Translate this page into:
Prevalence of cattle ticks in various agro-ecological zones of Khyber Pakhtunkhwa, and evaluation of botanical extracts against Hyalomma detritum
⁎Corresponding author at: Department of Entomology, Faculty of Agriculture, Gomal University, Pakistan. dr.faisal@gu.edu.pk (Muhammad Faisal Shahzad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Background
The occurrence of infestation by ticks on livestock is a significant challenge in several semi-tropical and tropical countries including Pakistan. Polluted environment and adapted tolerance by ticks against acaricide are the limiting factors that restrict the management of ticks globally.
Methods
In this study, we evaluated the infestation by ticks on cattle from numerous livestock farms present in different districts and agroecological areas of Khyber Pakhtunkhwa, a province in Pakistan. Furthermore, we compared the anti-tick efficacy of different botanicals using ethanolic and aqueous extracts to control Hyalomma detritum.
Results
The prevalence rate was 1.78, 35.37, 36.23, 8.15, 15.50, 0.29 and 2.67 percent for Amblyoma varigatum (A. varigatum), Hyalomma anatolicum (H. anatolicum), H. detritum, Hylomma rufipes (H. rufipes), Hyalomma truncatem (H. truncatum), Rhipicephalus microplus (R. microplus), and Hyalomma kashmirensis (H. kashmirensis), respectively. In terms of high mortality rate of ticks, extracts derived from Calotropis procera flower (93.33%), Citrullus colocynthis fruit (95.0%) and Calotropis procera flower (89.4%) showed significantly high efficancy (P < 0.05) than the extracts derived from other plants. In terms of their efficacy in causing mortality of H. detritum tick, Calotropis procera flower, Citrullus colocynthis fruit and Calotropis procera leaves extracts displayed non-significant variation.
Conclusion
This work revealed that the cattle tick H. detritum can be successfully controlled by employing both ethanolic and aqueous plant extracts. The intensive use of insecticides and chemical drugs are costly and may affect human health negatively due to residual effects in milk and animal meat.
Keywords
Acaricide
Cattle ticks
Citrullus colocynthis
Calotropis procera leaves
Calotropis procera flower
1 Introduction
Pakistan is mostly an agricultural nation, and many of its citizens also raise livestock. In rural regions, the agriculture industry accounts for 43% of the labor force. There are enormous herds of cattle, buffalo, sheep, and goats in Pakistan. In 2020–21, total milk production was 61,690 metric tons and meat production was 4,708 metric tons; exports of meat and meat products were 53.4 metric tons, earning around 3.1% of total foreign currency and accounting for 11.7% of GDP (GOP, 2021). Dairy animals are raised in large numbers by rural small farmers, for whom livestock is the primary means of subsistence (Khan et al., 2013). In addition to other diseases and difficulties, ticks and tick-borne infections are the biggest restrictions on the production of dairy animals (Ghafar et al., 2021).
Tick infestation on livestock plays a pivotal role in the transmission of several pathogenic microorganisms responsible for tick-related diseases in human and animals (Ntondini et al., 2008) that instigate high mortality and heavy morbidity resulting in reduced productivity as well as financial losses to poor and small owners of livestock (Jabbar et al., 2015).
The traditional techniques used to control ticks rely on utilization of chemicals such as manufactured pyrethroids, amitraz, macrocyclic lactones and organophosphates (Reshma and Prakasan 2020). Such chemicals proved to be less effective, more expensive, contaminate surrounding environment and induced increased tolerance in ticks (Klafke et al., 2010, Fernández-Salas et al., 2012). Among crucial factors that hamper ticks control worldwide, are the environmental pollution and resistance development against acaricide in ticks (Fernández-Salas et al., 2012) that result in significant economic losses (Sajid et al., 2017). In the recent past, the utilization of acaricides observed a substantial decrease due to their role in causing polluted environment and retention of their residues in the meat and milk (Ribeiro et al., 2011). Additionally, the continues decline in support by the Khyber Pakhtunkhwa government to small-holding farmers, led to employ several customary veterinary exercises for the treatment of diseases and ticks’ control.
