Translate this page into:
Probiotic potential of Lactobacillus agilis against oxidative, inflammatory and diabetic stresses
⁎Corresponding author. humaira.yasmin@comsats.edu.pk (Humaira Yasmin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nutrients lacking diet is responsible for major health problems like obesity, heart diseases, cancer, diabetes and inflammation. Microbial nutraceuticals can be the best alternative to resolve the drawbacks related to the phytochemical-based production of nutraceuticals. In vivo study was designed using mice to assess the anti-diabetic, antioxidant and anti-inflammatory potential of Lactobacillus agilis (L. agilis). Mice were put in control, toxic, standard and L. agilis dose-treated groups. The diabetics, oxidative stress and inflammation (in paw) were induced in mice. In alloxan-treated mice, the blood sugar level was elevated to 600 mg/dL and then it was decreased to 190 mg/dL after the L. agilis dose. Weight gain was increased from 51.48% to 68.56% in groups of diabetic and oxidatively stressed mice respectively as compared to the probiotic (L. agilis dose) group (25.99%) and standard drug-treated mice group (29.35%). The effect of L. agilis dose treatment on the alloxan-induced liver injury presented normal histology of hepatic cells with a well-preserved nucleus, cytoplasm, and hepatocytes in L. agilis dose group of mice. Antioxidant enzymes in L. agilis treated mice group were significantly improved as compared to alloxan treated mice with values 22.1 ± 0.18 μg/mg (super oxide dismutase), 8.9 ± 0.12 μg/mg (catalase), 4.1 ± 0.12 μg/mg (glutathione) and 20.8 ± 0.4 μg/mg (SOD), 7.2 ± 0.1 μg/mg (CAT), 3.7 ± 0.14 μg/mg (GSH) respectively. Paw size (thickness) of the treated mice was significantly reduced at T4 in mice group (L. agilis 1 mL dose) with value 2.1 ± 0.12 cm. Current in vivo study presented probiotic potential of L. agilis that can be used as nutraceutical.
Keywords
Lactobacillus agilis
Mouse Modules
Stresses
Probiotic
1 Introduction
The uncontrolled increase in random blood sugar and ROS (reactive oxygen species) may lead to diabetes Type 2, which causes cell dysfunction and insulin resistance (Asmat, Abad & Ismail, 2016). Free radicals, also known as reactive oxygen species (ROS), are a class of highly reactive molecules that comprise free radicals of oxygen (O2), hydroxyl (OH–), hydrogen peroxide (H2O2), and singlet oxygen (O1/2), which are often produced as consequences of biological reactions or from external sources (Shahid et al., 2014). It has been accepted with time that oxidative stress is a risky element for chronic diseases, and it has earlier been demonstrated to be inextricably linked to obesity (Shahid et al., 2014). Lactic acid bacteria can actively improve the nutritional and functional value inside gut that limit growth of pathogenic bacterial strains as well as reduce their toxin secretion capabilities (Bartkiene et al., 2018). Literature reported the probiotic usage of LAB in vaginal and gastrointestinal tract during animal infections as well (Pineiro and Stanton, 2007).
Inhibition of alpha-glucosidase activity by lactobacillus species have been found effective in regulation of glucose level (Hernández et al., 2021). Bacteriocins are the potent protein molecules with antagonistic potential that limit bacterial growth. Moreover, multiple studies have demonstrated that probiotics can be utilized as antibacterial agents as a replacement for of antibiotics to decrease antibiotic resistance in pathogenic Gram-positive and Gram-negative bacterial isolates like S. aureus, E. coli and P. aeruginosa (Sharafi et al., 2013). Through a variety of pathways, LAB aids in the induction of an immune response as well as the reduction/inhibition of infections. The study aimed to assess the antioxidant, anti-inflammatory, and anti-diabetic properties of L. agilis in disease-induced mice as novel work and validation of its biotherapeutic potential alternative to pharmaceuticals.
