Translate this page into:
Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology
⁎Corresponding author. zif_4@yahoo.com (Sumera Javad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Present study was aimed to optimize the microwave assisted extraction (MAE) of phytochemicals from Foeniculum vulgare Mill and its comparison with other conventional extraction methods. Biological activities of polar and nonpolar extracts (from MAE, Soxhlet and cold maceration) were also compared. For this purpose, seed powder of Foeniculum vulgare Mill was extracted by Microwave assisted extraction (MAE), Soxhlet extraction (SE) and Cold maceration (CM) using water as a solvent. MAE conditions (power level, time of microwave irradiation and particle size of plant matrix) for maximum phenolic content from F. vulgare, were optimized using central composite design (CCD) of response surface methodology. Thereafter, antioxidant activities and reducing power of all extracts was analyzed and compared. Nonpolar fractions of extracts were analysed with GCMS. Results showed that MAE gave higher extracts from F. vulgare in 4 min as compared to 20 and 24 h of SE and CM respectively. MAE extracted smaller particle size (40 µ) more efficiently while extraction yield of other two techniques was not significantly affected by particle size of seed powder. Optimal extraction conditions were found to be 600 W, 3 min and 80 µm of particle size. Antioxidant potential of MAE extracts was also higher (75.5%), comparable to the standard antioxidants used. Extracts were then fractionated into chloroform and n-hexane fractions and were subjected to GCMS analysis and antibacterial activity. On comparison it was concluded that both the fractions of MAE gave the maximum variety of nonpolar constituents and enhanced antibacterial activity. MAE proved to be a rapid and energy efficient method for plant based extractions for polar as well as non-polar components from F. vulgare without affecting their biological activities. In conventional herbal industry, MAE protocols can replace maceration and SE, to save time, energy and cost as well. This will also reduce the final cost of the medicine at consumer level
Keywords
Antioxidants
Foeniculum vulgare
Medicinal plants
Microwave assisted extraction
Phenolics
Phytochemicals
1 Introduction:
Foeniculum vulgare (fennel) is an aromatic herb native to Mediterranean areas, Asia and Europe belonging to family Umbelliferae and is also included in functional foods (Valussi, 2012). The biochemicals of fennel are utilized for various purposes like in food, alcoholic beverages, cosmetics and pharmacy industry and as flavoring agent in readymade products like candy, ice cream, toothpaste and non-alcoholic beverages (Hammouda et al., 2013). Fennel is also known for its phenolics and antioxidant activity. Phenolic compounds present in plant extracts act as antioxidants which control and ultimately lessen the oxidative damage in human bodies. They reduce inflammation, delay aging and act as anticancer agents. Antioxidants also increase the shelf life of food products as they delay the damage caused by Reactive Oxygen Species (Altemimi et al., 2017; Anwar et al., 2009).
Extraction is the first step for getting herbal product for commercial use. Efficacy of the final products depends upon the selection of extraction method (Azwanida 2015). SE and CM have been in use since a long time to extract phytochemicals due to their ease of use and availability of research data (Altemimi et al., 2017). Scientists and researchers are now showing concerns for these traditional techniques due to the requirement of significant amount of solvents, energy and time (Azwanida, 2015). The known methods of conventional extraction systems utilize extensive extraction time which enhances the exposure; leading to increased solubility of targeted compounds and the product exchange. But these requirements pose a severe harm to the phyto-constituents by thermal degradation. It also results in utilization of more energy and ultimately adding to the cost of final product (Shams et al., 2015).
Therefore some modern methods of extraction like Ultra High Pressure Assisted Extraction, Super Critical Fluid Extraction and Microwave Assisted Extraction have promising features to be future of extraction technology (Maric et al., 2018; Xiong et al., 2016).
MAE has been an innovative approach towards plant based extractions. Microwaves are the part of electromagnetic spectrum with wave length range of 300 MHz to 300 GHz. Microwaves are considered as a good option for the targeted extraction due to their advantages like effective heating inside a closed vessel, lesser or no solvent loss, no escape for metabolites, and shortened time of extraction. Yield of plant metabolites can be further optimized by increasing pressure and decreasing temperature inside the vessel for heat sensitive compounds (Kosar et al., 2007).
Present study was aimed to analyze the efficacy of Microwave assisted extraction for rapid recovery of phenolics, antioxidants and non-polar components from seeds of F. vulgare. Secondly, efficiency of MAE was also compared with conventional extraction methods i.e., Soxhlet extraction and cold maceration. Thirdly, comparison was made on basis of amount of extract, phenolic content, antioxidant activity, GCMS analysis and antibacterial activity. These results may depict the future of microwaves extraction in herbal industry and pharmaceutical research related to the extraction of F. vulgare at commercial level to save raw material, time, energies and cost.