Many studies have provided knowledge and understanding on the use of natural material in the form of traditional medicine, including both non-plants and plant treatments, to cure animal-related illnesses and diseases (Ndhlovu 2014). Poor livestock producers favor ethnoveterinary or traditional medicine over pricey and sophisticated animal health facilities because of limited and inadequate resources (Ghotge et al., 2002). The insecticidal properties of plant extracts provide a viable alternative that is safe for both people and the environment (Fernández-Salas et al., 2011). Because of their effectiveness against ticks, plant extracts have become a popular treatment option. Botanical extracts have been proposed as a practical method of dealing with ectoparasites like ticks (Ribeiro et al., 2011) since they are safe for animals, humans and environment (Rosado-Aguilar et al., 2017). To treat infection caused by the parasites, several ethnoveterinary materials derived from plants have successfully been utilized (Abbas et al., 2014). To control ectoparasites, around 55 species of botanical extracts belonging to different families of the plants were reported globally (Benelli et al., 2016). Although the ticks are the major ectoparasites that cause heavy financial losses to livestock especially the cattle, the information, about the volume of economic loss generated by ticks’ infestation on cattle in the Khyber Pakhtunkhwa, is still lacking.
This study was carried out in vitro to evaluate the efficacy of targeted plant-derived extracts to control H. detritum, a cattle tick. It may lead to develop an alternative future acaricide source for tick population control and rescue livestock from diseases caused by ticks. In addition, such kind of acaricide must be eco-friendly, degrade quickly, less toxic to mammals and minimize the resistance development in the ticks against them.
2 Materials and Methods
2.1 Ticks sample collection
From different agroecological areas in Khyber Pakhtunkhwa’s 13 districts, live ticks were collected from cattle infested naturally for period of two years between Jan. 2018 to Dec. 2019. A positive case for tick infestation was considered based on the one or more ticks infestation events on both female and/or male cattle. Blunt forceps were used to collect live ticks and supply of oxygen was maintained by placing them in the glass tubes which were covered with muslin cloth. Every single tube was properly labelled with all the information about collected sample.
2.2 Identification of ticks
The collected samples of ticks were moved to the Gomal University at the laboratory of Parasitology, Dera Ismail Khan, Pakistan. Tap water was used to rinse the collected samples of ticks and later distilled water treatment was done before drying using filter paper. For their identification based on the classification criterion for H. detritum, a stereomicroscope was used at 40X magnification scale (Miranpuri, 1979). After ticks’ identification, the selected H. detritum were further used to evaluate the lethal effects of the Botanical extracts were evaluated on the targeted H. detritum.
2.3 Plant materials
To prepare botanical extracts, different tissues, and plant parts of various botanicals (Table 1) such as leaves, fruit, flower, peel, milk, fruit, fruit peel, seeds, bulbs and rhizome were gathered from the local herbal market and were identified at the facility of Gomal University, Dera Ismail Khan. To collect clean tissues of the plant samples collected, all the activities were performed with utmost care. Table 1 shows the volume or weight for every botanical collected.
No.
Botanical
Parts used
Weight/Volume
1.
Alovera
Leaves
1000 gm
2.
Allium sativum
Bulb
1000 gm
3.
Azadirachta indica
Leaves
1000 gm
4.
Calotropis procera
leaves
1000 gm
Flower
1000 gm
Milk
1000 ml
5.
Citrullus colocynthis
Fruit
1000 gm
6.
Citrus sinensis
Fruit peel
1000 gm
7.
Datura alba
Leaves
1000 gm
8.
Eucalyptus camadulensis
Leaves
1000 gm
9.
Juglans regia
Leaves
1000 gm
10.
Melia azedarach
Leaves
1000 gm
11.
Mentha longifolia
Leaves
1000 gm
12.
Quercus berberidifolia
Leaves
1000 gm
13.
Peganum harmala
Seed
1000 gm
14.
Zingiber officinale
Rhizome
1000 gm
2.4 Preparation of plant extracts
The plant-derived material obtained was air-dried under the shade and were crushed and converted in the form of a fine powder by grinding using a stainless-steel pestle mortar. An appropriate amount of individual botanical (Table 2) was socked for at least 24 h in 70 % ethanol or 2.0 L of distilled water and maceration method at room temperature was used to process for extraction. Once extraction process was completed, rotary vacuum evaporator at 20 rpm was utilized to dry the extracted products at 40 °C. The solution preparation for the ticks dipping, each dried extract was converted into a fine powder by softly grinding in a porcelain pestle mortar.
No.
Botanical
Dry Weight
The volume of aqua distilled
Volume ethanol
1.
Aloe vera
700 g
1000 ml
1000 ml
2.
Allium sativum
600 g
1000 ml
1000 ml
3.
Azadirachta indica
800 g
1000 ml
1000 ml
4.