2 Materials and method
2.1 Bioassay on diseased induce mice
Lactobacillus. agilis NMCC-15 (MK614016) samples were used as a probiotic dose as already reported in our previous study (Khan et al., 2021). Laboratory mice were obtained from National Institute of Health (NIH) Islamabad and the studies were conducted in NIH and National Agricultural Research Center (NARC) Islamabad. Ethical approval for the current study was taken from the National Agricultural Research Center (NARC, Islamabad, Pakistan) Ethical and Biosafety Committee under IBC-NARC 2020–1 (Approval No.) dated 15–12-2021. Entire approved protocols of National Agricultural Research Center (NARC, Islamabad, Pakistan) Ethical and Biosafety Committee were used in experiments. The standard protocols and guidelines of Animal Research: Reporting of in vivo Experiments (ARRIVE) was followed in animal research model experiments. Diseases were induced in all the toxic control group of mice. Both the tested and control group were provided same nutrients and proper living condition in animal house of NIH Islamabad. The temperature of 21 ± 2 °C and 12 h. Light-dark cycling were provided to all the groups. The mice had free access to standard food and distill water and were grouped as shown in Table 1.
Group
Antidiabetic
Anti-oxidant
Anti-inflammatory
A
Normal control group
Normal control group
Normal control group
B
Alloxan treated group (toxic control)
Alloxan treated group (toxic control)
Formalin treated group
C
Alloxan + glibenclamide treated group (reference drug)
–
Formalin + diclofenac sodium treated group (reference drug)
D
Alloxan + L. agilis Dose (tested sample)
Alloxan + L. agilis Dose (tested sample)
Formalin + L. agilis Dose (tested sample)
E
–
Alloxan + ascorbic acid group (reference drug)
–
2.1.1 In vivo antidiabetic assay
Glucose level of each group was examined prior to treatment. Diabetes was induced in overnight fasted mice (12 h) through 120 mg/kg alloxan monohydrate intra-peritoneal injections that was mixed in 0.9 % w/v cold normal saline (Fireman et al., 2012). Measurement of fasting blood glucose level was done after 72 h of injection. Animals with less than 200 mg/dL glucose value were excluded from the current study, until the state of the diabetic was confirmed by means of the glucose dehydrogenase technique i.e Accu-Chek glucometer (Roche Diagnostics, Germany), once before the injection of alloxan. Mice with glucose level exceeding 300 mg/dL were considered diabetic. As shown in Table 3.1, each group contains 5 mice having average weight of 30 g and average age 2 months. Group A indicate normal control mice that were orally fed with 1 mL distill water through feeding tube. The Group B include alloxan injected diabetic mice that every morning fed on 1 mL distill water while Group C include alloxan injected diabetic positive mice treated every morning with glibenclamide; a reference drug (5 mg/kg a day). In group D diabetic mice, the tested bacterial dose (1 mL) was used to administered orally each morning. These in vivo treatments carried out for two consecutive weeks.
2.1.2 Blood sugar assessment
Prior to blood collection; Alloxan were injected into the mice on day (0), days 1, 3, 5, 7, and 14 subsequently. Blood sugar analysis was carried out from the blood samples of tail vein of overnight fasted mice (12 h). After successful blood collection from the tail vein, the first drop of blood was discarded while the second drop of blood was fall down on Accu-Check glucometer strips for glucose (Roche Diagnostics, Germany) and reading was noted. Each time mice tail was then cleaned with ethanol to avoid infection and similarly body weight of the mice was also measured.