2 Materials and methods
Seeds of a local variety of F. vulgare (sweet fennel) were taken from NARC (National Agricultural Research Institute) Islamabad, Pakistan. These seeds were made into fine powder with the help of electric grinder and divided into different particle sizes by using sieves of different sizes (40, 80 and 120 µ).
2.1 Microwave assisted extraction (MAE)
For MAE, 10 g of plant material was added with 50 mL of distilled water to each vial every time. The vials were loaded into Teflon sample vials of Microwave assisted extractor (MDS-6G). Distilled water was used as a standard for temperature and pressure sensing of reaction vessel. For the optimization of MAE, the selected parameters were, power level (200 W, 600 W and 1000 W), time used for microwave irradiation (1, 2, 3, 4 and 5 min) and particle size (40, 80 and 120 µ). A Buchner funnel lined with Whatman #1 filter paper was used to filter extract. Filtrate was dried in rotary evaporator, collected in labelled vials and was stored for further analysis.
2.2 Soxhlet extraction (SE)
Soxhlet apparatus was used for the extraction of powdered plant material. 20 g of seed powder was taken each time in thimble. Parameters optimized for soxhlet extraction were time (2, 8, 16 and 24 h) and particle size. Each time 250 mL of water was used as solvent. For each time of extraction the extract was separated as mentioned in MAE Section 1.1.
2.3 Cold maceration (CM)
Cold maceration of seed powder of F. vulgare was done at room temperature using water as a solvent. For this purpose solvent amount was kept constant (250 mL). Each time 20 g of seed powder was soaked in solvent, covered with lid (to avoid evaporation) and placed at room temperature on shaker (30 rpm) for 2, 8, 16 and 24 h. For each time of extraction the extract was separated as mentioned in MAE Section 1.1.
2.4 Total phenolic content
To estimate the phenolics, Spectrophotometric assay was used. 1 mg of aqueous plant extract was well mixed with 1 mL of FC (Folin and Ciocalteu’s) reagent, 1 mL of saturated sodium carbonate was added to the prepared mixture after 5 min and distilled water was used to adjust the total volume up to 10 mL. After 90 min in dark, absorbance was checked at 725 nm. Gallic acid was used as a standard phenolic to construct standard curve. The values of phenolics were expressed as µg of Gallic acid equivalent per mg of extract.
2.5 Response surface methodology (RSM)
Design Expert software (Version 11) was used to apply RSM. CCD (Central composite design) was used which generated a design with a set of 20 experimental runs. Three independent variables i.e., power level (A), time of irradiation (B) and matrix size (C) at their low, medium and high levels were selected to study their combined effect on phenolic content (dependent variables), to optimize the MAE conditions. A quadratic polynomial regression model was contrived to explain the relationship between dependent and independent variables. Where predicted response was estimated by using the relation
2.6 Antioxidant activity and reducing potential of extracts:
Antioxidant assay and reducing potential was measured for MAE, SE and CM extract of F. vulgare with maximum amount of phenolics. For antioxidant activity assay, 1 mL of seed extract (1 mg/mL) was mixed with 1 mL of 1 mM 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) solution. The mixture was incubated for half an hour at 37 °C. Reaction mixtures were checked for absorbance at 517 nm. The percentage of DPPH inhibition was evaluated using eq. where Ac is the absorption of water + DPPH, As is the absorption of sample + DPPH. Seed powder extract with maximum phenolics were selected for the study of reducing power. Range of concentrations i.e., 50–250 µg were mixed with 1 mL of water, 2.5 mL of phosphate buffer (pH 6.6 and 0.2 M) and 2.5 mL of 1% potassium ferricyanide separately. Mixture was incubated for 25 min at 50° C. 10% trichloro-acetic acid (2.5 mL) was added then and centrifuged at 3000 rpm for 15 min. Supernatant was removed and mixed with 2.5 mL of double distilled water and 0.5 mL iron chloride (0.1%). Absorbance of each solution was then measured at 700 nm. There is a direct relationship between absorbance and reducing power of extract.