Calotropis procera
800 g (leaves)
1000 ml
1000 ml
700 g (flowers)
1000 ml
1000 ml
1000 ml (milk)
1000 ml
1000 ml
5.
Citrullus colocynthis
600 g
1000 ml
1000 ml
6.
Citrus sinensis
700 g
1000 ml
1000 ml
7.
Datura alba
700 g
1000 ml
1000 ml
8.
Eucalyptus camadulensis
800 g
1000 ml
1000 ml
9.
Juglans regia
800 g
1000 ml
1000 ml
10.
Melia azedarach
1000 ml
1000 ml
11.
Mentha longifolia
700 g
1000 ml
1000 ml
12.
Quercus berberidifolia
800 g
1000 ml
1000 ml
13.
Peganum harmala
700 g
1000 ml
1000 ml
14.
Zingiber officinale
600 g
1000 ml
1000 ml
2.5 Preparation of dip/solution for adult immersion test (AIT)
For both aqueous and ethanolic extracts of 14 selected botanicals, 100 mg/ml concentration was prepared by dissolving 2.0 g of ethanolic and aqueous extract of every single botanical into 20 ml of distilled water (Table 3).
S.No.
Botanical
Dry Weight of extract
Volume of aqua distilled
Volume of
70% ethanol
1.
Aloe vera
2 g
20 ml
20 ml
2.
Allium sativum
2 g
20 ml
20 ml
3.
Azadirachta indica
2 g
20 ml
20 ml
4.
Calotropis procera
2 g
Leaves20 ml
20 ml
2 g
Flower20 ml
20 ml
100 ml (milk)
20 ml
20 ml
5.
Citrullus colocynthis
2 g
20 ml
20 ml
6.
Citrus sinensis
2 g
20 ml
20 ml
7.
Datura alba
2 g
20 ml
20 ml
8.
Eucalyptus camadulensis
2 g
20 ml
20 ml
9.
Juglans regia
2 g
20 ml
20 ml
10.
Melia azedarach
2 g
20 ml
20 ml
11.
Mentha longifolia
2 g
20 ml
20 ml
12.
Quercus berberidifolia
2 g
20 ml
20 ml
13.
Peganum harmala
2 g
20 ml
20 ml
14.
Zingiber officinale
2 g
20 ml
20 ml
The number of dips and number of adult ticks (H. detritum) used were, no. of dip for H. detritum = no. of aqueous extract + no. of alcoholic extract = 16 + 16 = 32 (dip), the volume of distilled water for each dip = 20 ml, total volume used for preparation of dips for H. detritum = 20 × 32 = 640 ml, total no. of dips = 62, no. of H. detritum in each dip = 30, and total no. of H. detritum used = 32 × 30 = 960.
2.6 Adult immersion test (AIT)
Thirty adult ticks including Hyalomma anatolicum and H. detritum were separately dipped for five minutes in each aqueous and 70% ethanolic extract to determine efficacy of selected botanicals against ticks.
After immersion, ticks were transferred to the Petri plates which were covered by muslin cloth. The incubation temperature of Petri plates was 28 ± 2 °C with 85 ± 2% relative humidity. After incubating Petri plates for 48 h, another five minutes bath was given to the ticks using the same solution and once again transferred to Petri plates.
3 Results
3.1 Prevalence of cattle ticks in various agro-ecological zone/districts of Khyber Pakhtunkhwa. The rate of infestation by the ticks in the Southern Piedmont plains, Central valley Plains, Eastern wet mountains, Western dry mountains, Northern dry mountains of Khyber Pakhtunkhwa was 88.76%, 84.76%, 72.78%, 62.71% and 60.49% respectively (Table 4). According to the data, Southern Piedmont plains was the highest affected zone and Northern dry mountains was the lowest affected zone. With respect to the number of cattle infested with ticks, Dera Ismail Khan was the highly affected district whereas Swat was the lowest affected district. Amblyoma varigatum = A. varigatum, Hyalomma Anatolicum = H. anatolicum, Hyalomma detritum = H. detritum, Hyalomma rufipes = H. rufipes, Hyalomma truncatum = H. truncatum, Rhipicephalus microplus = R. microplus, Haemaphysalis kashmirensis = H kashmirensis.