2.1.3 In vivo antioxidant activity
Oxidative stress in form of free radical [ROS] was also generated in mice of group B, D and E along with alloxan induced diabetics. After 72 h of diabetic’s induction in Group E, standard antioxidant drug ascorbic acid (5 mg/kg/day) was orally administered. Blood samples from Group A, B, D and E was taken out via injection and collected in anticoagulant containing tubes from the retro-orbital venous plexus of the animals. Immediately after the blood collected (within 20 min), the sera were centrifuged for 10 min at 3500 rpm at 4 °C and the concentration of antioxidative enzymes like CAT (catalase) by ready-to-use colorimetric catalase activity microplate kit, SOD (superoxide dismutase) by the cayman chemical SOD assay kit and the contents of GSH (Glutathione reductase) by glutathione reductase assay kit (ab83461) in supernatants were examined.
2.1.4 Examination of diseased induced mice
After the induction of diabetes in mice via alloxan, each day the mice groups were examined for weight gain, specific growth rate, feed conversion ratio and feed conversion efficiency. Later, on 15th day, all the mice groups were sacrificed for the examination of complete blood count (CBC) and histopathology. Blood samples from each group were collected in labeled EDTA containing anticoagulant purple cap vacutainer tubes and vacutainer tubes were kept at 2 °C. Complete blood count for each group were analyzed using blood analyzers and H&E staining to assess histological changes in stomach, hepatic, and renal tissues of the sacrificed mice. Pathological changes in the tissues were observed by fixing the samples in formalin (10 %) solution and paraffin-embedded blocks. The samples were cut into thick sections of 5 µm using a microtome (Model: Leica® RM2135) and stained with H&E solution according to the published data of Shal et al. (2020). The slides were examined under a light microscope and compared to the control group.
2.1.5 In vivo anti- inflammatory assays
Formalin was used as empathogenic agent (to induce paw edema in mice) according to the modified protocol of (Winter, Risley, & Nuss, 1963). Formalin was injected in tissue of hind paw of each mouse. Mice was grouped in such way that each group contain 5 mice. Group A (control group) was not given any treatment, group B was treated with formalin (200 µL/kg), group C received formalin and diclofenac sodium (150 mg/kg) as control treatment and group D received formalin and L. agilis dose (1 mL orally). Size (thickness) of the mice paw was measured by vernier caliper before and after treatment with formalin, diclofenac sodium and L. agilis dose at 1 h., 2, 4 and 4 h. Anti-inflammatory (% inhibition of edema) activity was measure by the following formula.
2.2 Statistical analysis
Statistical analysis was carried out by feeding of data into Statistix software (ver. 8.1). One-way ANOVA was used for analysis of variance and variations in the data was identified followed by calculation of standard error for triplicates treatment experiment. The probability value (P-value) less than 0.05 was used for comparison between the treatments.
3 Results
3.1 Antidiabetic assay
Dose of L. agilis acted similar to standard glibenclamide drug by effectively controlling blood glucose level of mice group treated with L. agilis dose as shown in Fig. 1. Mice group with only alloxan (toxic control) having increase level of blood glucose due to either necrosis of beta pancreatic cell by free radical formation or inhibition of glucokinase, glucose sensor of beta cells. In alloxan treated mice the blood sugar level was elevated to 600 mg/dL and was decreased after L. agilis dose to 190 mg/dL.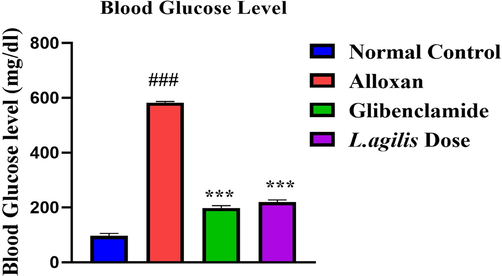
In vivo antidiabetic potential of L. agilis. Legend: Data of blood glucose level was calculated of 14 consecutively days after induction diabetes in mice. ≠ ≠ ≠ shows significance (p < 0.05) of toxic control group to normal control group. *** shows significance (p < 0.001) of L. agilis dose to standard glibenclamide treated mice groups. ≠ ≠ ≠ p < 0.05 vs control group, ***p < 0.001 vs negative group.