2.7 GCMS for non-polar components
Three maximum yielding extracts from each extraction methods were selected for fractionation separately. Extracts were mixed in water. According to the polarity differences they were fractionated with n-hexane and chloroform. GCMS analysis was done to check the presence of non-polar components in the plant extract. Helium was the carrier gas with flow rate of 1 mL\min. The temperature of injector was set at 250 °C. The temperature of the GC column was fixed at 40 °C for starting 5 min, then elevated to 140 °C at the rate of 5 °C per minute and these conditions were set to be maintained for further 5 min. Then temperature was increased to 280 °C (9 °C per min) and maintained for 5 min. Detector temperature was fixed at 245 °C. Mass spectrometer detector was used at a potential of 70 eV (Hammouda et al., 2013). The nonpolar components of chloroform and n-hexane fractions of MAE, SE and SM extracts were studied with their GC retention times, mass spectra, retention indices, comparison with literature and library search of mass spectra (The National Institute of Standards and Technology NIST).
2.8 Antibacterial assay
Antibacterial assay of non-polar components was carried out and 6 pathogenic bacterial strains were used namely Staphylococcus aureus, Klebciella pneumonia (K92), Eshercia coli (E909), Pseudomonas aeruginosa (P23), Bacillus cereus (B33), Brucella melitensis (Bm42). Sterilized Mueller & Hinton Agar medium was poured into petri-plates and 100 μL of bacterial inoculum was spread. Wells of 4 mm diameter were pierced and were filled with standard antibiotic (gentamycin) as a positive control, solvent as a negative control, n-hexane and chloroform fractions of MAE, CM extract and SE sample. The plates were incubated at 37 °C for 24 h. Inhibition zones were measured in millimeters.
2.9 Statistics
SPSS software was used to analyze the data statistically by comparing the means with one way ANOVA (Analysis of variance) and significance of results was checked at 5% level of significance by applying Duncans New Multiple Range test. Furthermore, Design Expert software, version 11 was used for applying RSM.
3 Results and discussion
3.1 Comparison of MAE, SE and CM
MAE is reported to be an efficient extraction method as compared to conventional methods for a number of plants. Time and power levels are the two main parameters for MAE of plant metabolites. Adequate time of exposure to microwaves is requirement for the good extraction yield due to higher contact of plant matrix and solvent, softening of plant tissue and weakening of cell wall integrity (Liu et al., 2016). Effect of time, power and particle size on MAE is shown in Fig. 1(a–c). Maximum amount of extract was recorded as 149.35 mg/g of plant material after 4 min of MAE at 1000 W and 40 µ particle size. Results indicated that on increasing the time of extraction and power level of microwaves amount of extract increased. But on exceeding the exposure to microwaves at 4 min, amount of extract started to decrease at 1000 W. Earlier (Calinescu et al., 2017) reported higher extraction efficiency of microwaves for fennel as it took only 10 min to extract same amount of essential oils as 150 min of traditional hydro-distillation technique. (Hammouda et al., 2011) also reported higher yield of oil from fennel with 28% fenchone and 72% anethole while using MAE. Other researchers have also showed the higher efficiency of microwaves for plant based metabolites (Liazid et al., 2011; Hayat et al., 2009). Optimum combination of time and power level can enhance the extraction of targeted molecules in MAE. Higher power levels cause sudden rupture of cells which may be more suitable to get a targeted compound. Moisture present inside the cells absorbs the microwaves which causes the internal heating of the cell environment creating extra pressure on the cell wall. This in turn bursts to release the exudates earlier as compared to conventional methods of heating which rely on the surface heating only (Akhtar et al. 2019). Particle size of plant matrix is another parameter which needs to be optimized for the rapid recovery of phyto-constituents from plants. Smaller is the particle size, greater is the surface area. Greater surface area enhance the contact between the plant surface and microwaves, thus increasing the chance of penetration of the microwaves into the cells and causing more plant exudates to come out of the cell (Huie, 2002).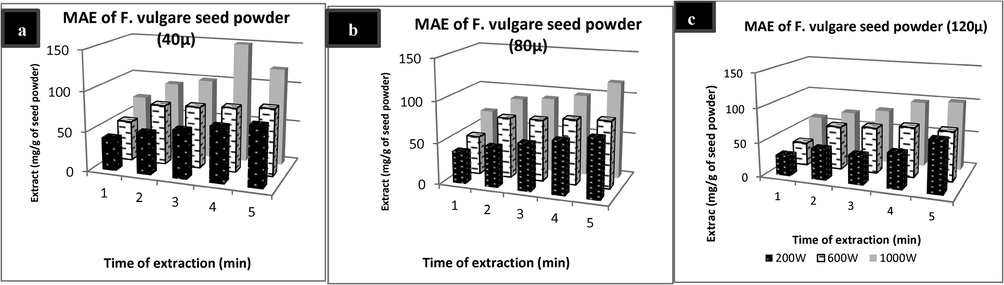
a) MAE of 40 µ seed powder of F. vulgare; b) MAE of 80 µ seed powder of F. vulgare; c) MAE of 120 µ seed powder of F. vulgare.
