
AC
63325.21
1
63325.21
1.80
0.2219
BC
2.417E+05
1
2.417E+05
6.86
0.0344
A2
3.354E+05
1
3.354E+05
9.52
0.0177
B2
5.792E+05
1
5.792E+05
16.44
0.0048
C2
1.616E+05
1
1.616E+05
4.59
0.0695
Residual
2.466E+05
7
35232.16
Lack of Fit
39291.71
3
13097.24
0.2527
0.8562
Not significant
Pure Error
2.073E+05
4
51833.35
Cor Total
3.838E+06
16
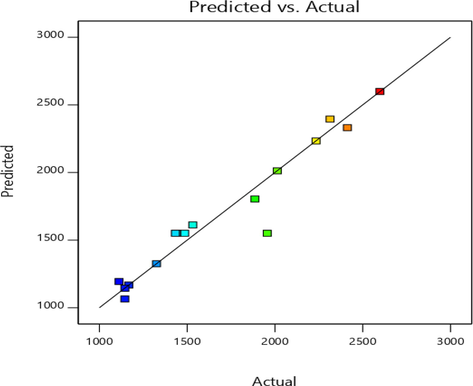
Distribution of experimental/actual and predicated values of xylanase activity by Parity plot.
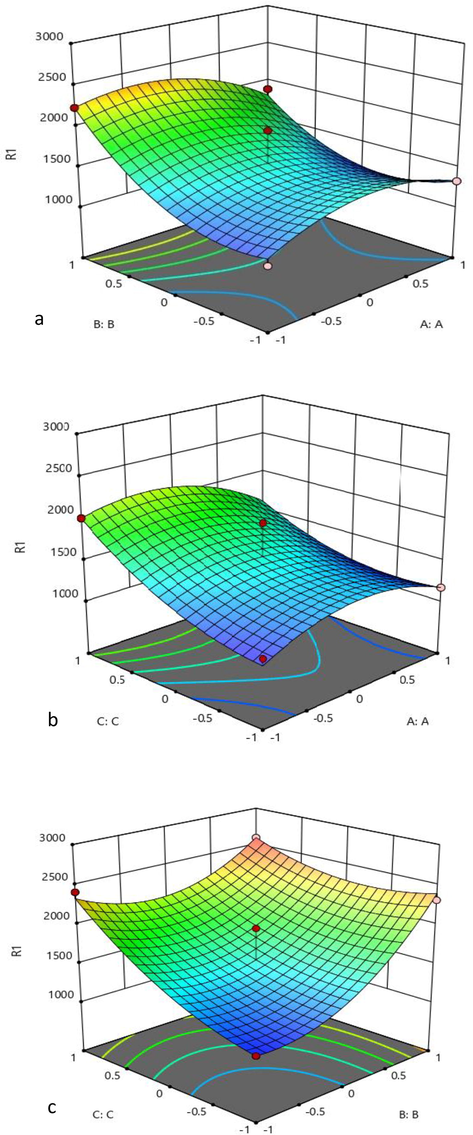
3D plot showing interaction effect between (a) moisture (A:A) and birchwood xylan (B:B), (b) moisture (A:A) and yeast extract (C:C), and (c) birchwood xylan (B:B) and yeast extract (C:C) on xylanase activity (R1).
3.5 Xylanase purification and characterization
Table 5 shows the purification steps and summary of extracellular enzyme. The SDS-PAGE showed molecular mass of 40 kDa (Figure not shown). Xylanase was stable from slight acidic to alkaline conditions for 4 h (Fig. 3a). Xylanase revealed stability till 65 °C with 25.12% of residual activity (Fig. 3b). Xylanase exhibited stability towards various metal ions, showing activities of 93.32 ± 3.3 to 20.22 ± 1.3% (Fig. 3c). Likewise, xylanase depicted stability towards varied solvents with residual activities of 80.16 ± 2.3 to 28.12 ± 1.3% (Fig. 3d).
Purification Step
Protein (mg/g)
Enzyme (U/g)
Specification (U/mg)
Purification fold
Crude
8.5
2598.14
305.6
1
Ammonium sulphate
4.4
1913.16
434.8
1.4
Dialysis
2.2
12,752
579.6
1.9
Ion-exchange chromatography
1.3
984.3
757.2
25
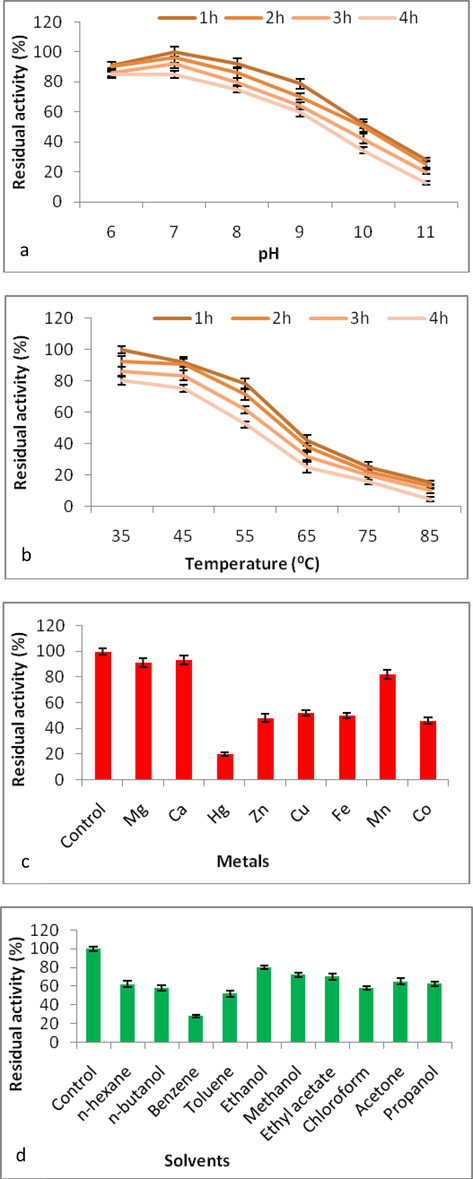
Effect of (a) pH, (b) temperature, (c) various metal ions and (d) various solvents on the stability of xylanase.