Agro-ecological zone/district
No. of cattle observed
Cattle infested
(%)
Species of tick found
A. varigatum
H. Anatolicum
H. detritum
H. rufipes
H. truncatum
R. microplus
H. kashmirensis
Northern dry mountains
2058
1245 (60.49)
24
535
357
109
220
–
Dir
697
446 (63.99)
–
140
120
59
127
–
–
Swat
653
349 (53.45)
–
238
91
–
20
–
Shangla
708
450 (57.21)
24
157
146
50
73
–
–
Eastern wet mountains
1933
1407 (72.78)
–
493
673
66
175
–
Mansehra
559
405 (72.45)
–
177
153
–
75
–
–
Abbottabad
776
530 (68.29)
–
149
272
66
43
–
–
Haripur
598
472 (78.92)
–
167
248
–
57
–
–
Central valley Plains
1799
1525 (84.76)
02
523
568
147
285
–
Kohat
508
406 (79.92)
–
106
88
98
114
–
–
Peshawar
738
657 (89.02)
–
198
352
38
69
–
–
Charsadda
553
462 (83.54)
02
219
128
11
102
–
–
Southern Piedmont plains
2679
2378 (88.76)
71
835
797
203
288
13
171
Karak
556
475 (85.43)
71
109
124
57
101
13
–
Bannu
807
685 (84.88)
–
169
176
85
84
–
171
D.I. Khan
1316
1218 (92.55)
–
557
497
61
103
–
–
Western dry mountains
480
301 (62.71)
25
39
89
34
95
07
12
North Waziristan
480
301 (62.70)
25
39
89
34
95
07
12
Total
8949
6856 (76.6%)
122
2425
2484
559
1063
20
183
The prevalence of H. kashmirensis, R. microplus, H. truncatum, H. rufipes, H. detritum, H. anatolicum and A. varigatum was 2.67%, 0.29%, 15.50%, 8.15%, 36.23%, 35.37% and 1.78% respectively. This data highlighted that the prevalence of H. detritum and H. anatolicum was in 36.23% and 35.37% cows respectively and cattle is the most preferred host for infestation as compared to other hosts.
Fig. 1 presents graphical data about number of the cattles infested by seven tick species in the five agroecological areas of Khyber Pakhtunkhwa. Fig. 2 presents graphically ticks prevalence in percentage at different agroecological zone/district of Khyber Pakhtunkhwa.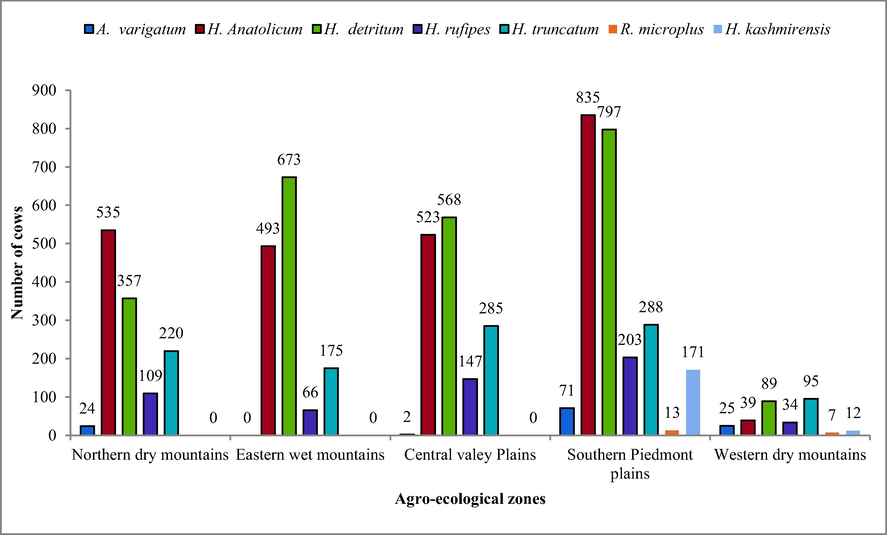
Number of cattle infested by various species of tick in agro-ecological zones of KPK.

Percent cattle infested by various tick species.
3.1 In vitro efficacy of various botanical extracts against H. Detritum
Data in Table 5 highlights the number of ticks H. detritum killed because of immersion in ethanolic and the aqueous solutions having 100 mg/ml ethanolic and/or aqueous extract of plants i.e. Allium sativum bulb, Aloe vera leaves, Calotropis procera leaves, Azadirachta indica leaves, Datura alba leaves, Calotropis procera flowers, Calotropis procera milk, Citrullus colocynthis fruit, Zingiber officinale rhizome, Melia Azedarach leaves, Juglans regia leaves, Quercus berberidifolia leaves, Peganum harmala seed, Mentha longifolia leaves, Peganum harmala seed, Eucalyptus camadulensis leaves and Citrus sinensis peel. Figures that do not share a letter are significantly different within a column while different letters indicate significant differences at p value < 0.05.