Effect of time on SE and CM of the seeds of F. vulgare is shown in Fig. 2(a-b). The maximum amount of extract was obtained as 83.05 mg/g and 53.41 mg/g after 16 and 24 h of SE and CM respectively. It was significantly lesser than the 4 min extract of MAE. Low temperature during CM decreases the kinetics of reaction mixture. On comparison of these results with MAE, it becomes clear that microwaves are more efficient to give per g extract of plant material in smart timing of extraction. MAE has proved its simplicity of work, cost effectiveness; reduced power and energy consumptions for the extraction of plant metabolites (Zheng et al., 2009).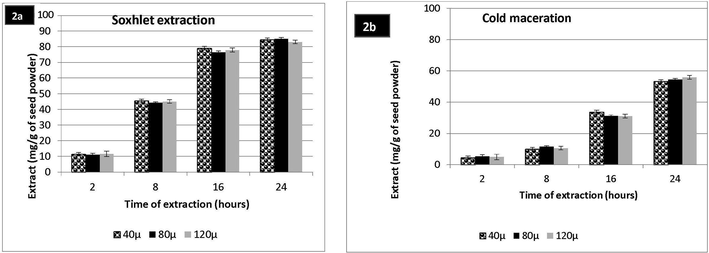
(a): Soxhlet extraction of F. vulgare seed powder (b): Cold Maceration of F. vulgare seed powder.
3.2 Phenolic content
Three main factors namely power level, time of irradiation and particle size of plant material were selected to be studied by response surface methodology by using CCD. RSM is basically a method of optimization of different variable conditions. The second order regression model was carried on coded values of three independent variables with 6 axial and central points and with 20 runs in design (Table 1). Response for the phenolic content is represented in the equation below
Where A is power level in Watts, B is Time in minutes, C is plant particle size and Y is response i.e., phenolic content.
Run order
A
B
C
Actual Y
Predicted Y
1
800
2
40
141.00
123.82
2
600
3
80
240.00
244.52
3
800
4
120
190.00
175.61
4
600
1.318
80
132.00
152.48
5
600
3
147.272
5.00
22.17
6
800
2
120
121.00
112.15
7
263.641
3
80
150.00
163.55
8
600
4.68
80
220.00
228.09
9
600
3
80
240.00
244.52
10
400
2
40
128.00
122.19
11
936.359
3
80
195.00
210.02
12
600
3
80
245.00
244.52
13
600
3
12.72
43.00
54.41
14
600
3
80
250.00
244.52
15
400
4
120
125.00
121.97
16
400
4
40
160.00
148.65
17
400
2
120
99.00
80.02
18
800
4
40
173.00
171.78
19
600
3
80
245.00
244.52
20
600
3
80
252.00
244.52
Fisher’s test was applied and p values were calculated to establish the statistical significance of model. The F value of model was 40.09 which indicate that the model is significant. To evaluate the coefficients, p values were studied whose values if lesser than 0.05, showed the significance of that factor or combination of factors for the yield of dependent factor. Analysis of variance results showed that the linear coefficient for A, B and C; interactive coefficient AC and quadratic coefficients for A2, B2, C2 were significant, thus indicating that phenolic content yield from F. vulgare is significantly influenced by the above said factors. Furthermore value of R2 (0.973) designates the fitness of the model. The predicted R2 value of 0.8004 is also in reasonable adjustment with the adjusted R2 value (0.9488). Value of Adequate precision is 19.6483 which is a ratio of signal to noise, which suggests that this model can be used to navigate the design space. The CV % also suggests good reproducibility.
3D graphs were used to study the effect of each factor on response i.e., phenolic content of F. vulgare seeds (Fig. 3). Power level of microwaves, irradiation time and particle size of plant material has a significant effect on the yield of phenolics as explained in each graph. Each graph is representative of effect of 2 independent factors on the response of dependent variable and its maximum response. Optimal conditions were found to be power level as 600 W, time as 3 min and particle size as 80 µm. Furthermore values were closer to predicted values so model is reliable (Table 1). The independent variables i.e., power level, time of extraction and matrix particle size had a significant effect on recovery of phenolics from fennel seed powder.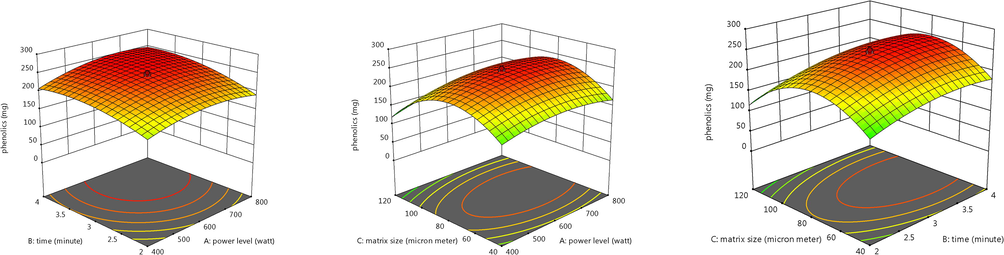
The 3D response plots showing the interactive effects of power level of microwaves (A), time of microwave irradiation (B) and particle size of plant matrix (C) on the yield of phenolics from F. vulgrare.