3.2 Mice physiology
Physiology of mice (L. agilis dose treated) showed different readings in comparison with control, reference, diabetic and antioxidant groups. Weight gain was increased from 51.48 % to 68.56 % in group of diabetic and in mice with oxidative stress respectively as compared to probiotic (L. agilis dose) group (25.99 %) and standard drug treated mice group (29.35 %). Feed conversion ration and feed conversion efficiency increased in probiotic (L. agilis dose) and standard drug treated mice group with 1.08, 92.59 and 0.99 and 101 respectively (Table 2). Lower FCR values indicate that a feed is efficiently converted into mice weight gain, specific growth rate is the expression of FCR and FCE.
Parameters
Control group
Diabetic model group
Reference group
Probiotic group (L. agilis dose)
Oxidative stressed group
Weight Gain
18.23 %
51.48 %
29.35 %
25.99 %
68.56 %
Specific Growth Rate
4.63 %
11.76 %
37.32 %
6.70 %
14.90 %
Feed Conversion Ratio (FCR)
1.75
0.625
0.99
1.08
0.65
Feed Conversion Efficiency (FCE)
57.14
160
101
92.59
153.8
3.3 Effect of various treatments on mice hematology
Hematological examination showed that group B possessed significant WBC concentration (mean value 134 ± 0.19) due to stress and injuries of the tissues as compared to group A (34 ± 0.14) and group D (94 ± 0.12) while in group B and C, RBC count decreased significantly to 0.34 ± 0.19 and 0.24 ± 0.13 respectively. Platelets count increased in toxic group of mice with value 11904 ± 0.43 and showed improvement in group D (5034.2 ± 0.33) as well as significantly lowered in group E having L. agilis dose with value 9694.1 ± 0.42 (Table 3). HGB: Hemoglobin; PLT: Platelets; PCV: Packed Cell Volume, also called Hematocrit value (HCT), it is fraction of whole blood volume that consists of red cells; MCH: Mean corpuscular hemoglobin, it measures average mass of hemoglobin per red blood cell in picograms; RDW: Red cells distribution width, it measures variations in RBC size or volume; MCV: Mean corpuscular volume, it measures average volume of red cells in femtoliters. *P < 0.05 when compared with toxic control group.
Mice Groups
WBC
RBC
HGB
HCT
MCV
PLT
Group A (normal)
34E ± 0.14
7.54A ± 0.19
13.44A ± 0.12
37.64A ± 0.12
48.74CD ± 0.12
3954.2D ± 0.43
Group B (alloxan stress)
134A ± 0.19
0.34C ± 0.19
5.44D ± 0.23
2.64D ± 0.11
69.74A ± 0.13
11904.3A ± 0.43
Group C (alloxan + ascorbic acid)
94B ± 0.12
0.24C ± 0.13
6.14BC ± 0.21
2.34D ± 0.13
45.14D ± 0.12
9004.1B ± 0.23
Group D (alloxan + Glibenclamide)
42.54D ± 0.14
0.094D ± 0.2
10.84B ± 0.11
19.1C ± 0.14
59.2C ± 0.16
5034.2D ± 0.33
Group E (alloxan + L. agilis dose)
50.44C ± 0.15
3.464B ± 0.3
9.64C ± 0.19
22.14B ± 0.17
63.9B ± 0.12
9694.1B ± 0.42
3.4 In vivo antioxidant activity of L. agilis
In vivo antioxidant activity for mice liver was conducted after its slaughtering. Study was divided in four groups (A, B, C, D) such as group A was normal (control group) and group B was toxic group (alloxan treated), group C mice treated with L. agilis dose (as anti-oxidant) plus alloxan, and fourth group (D) was the treated group of ascorbic acid plus alloxan (Table 4). Results showed a significant rise in anti-oxidant enzymes level (SOD, CAT, GSH) after the administration of L. agilis dose to detoxify the effect of free radicals. Levels of anti-oxidant enzymes in group C were significantly improved as compared to toxic control group B with values 22.1 ± 0.18 μg/mg (SOD), 8.9 ± 0.12 μg/mg (CAT), 4.1 ± 0.12 μg/mg (GSH).