3.6 Pre-digestion of feed
Poultry feed were supplemented with purified xylanase of strain LLD06. The purified xylanase showed increment in the total reducing sugar content (88%) with respect to control (Fig. 4).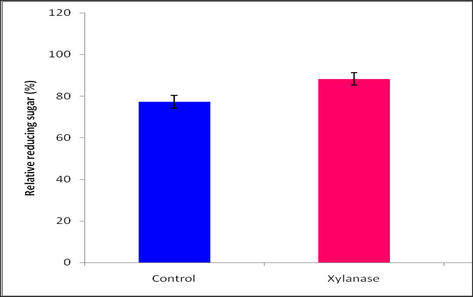
Pre-digestion of poultry feed with purified xylanase on reducing sugar production. Higher reducing sugar was obtained when poultry feed was treated with purified xylanase.
4 Discussion
Bacteria produce plethora of metabolites (enzymes and proteins) during different phases of growth (Khusro et al., 2020). Among those metabolites, xylanases are receiving increasing attentions because of their great importance in various industrial applications (Khusro et al., 2016). In the present investigation, xylanolytic bacteria, namely P. nitroreducens was isolated from P. giganteus faeces.
Bacillus spp. are considered prominent sources of extracellular xylanases (Gupta et al., 2015). Surprisingly, there is lack of report demonstrating xylanase production from P. nitroreducens. Previously, Dhivahar et al. (2019) reported xylanolytic Bacillus spp. from P. giganteus. Likewise, Anand and Sripathi (2004) isolated cellulase and xylanase producing microbes such as Proteus spp., Citrobacter sp., Serratia sp., and Klebsiella sp. from P. giganteus. This investigation indicated the first study on the isolation of P. nitroreducens as xylanolytic strain.
Agro wastes, poultry wastes, and other organic wastes are implied as cheap substrate for producing different types of enzymes (Bhardwaj et al., 2019). In this context, P. giganteus faeces were used for producing extracellular xylanase from strain LLD06. P. giganteus consumes leaves of different groups of plants (Bhat, 1994; Ruby et al., 2000; Elangovan and Marimuthu, 2001) which are mostly composed of polysaccharides rich in cellulose and xylan (Stryer, 1996; Salisbury and Ross, 1985), thereby enhancing increased yield of xylanase production.
Strain LLD06 exhibited optimum production of xylanase at 100% moisture level. Lower moisture content reduced the enzyme activity. This may be because of the reduced swelling of faeces at low moisture level, thus, decreasing xylanase yield. The strain depicted increased xylanase yield in the presence of xylan and yeast extract. Xylan acted as inducer for improving xylanase productivity. On the other hand, nitrogenous components as well as growth factors present in yeast extract played prominent role in the enhanced yield of xylanase (Khusro and Aarti, 2015; Aarti et al., 2018). The isolate revealed increased xylanase production at pH 7.0 and 37 °C. Strain LLD06 exhibited prominent yield of enzyme at 48 h. The reduction in xylanase yield afterwards may be because of the nutritional components depletion in the medium. (Khusro et al., 2016).
The statistical optimization showed enhanced production of xylanase which revealed mutual interconnection between selected parameters. Xylanase was purified using chromatographic techniques which revealed molecular mass of about 40 kDa. Previously, xylanases of molecular mass ranging from 20 to 50 kDa were obtained from Streptomyces sp. (Meryandini et al., 2009), Scytalidium sp. (Kocabaş et al., 2015), and Streptomyces sp. (Ninawe et al., 2008).
In this study, strain LLD06 exhibited significant digestion of feed which can certainly correspond to the growth and performance of the poultry. The degradation of non-starch polysaccharides by xylanase showed huge potentiality in poultry industries because of the increased nutritional aspects for energy (Maheswari and Chandra, 2000).