Botanicals
Ticks treated by each treatment (n = 90)
Mean
efficacy (%)
Aqueous Extract
Alcoholic Extract
No. of Ticks killed
Efficacy (%)
No. of Ticks killed
Efficacy (%)
Aloe vera
15
16.66
21
23.33
19.99f
Allium sativum
27
30
39
43.33
36.66def
Azadirachta indica
42
46.66
64
71.11
58.9bc
Calotropis procera leaves
78
86.66
90
100.00
93.33a
Calotropis procera flower
72
80
89
98.89
89.44a
Calotropis procera milk
18
19
24
26.67
22.84f
Citrullus colocynthis fruit
81
90
90
100.00
95.00a
Citrus sinensis peel
45
50
60
66.67
58.34bc
Datura alba leaves
51
56.66
69
76.67
66.7b
Eucalyptus camadulensis leaves
30
33.33
48
53.33
43.3cde
Juglans regia leaves
33
36.66
54
60.00
48.3b
Melia azedarach leaves
36
43.33
57
63.33
53.3bcd
Mentha longifolia leaves
27
30.66
39
43.33
36.99def
Quercus berberidifolia leaves
32
36.66
48
53.33
44.99cde
Peganum harmala seed
46
56.66
69
76.67
66.7b
Zingiber officinale rhizome
17
20.0
30
33.33
26.66ef
Total ticks killed
650
45.14
891
61.88
53.51
The number of killed ticks H. detritum due to the aqueous solution derived from the aqueous extract of above mentioned plants were 27, 15, 78, 42, 51, 72, 18, 81, 17, 36, 33, 32, 46, 27, 30 and 45, and the efficacy was 30.0%, 16.66%, 86.66%, 46.66%, 56.66%, 80.0%, 19.0%, 90.0%, 20.0%, 43.33%, 36.66%, 36.66%, 56.66%, 30.66%, 33.33% and 50.0% respectively. Whereas, ticks killed in numbers due to 70% ethanolic solution derived from ethanolic extracts of the, Zingiber officinale rhizome, Azadirachta indica leaves, Allium sativum bulb, Mentha longifolia leaves, Calotropis procera milk, Calotropis procera flowers, Citrus sinensis peel, Citrullus colocynthis fruit, Eucalyptus camadulensis leaves, Datura alba leaves, Melia Azedarach leaves, Juglans regia leaves, Calotropis procera leaves, Peganum harmala seed, Quercus berberidifolia leaves and Aloe vera leaves were 30, 64, 39, 39, 24, 89, 60, 90, 48, 69, 57, 54, 90, 69, 48, and 21, and the efficacy of these botanicals was 33.33%, 71.11%, 43.33%, 43.33%, 26.67%, 98.89%, 66.67%, 100%, 53.33%, 76.67%, 63.33%, 60.0%, 100%, 76.67%, 53.33% and 23.33%, respectively, at incubation time of 120 h during two immersion at 0 and 48 h’ time points of the experiment. No mortality of ticks was observed after both ethanolic and aqueous control immersions with no extracts of any botanicals. Hence, the tick’s data generated by immersing in ethanolic, or aqueous extracts of selected plants was significantly different than in control dips.
Statistics of the available data showed significantly high efficacy (P < 0.05) of the extracts derived from Citrullus colocynthis fruit, Calotropis procera flower and Calotropis procera leaves, in high ticks mortality than the extracts derived from Mentha longifolia leaves, Melia Azedarach leaves, Peganum harmala seed, Quercus berberidifolia leaves, Allium sativum, Alove vera, Calotropis procera milk, Azadirachta indica, Datura alba leaves, Citrus sinensis peel, Eucalyptus camadulensis leaves, Zingiber officinale rhizome, and Juglans regia leaves. It was observed that there was a non-significant variation between Calotropis procera flower, Citrullus colocynthis fruit and Calotropis procera leaves extracts when it comes to their efficacy in causing mortality against ticks H. detritum.