Groups
Super oxide dismutasea (μg/mg)
Catalaseb(μg/mg)
Glutathionec GSH (μg/mg)
Group A (normal)
8.2D ± 0.3
4.1C ± 0.12
2.5C ± 0.1
Group B Toxic control (oxidative stress)
20.8B ± 0.4
7.2B ± 0.1
3.7B ± 0.14
Group C oxidative stress + ascorbic acid
11.2C ± 0.2
7.4B ± 0.12
4.5A ± 0.13
Group D oxidative stress + dose (L. agilis fed)
22.1A ± 0.18
8.9A ± 0.12
4.1B ± 0.12
3.5 Effect of L. agilis on the histology of renal, stomach and hepatic tissues of stressed and non-stressed mice
Histological analysis of diabetic mice revealed injury in the stomach, kidneys, and liver. Kidney tissues showed massive tubular cell necrosis, fibrin infiltration, and atrophy. L. agilis dose treatment improved liver injury, while alloxan-induced groups showed morphological changes. Treatment of mice with L. agilis dose and glibenclamide improved stomach histological features (Fig. 2, Table 5). (+) = Damaged tissues, (-) = Normal/ recovered tissues.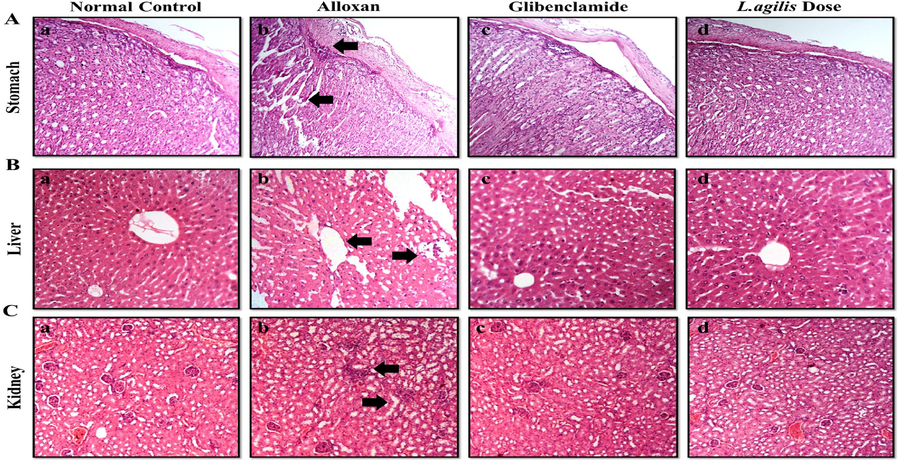
Histopathological examination of stress induced sacrificed mice tissues.
Tissue
Parameters
Normal control
Alloxan treatment
Glibenclamide + alloxan treatments
L.agilis Dose + alloxan treatments
Stomach
Inflammation
–
+
–
–
Necrosis
–
+
–
–
Liver
Hepatic cytoplasmic inflammation
–
+
–
–
Centrilobular necrosis
–
+
–
–
Cellular hypertrophy
–
+
–
–
Kidney
Tubular necrosis
–
+
–
–
Hyaline cast
–
+
–
–
Fibrinous exudates
–
+
–
–
The arrows denote necrosis, inflammation hyaline cast and fibrinous exudates.