Comparison of phenolic content of MAE, SE and CM Also showed the efficiency of MAE (Fig. 4a). It clearly shows that for MAE of all particle sizes of fennel seed powder, 4 min was the optimum time of phenolic extraction. While for SE and CM, 16 and 24 h were required to get maximum phenolics. Furthermore, 225.35 µg equivalent of phenolic content was calculated for optimized MAE sample of fennel while 154.43 and 58.76 of phenolic content were extracted at maximum by SE and CM respectively. Earlier Liazid et al. (2007) reported stability of phenolics under microwave heating. Biesaga, 2011 compared MAE, CM, reflux heating and sonication for extraction of flavonoids. He concluded that MAE and reflux heating had least effects on stability of flavonoids but time required for MAE was significantly lesser.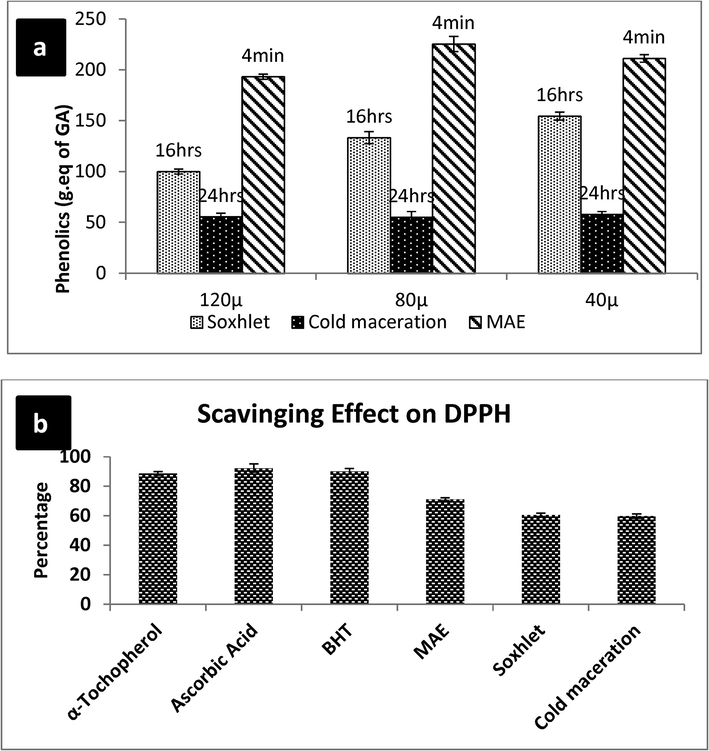
a) Comparison of efficiency of MAE, SE, CM for extraction of phenolics b) antioxidant studies of different extraction methods and their comparison with standard antioxidants.
3.3 Antioxidant activity
DPPH is a free radical and it is easily reduced by accepting an electron or hydrogen ion and forms a diamagnetic molecule (Soaras et al., 1997). Degree of reduction of DPPH radicals is determined from the decrease in the absorbance values of the reaction mixtures with plant extracts or any other anti-oxidants. MAE, SE and CM extracts of F. vulgare with maximum phenolic content were used to find the effect of extraction method on the antioxidant activity of extracts. Results of DPPH assay of extracts of F. vulgare are shown in Fig. 4b. Out of three extraction techniques used, MAE extract gave highest antioxidant activity (75.5%) which is also comparable to the standard antioxidants. Burkhard et al. (2015) reported higher distillation time requirement for higher antioxidant activity of fennel extracts. Lovric et al. (2017) also reported higher antioxidant capacity of Blackthorn flowers using ethanol and MAE.