5 Conclusions
Strain LLD06 showed hyper-xylanase production of 2598.14 U/g using frugivorous P. giganteus faeces as ideal substrate under SSF. Xylanase was purified using chromatographic techniques which revealed molecular mass of approximately 40 kDa. Strain LLD06 digested poultry feed which can certainly play pivotal role towards the improved growth and performance of the poultry.
Conflict of interest
None.
Acknowledgement
Authors acknowledge Department of Plant Biology and Biotechnology, Loyola College, Chennai for the support. Authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/144), King Saud University, Riyadh, Saudi Arabia.
References
- Goat dung as a feedstock for hyper-production of amylase from Glutamicibacter arilaitensis strain ALA4. Bioresour. Bioprocess.. 2017;4
- [CrossRef] [Google Scholar]
- Carboxymethyl cellulase production optimization from Glutamicibacter arilaitensis strain ALA4 and its application in lignocellulosic waste biomass saccharification. Prep. Biochem. Biotechnol.. 2018;48:853-866.
- [Google Scholar]
- Characterization of Staphylococcus aureus isolates from faecal samples of the straw-coloured fruit bat (Eidolon helvum) in Obafemi Awolowo University (OAU), Nigeria. BMC Microbiol.. 2012;12
- [CrossRef] [Google Scholar]
- Isolation and characterization of cellulose-degrading and xylanolytic bacteria from the short-nosed fruit bat Cynopterus sphinx. Acta Chiropterol.. 2012;14:233-239.
- [Google Scholar]
- Digestion of cellulose and xylan by symbiotic bacteria in the intestine of the Indian flying fox (Pteropus giganteus) Comput. Biochem. Physiol. A Mol. Integr. Physiol.. 2004;139A:65-69.
- [Google Scholar]
- A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour. Bioprocess.. 2019;6
- [CrossRef] [Google Scholar]
- Observations on the food and feeding behaviour of Cynopterus sphinx Vahl (Chiroptera, Pteropodidae) at Pune, India. Mammalia. 1994;58:363-370.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Dietary strategies of the Old World fruit bats (Megachiroptera, Pteropodidae): how do they obtain sufficient protein? Mammal. Rev.. 1998;28:185-194.
- [Google Scholar]
- Isolation and characterization of hyper-xylanase producing Bacillus spp. from faeces of the Indian flying fox (Pteropus giganteus) Acta Chiropterol.. 2019;21:229-236.
- [Google Scholar]
- Survey on distribution and conservation status of the Indian flying fox Pteropus giganteus: a bioindicator of wetland ecosystem. Res. J. Chem. Environ. Sci.. 2018;6:75-80.
- [Google Scholar]
- Effect of moonlight on the foraging behaviour of a megachiropteran bat Cynopterus sphinx. J. Zool.. 2001;253:347-350.
- [Google Scholar]
- Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of kraft pulp and deinking of waste paper. Bioprocess. Biosyst. Eng.. 2015;38:947-956.
- [Google Scholar]
- Sources of protein in two species of phytophagous bats in a seasonal dry forest: evidence from stable-isotope analysis. J. Mammal.. 2001;82:352-361.
- [Google Scholar]
- Production and purification of anti-tubercular and anticancer protein from Staphylococcus hominis under mild stress condition of Mentha piperita L. J. Pharm. Biomed. Anal.. 2020;182:113136
- [CrossRef] [Google Scholar]
- Statistical optimization of thermo-alkali stable xylanase production from Bacillus tequilensis strain ARMATI. Electron. J. Biotechnol.. 2016;22:16-25.
- [Google Scholar]
- Molecular identification of newly isolated Bacillus strains from poultry farm and optimization of process parameters for enhanced production of extracellular amylase using OFAT method. Res. J. Microbiol.. 2015;10:393-420.
- [Google Scholar]
- Purification strategies and properties of a low-molecular weight xylanase and its application in agricultural waste biomass hydrolysis. J. Mol. Catal. B-Enzym.. 2015;115:66-75.
- [Google Scholar]
- Hibernation in bats (Mammalia: Chiroptera) did not evolve through positive selection of leptin. Ecol. Evol.. 2018;8:12576-12596.
- [Google Scholar]
- Production and potential applications of a xylanase from a new strain of Streptomyces cuspidosporus. World. J. Microb. Biotechnol.. 2000;16:257-263.
- [Google Scholar]
- Characterization and purification a specific xylanase showing arabinofuranosidase activity from Streptomyces spp.234P-16. Biodiversitas. 2009;9:115-119.
- [Google Scholar]
- Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process. Biochem.. 2012;47:1402-1410.
- [Google Scholar]
- Purification and characterization of extracellular xylanase from Streptomyces cyaneus SN32. Bioresour. Technol.. 2008;99:1252-1258.
- [Google Scholar]
- Chemical composition of fruits and leaves eaten by short nosed fruit bat, Cynopterus sphinx. J. Chem. Ecol.. 2000;26:2825-2841.
- [Google Scholar]
- Plant physiology (3rd ed.). Belmont, CA: Wadsworth Publishing Company; 1985. p. :540.
- Biochemistry (4th ed.). NY: W. H. Freeman and Company; 1996. p. :473-474.