3.6 In vivo anti-inflammatory activity of L. agilis
In vivo anti-inflammatory assays of L. agilis revealed that the inflammation induced in mice paw was prevented (Table 6). Thickness in mice paws were observed after formalin (200 µL/kg) injection in group B, C and D mice. Inflammation in paw of group D mice (L. agilis dose treated) was remained same at, T1, T2 with values 3.3 ± 0.5 cm, but after 3 and 4 h (T3 and T4) notable reduction in inflammation was observed. Paw size (thickness) of the treated mice was significantly reduced at T4 in group D (L. agilis 1 mL dose) with value 2.1 ± 0.12 cm, comparing it with the group A (2.3 ± 0.12 cm) and group B (3.1 ± 0.23 cm). Nearly similar effects were observed in mice of group C (diclofenac sodium treated) and group D (L. agilis treated) at T4 with values 2.2 ± 0.13 cm and 2.1 ± 0.12 cm respectively. These results showed a significant anti-inflammatory behavior of L. agilis.
Groups
1 ha
2 hb
3 hc
4 hd
Group A (normal group)
2.3B ± 0.12
2.3C ± 0.12
2.3B ± 0.12
2.3B ± 0.12
Group B formalin (stress)
3.5A ± 0.7
3.9A ± 0.42
3.7A ± 0.9
3.1A ± 0.23
Group C formalin stress + diclofenac sodium (standard)
3.3A ± 0.5
3.3B ± 0.5
3.5A ± 0.7
2.2B ± 0.13
Group D formalin stress + L. agilis fed
3.3A ± 0.5
3.3B ± 0.5
2.3B ± 0.21
2.1B ± 0.12
4 Discussion
Lactic acid bacteria showed a significant role as probiotics that can prevent the growth of pathogenic bacteria in as study of Manguntungi et al. (2020). As noted by Everard and Cani (2013), diabetic is a global epidemic, primarily caused by obesity, high-calorie diets, and physical activity in individuals with a genetic predisposition to type 2 diabetes. Current study is correlated to the study of Honda et al. (2012) that described the ability of LAB bacteria as antidiabetic. In another study, the enzyme glucosidase is known to be inhibited by polysaccharides released by Lactic acid bacteria successfully (Reuben et al., 2020) which resembled current in vivo study, showed that L. agilis strain effectively prevented mice from developing diabetes after receiving alloxan treatment. The results obtained in recent study of histopathological analysis of mice groups correlated with findings of Shal et al. (2020), Zhang et al. (2015) and Zolali et al. (2020). The alloxan-treated group showed marked injuries in the tissues of gastric, hepatic and kidney tissues. However, significant improvement was observed in gastric, liver and kidney L. agilis and glibenclamide treated mice after alloxan treatment. The current findings obtained from in vivo anti-oxidative assays revealed that L. agilis can inhibit the free radicals as well as elevate the levels of anti-oxidative enzyme to break the free radicals produced. The results of present study were correlated with study of Li et al. (2012) in which L. plantarum AR113, AR269, AR300, AR501, and P. pentosaceus AR243 showed significant tolerance to hydrogen peroxide, which corelate current findings. L. agilis treated in vivo for anti-inflammatory assay on mice, revealed that L. agilis can reduce inflammation in mice paw.
These results are in accordance with a previous report where L. casei and L. acidophilus demonstrated very consistent anti-inflammatory effect, resulting in a significant reduction in rat paw thickness (Amdekar et al., 2012). The findings of this study suggest that L. agilis could be employed as an anti-inflammatory agent which had very reliable anti-inflammatory activity and thus showed momentous decrease in the paw thickness of mice after treatment with formalin. The presence of such variable compounds secreted by L. agilis may justify that it can be used as probiotic or nutraceutical.