3.4 GC–MS analysis
The major chemical constituents of n-hexane and chloroform fraction of MAE and soxhlet extract of F. vulgare are given in (Fig. 5a-d). GC–MS analysis of n-hexane fraction of MAE identified fifteen (15) compounds. The major constituents were identified as α-pinene, β-pinene, α-fenchene and terpinene-4-ol, fenchone, Anethol, Elamicin, Dilapiol, Apiol, z-Liguistelide, Falcarinol and fernecyl acetone. While hexane fraction of CM showed (9) compounds and that SE showed (11) compounds (Table 2). Results clearly showed that MAE is more effective towards extraction of non-polar components from fennel as well. Non-polar components are usually volatile components which are evaporated easily or degraded under the effect of heat. MAE is a solution to both problems with closed container action and controlled temperature option without boiling of the solvent. In conventional methods time of extraction directly affects the quality of oils obtained particularly compounds with low boiling points suffer more (Moser et al., 2014; Zheljazkov et al., 2013). Time is shortened in MAE which avoids the degradation of main components.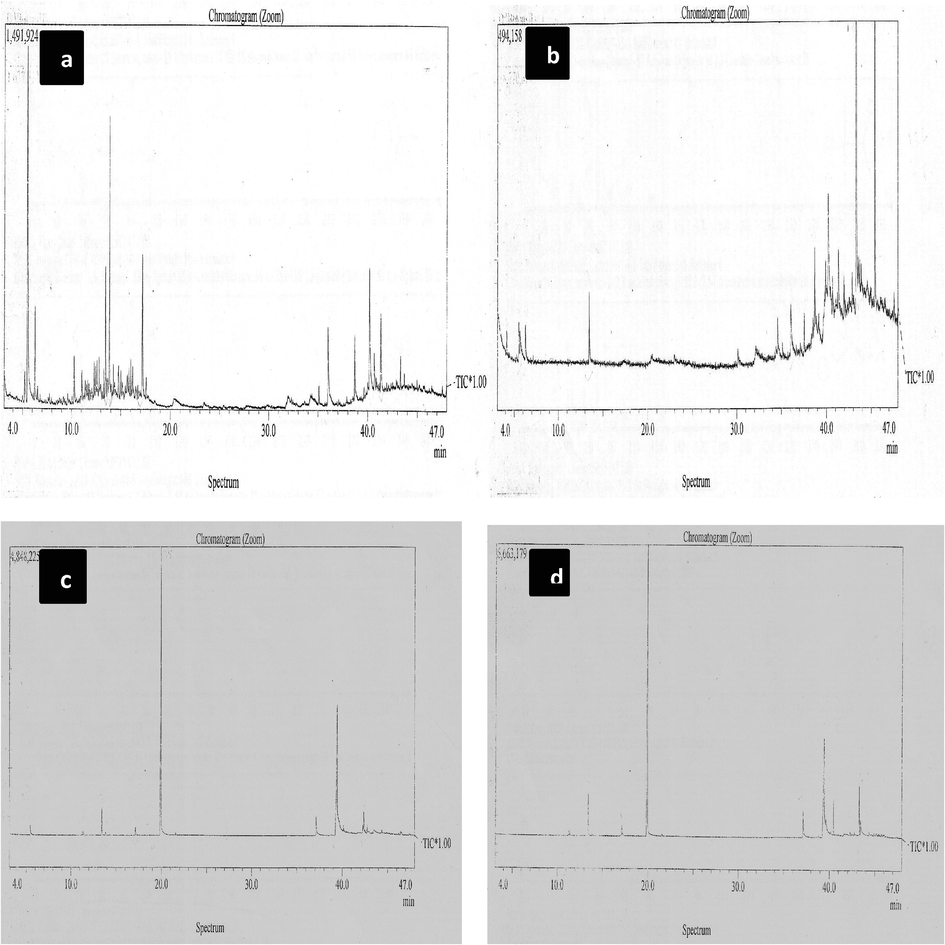
Mass spectra of F. vulgare a) n-hexane fraction of MAE b) chloroform fraction of MAE c) n-hexane fraction of SE d) chloroform fraction of SE.