5 Conclusion
In this study, the strain L. agilis NMCC-15 has been characterized as a safe rhizobacterium, demonstrating significant potential for antioxidant, anti-inflammatory, and anti-diabetic activities in disease-induced mice models. Through rigorous in vivo experimentation, L. agilis NMCC-15 has shown promise as a potent bio-therapeutic agent, suggesting its capability to mitigate various disease conditions effectively. The observed biological activities of L. agilis NMCC-15 underscore its potential in contributing to the development of novel therapeutic strategies, particularly in the management and treatment of chronic diseases that are often characterized by oxidative stress, inflammation, and glucose metabolism disorders. Future research should focus on detailed mechanistic studies to fully understand the pathways through which L. agilis NMCC-15 exerts its therapeutic effects. Additionally, comprehensive clinical trials are warranted to evaluate its safety and efficacy in human populations. These steps are crucial for transitioning L. agilis NMCC-15 from a promising laboratory discovery to a viable clinical bio-therapeutic option. The integration of such bio-therapeutic agents into disease management protocols could revolutionize current treatment modalities, offering safer, more natural alternatives to conventional chemical drugs. Thus, L. agilis NMCC-15 represents not just a significant advancement in bio-therapeutic agents but also highlights the untapped potential of rhizobacteria in medical science.
Declarations.
Ethics approval.
Not applicable.
Consent to participate.
All authors consent to participate in the manuscript publication
Consent for publication
All authors approved the manuscript to be published.
CRediT authorship contribution statement
Allah Nawaz Khan: Literature Review, Analysis, Data Collection, Methodology, Software, Investigation, Resources, Writing – original draft, Visualization. Shakira Ghazanfar: Writing – review & editing, Visualization, Supervision. Muhammad Nadeem Hassan: Analysis, Data Collection, Methodology, Software. Ajaz Ahmad: Analysis, Data Collection, Software, Writing – review & editing. Naeem Khan: Methodology, Investigation, Resources, Writing – original draft, Supervision. Sharjeel Khalid: Data Collection, Methodology. Humaira Yasmin: Conception, Investigation, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Funding.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R350), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory activity of lactobacillus on carrageenan - induced paw edema in male wistar rats. Int J Inflam.. 2012;2012
- [CrossRef] [Google Scholar]
- Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J.. 2016;24(5):547-553.
- [Google Scholar]
- Lactobacillus plantarum LUHS135 and paracasei LUHS244 as functional starter cultures for the food fermentation industry: characterisation, mycotoxin-reducing properties, optimisation of biomass growth and sustainable encapsulation by using dairy by-products. Lwt.. 2018;93:649-658.
- [Google Scholar]
- A protocol for active surveillance of acute myocardial infarction in association with the use of a new antidiabetic pharmaceutical agent. Pharmacoepidemiol Drug Saf.. 2012;21:282-290.
- [Google Scholar]
- Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J Clin Biochem Nutr J CLIN BIOCHEM NUTR.. 2012;51(2):96-101.
- [Google Scholar]
- Antagonistic, anti-oxidant, anti-inflammatory and anti-diabetic probiotic potential of lactobacillus agilis isolated from the rhizosphere of the medicinal plants. Saudi J. Biol. Sci. 2021;28(11):6069-6076.
- [Google Scholar]
- Metagenomic analysis and biodiversity of lactic acid bacteria (LAB) on masin (Fermented Sauce) from Sumbawa, West Nusa Tenggara, Indonesia. Biodivers. J.. 2020;21(7)
- [Google Scholar]
- Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci.. 2020;103(2):1223-1237.
- [Google Scholar]
- Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol.. 2014;232:1-44.
- [Google Scholar]
- Neuroprotective effect of 25-methoxyhispidol a against CCl(4)-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci.. 2020;253:117684
- [Google Scholar]
- Antibacterial activity and probiotic potential of lactobacillus plantarum HKN01: a new insight into the morphological changes of antibacterial compound-treated escherichia coli by electron microscopy. Microbiol. Biotechnol.. 2013;23(2):225-236.
- [Google Scholar]
- Anti-inflammatory and antipyretic activities of indo-methacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-indole-3-acetic acid. J. Pharmacol. Exp. Ther.. 1963;141(3):369-376.
- [Google Scholar]
- Metformin had potential to increase endocan levels in STZ-induced diabetic mice. Pharm. Sci.. 2020;26:133-141.
- [Google Scholar]