RT
ERI
RRI
Compound
Mode of Identification
Percentage of extract in
MAE fraction
Cold macerated fraction
Soxhlet extract fraction
n-hexane
Chloroform
n-hexane
Chloroform
n-hexane
Chloroform
4.150
907
902
Heptanal
MS Detector, RI
–
1.80
–
–
–
–
5.217
924
926
α-Pinene
MS Detector, RI, Standard
2.00
–
–
–
–
–
5.450
942
943
α-Fenchene
MS Detector, RI, Standard
22.69
2.70
1.50
Tr
Tr
–
6.283
982
982
β-Pinene
MS Detector, RI, Standard
5.81
2.70
–
–
–
–
10.275
1087
1078
Fenchone
MS Detector, RI, Standard
3.43
–
–
–
–
–
11.058
1107
1108
2,4-Hepta dienal
MS Detector, RI
2.11
–
–
–
–
–
11.345
1112
1114
Mentha 1,3,8-triene
MS Detector, RI
11.10
–
Tr
Tr
–
2.10
13.45
1161
1161
2-nonenal
MS Detector, RI
7.90
6.40
–
–
–
–
13.852
1182
1182
Terpinene-4-ol
MS Detector, RI
19.3
–
6.30
7.80
Tr
Tr
17.175
1250
1255
Anethol
MS Detector, RI
8.90
–
1.50
1.50
0.5
0.40
19.925
1306
1307
Vinyl guaiacol
MS Detector, RI
–
–
57.1
53.1
68
71.09
30.332
1557
1557
Elamicin
MS Detector, RI
–
1.38
–
–
–
–
36.008
1647
1645
Dill apiol
MS Detector, RI
4.70
3.70
–
–
–
–
36.003
1683
1682
Apiol
MS Detector, RI
–
4.60
–
–
Tr
0.90
37.525
1722
1719
Sedanenolide
MS Detector, RI
–
3.70
3.10
4.60
–
–
38.683
1753
1750
z-Liguistelide
MS Detector, RI
4.70
9.20
23.8
17.2
12.1
9.78
40.158
1788
1783
e-Liguistelide
MS Detector, RI
7.90
11.98
1.50
6.20
10.9
8.12
42.255
1853
1855
Fernesyle acetone
MS Detector, RI
2.11
3.60
3.10
–
1.5
1.11
43.308
1879
1890
Methyl hexadeconate
MS Detector, RI
0.05
1.80
1.50
9.30
3.2
2.29
45.408
1940
1940
Hexadecanoic acid
MS Detector, RI
–
25.8
–
–
1.11
1.54
47.515
2011
2037
Falcarinol
MS Detector, RI
1.1
2.80
–
–
0.5
0.30
Total compounds identified
15
14
09
09
11
11
%age of Monoterpenoid
63.23
8.58
9.30
9.30
0.50
0.4
3.5 Antibacterial assay
The antibacterial activity of the nonpolar components of F. vulgare extracts were evaluated against six pathogenic bacterial strains. The results of antibacterial activity are summarized in Table 3. In terms of clear area of inhibition (AOI), the n-hexane fraction of MAE sample exhibited significant antibacterial activity against all strains (14.4–20.8 mm). Least AOI for n-hexane fraction of MAE sample was recorded for P. aeruginosa. AOI recorded for MAE fractions were significantly higher as compared to fractions of all other extraction methods and even comparable to standard antibiotic used. Chloroform fraction of all extracts showed lesser antibacterial activity over all. Therefore, it is obvious and clear from the study that MAE fraction had more number and quantity of bioactive compounds without any loss of their bioactivity
Extracts by
Inhibition zones in mm against
S. aureus
E. coli
P. aeruginosa
B. cereus
B. melitensis
K. pneumonia
MAE
n-hexane fraction
20.8a ± 0.8
14.4b ± 0.5
5.53b ± 0.5
19.5b ± 0.5
20.4a ± 0.5
20.3b ± 0.6
Chloroform fraction
16.4c ± 0.7
13.8b ± 1.4
5.00b ± 1.1
11.3c ± 1.0
0.00d ± 00
5.60f ± 0.3
Cold maceration
n-hexane fraction
13.8de ± 0.3
8.40c ± 0.5
0.00c ± 00
9.6 cd ± 0.7
3.90c ± 0.4
7.40e ± 0.4
Chloroform fraction
13.0e ± 0.9
7.90c ± 0.5
0.00c ± 00
8.8 cd ± 0.4
0.00d ± 00
5.50f ± 0.5
Soxhlet extraction
n-hexane fraction
14.8 cd ± 0.8
8.50c ± 0.6
0.00c ± 00
9.9 cd ± 1.0
7.90b ± 0.36
11.4c ± 0.51
Chloroform fraction
13.8de ± 1.0
7.90c ± 0.2
0.70c ± 0.1
8.30d ± 0.5
0.00d ± 00
9.60d ± 0.6
Gentamycin
—
24.8a ± 1.4
23.9a ± 2.0
23.5a ± 1.0
24.5a ± 2.13
20.3a ± 0.6
24.9a ± 0.1
4 Conclusion
Present study suggested statistically significant advantages of MAE for extraction of polar and nonpolar components from fennel seed powder as compared to the conventional methods of extraction. MAE gave higher extract in lesser time. MAE also gave the higher phenolic content in comparison to SE and CM which suggest that it does not degrade the phenolics due to lesser but effective exposure time. Plant matrix size, time of exposure and power level has an interactive effect on final phenolic content. MAE extracts also gave 75.5% antioxidant activity in contrast to SE (61%) and CM (58.8%). GCMS analysis also shows higher extraction efficiency of MAE with 16 compounds and higher antibacterial activity showing no loss to the bioactivity of extracts due to microwave heating. It takes lesser time, uses lesser energy and has strong penetration force into the plant matrix to get more extract and antioxidants from fennel seed powder. It can reduce the cost of production too on the basis of above said parameters. This can finally increase the quality of final product as well as can reduce the product price at consumer level. This method may be suggested to the herbal industry for extraction of fennel oil as well as other pharmaceutically important plant components like phenolics.
Acknowledgements
Research work was done under the research project of Higher Education Pakistan, NRPU program, funding number 20-3545/NRPU/R&D/HEC/14/883.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Microwave assisted extraction of phytochemicals an efficient and modern approach for botanicals and pharmaceuticals. Review. Pak. J. Pharm. Sci.. 2019;32(1):223-230.
- [Google Scholar]
- Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(42):1-23.
- [Google Scholar]
- Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. J. Flav. Frag.. 2009;24:170-176.
- [Google Scholar]
- A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Arom. Plants. 2015;4(3):1-6.
- [Google Scholar]
- Influence of extraction methods on stability of flavonoids. J Chromatogr. A. 1218(18), 2505-2512.. 2011;1218(18):2505-2512.
- [Google Scholar]
- Method for attaining fennel (Foeniculum vulgare Mill.) seed oil fractions with different composition and antioxidant capacity. J. Appl. Res. Med. Arom. Plants. 2015
- [CrossRef] [Google Scholar]
- Integrating microwave assisted extraction of essential oils and polyphenols from rosemary and thyme leaves. Chem. Eng. Commun.. 2017;204(8):965-973.
- [Google Scholar]
- Evaluation of the Essential oil of Foeniculum vulgare Mill (Fennel) fruits extracted by three different extraction methods by GC/MS. Afr. J. Trad. Comp. Alt. Med. 2013;11:277-279.
- [Google Scholar]
- Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules. 2011;16:1366-1377.
- [Google Scholar]
- Optimized microwave-assisted extraction of phenolic acids from Citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol.. 2009;70:63-70.
- [Google Scholar]
- A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Int. J. Sci. Res. Manag.. 2002;1–2:23-30.
- [Google Scholar]
- Comparison of microwave-assisted hydrodistillation and hydrodistillation methods for the fruit essential oils of Foeniculum vulgare. J. Essen. Oil Res.. 2007;19(5):426-429.
- [Google Scholar]
- Integrated traditional Chinese and Western medicine for menopausal syndrome: meta-analysis of randomized controlled trials. Afr. J. Trad. Comp. Alt. Med.. 2016;13(1):157-168.
- [Google Scholar]
- Microwave assisted extraction of anthocyanins from grape skins. Food Chem.. 2011;124(3):1238-1243.
- [Google Scholar]
- Effect of Microwave assiated extraction on the phenolic compounds and antioxidant capacity of blackthorn flowers. Food Technol. Biotechnol.. 2017;55:243-250.
- [Google Scholar]
- An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol.. 2018;76:28-37.
- [Google Scholar]
- Method for obtaining three products with different properties from fennel (Foeniculum vulgare) seed. Ind. Crops Prod.. 2014;60:335-342.
- [Google Scholar]
- Green extraction techniques: effect of extraction method on lipid contents of three medicinal plants of Apiaceae. J. Chem. Pharm. Res.. 2015;7:1080-1088.
- [Google Scholar]
- Antioxidant activity of some extracts of Thymus zygis. Free Radical Res.. 1997;26:469-478.
- [Google Scholar]
- Functional foods with digestion enhancing properties. Int. J. Food Sci. Nut.. 2012;63:82-89.
- [Google Scholar]
- Optimization of microwave-assisted extraction of bioactive alkaloids from lotus plumule using response surface methodology. J. Pharm. Ana.. 2016;6:382-388.
- [Google Scholar]
- Distillation time modifies essential oil yield, composition, and antioxidant capacity of fennel (Foeniculum vulgare Mill) J. Oleo Sci... 2013;62(9):665-672.
- [Google Scholar]
- Application of response surface methodology to optimize microwave-assisted extraction of silymarin from milk thistle seeds. Sep. Purif. Technol.. 2009;70:34-40.
- [Google Scholar]