Likewise, differences were non-significant between Citrus sinensis peel, Azadirachta indica, Juglans regia leaves, Datura alba leaves, and Peganum harmala seed, and Melia Azedarach leaves extracts regarding their efficacy in causing the mortality of the H. detritum ticks. Calotropis procera milk and Aloe vera leaves displayed lowest efficacy in ticks H. detritum mortality. When it comes to the mortality of the ticks H. detritum, there was non-significant variation between Eucalyptus camadulensis leaves, Allium sativum bulb, Quercus berberidifolia leaves, Zingiber officinale rhizome and Mentha longifolia leaves.
Fig. 3 represents graphically the effectiveness of ethanolic and aqueous solutions that contain 100 mg/ml of extract derived from selected botanicals in causing mortality of the ticks H. detritum. In the graph, moving average lines revealed the shift in ticks’ mortality by ethanolic and aqueous extracts and the correlation among the ethanolic and aqueous extracts derived from various plants.
The mortality of Hyalomma detritum during in vitro application of aqueous and alcoholic extract of various botanicals.
4 Discussion
4.1 Predominance of livestock ticks in agro-ecologically different regions of Khyber Pakhtunkhwa.
The current results are consistent with those of Rehman et al. (2017), who mentioned the existence of Rhipicephalus turanicus, Hyalomma anatolicum, Hyalomma dromedarii and Rhipicephalus microplus tick species in cattle farms in Pakistan's arid and semi-arid agro-ecological areas (Rehman 2017, Khan et al., 2022). Total proportion of tick-infested ruminants was 78.3%, with the occurrence of H. anatolicum in buffaloes being 81.4%, 60.0% in goats, 89.9% in cattle, and 11.1% in sheep. The current study identified seven species of the ticks which includes H. rufipes, H. anatolicum, Haemaphysalis kashmirensis, H. truncatum, H. detritum, A. varigatum and R. microplus from different regions of Khyber Pakhtunkhwa province and their occurrence was 2.67%, 35.37, 36.23, 8.15, 15.50, 0.29 and 1.78%, respectively. According to the available data, H. detritum and H. anatolicum were observed in 36.23% and 35.37% of cows, respectively, and prefer cattle as a host for infestation over other hosts. Wanzala (2017) also stated that Hyalomma was one among the most important ticks in tropical countries. Singh and Rath (2013) conducted a study in East Punjab and found highest percentage occurrence of H. anatolicum being 58.08%, while 11.34% for Hyalomma anatolicum, 50.16% for R. microplus, Ixodid ticks and mixed infestation being, 58.06% and 3.45% respectively. It was also stated that the prevalence of H. anatolicum was 79.36% in the sub-mountain undulating region, highlighting its preference for moderately arid region and the overall occurrence of Ixodid ticks was maximum during the monsoon (83.74%), preceded by summer (69.01%) and lowest during winters (31.64%).
Hyalomma species are the most frequently observed ticks on domestic animals in Pakistan (Ali et al., 2022). The most commonly found species are H. dromedarii, H. marginatum rufipes, H. anatolicum, H. truncatum, and H. detritum. The first one take food from camels while the last four species feed on domesticated animals. Hyalomma spp. can inhabit locations with a varied range of temperature fluctuations and precipitation (Rehman 2017). Hyalomma species of tick serve as a vector for the transmission of many bacterial, viral, and parasitic diseases throughout the world, attempting to make them significant ixodids from economic point of view (Mehlhorn, 2012). The infestation rate of the ticks within the Eastern wet mountains, Western dry mountains, Southern Piedmont plains, Central valley Plains, and Northern dry mountainous range of Khyber Pakhtunkhwa was, 72.78%, 62.71%, 88.76%, 84.76%, and 60.49%, respectively. Following the data, the Southern Piedmont plains were the most affected region, while the Northern dry mountains were the least affected. In terms of the total number of ticks infested cattle, Dera Ismail Khan was the most affected region, while Swat was the least affected. A recent study performed in temporal regions of Khyber Pakhtunkhwa, identified the occurrence of ticks being 10.33%, 0.42%, 10.08%, 78.50%, and 0.67% for Heamaphysalis, Amblyomma, Hyalomma, Rhepicephalus and Dermacenter respectively (Farooqi et al., 2017).
The current study's findings are consistent with Singh and Rath (2013), who mentioned that lower rainfall and higher temperatures promote the growth of H. anatolicum. Previously, H. anatolicum was found to be the most common cattle tick throughout the Punjab. The decline in predominance may be attributed to adaptation under improved farm animal management system, which seems to be a threat to the longevity of multi-host ticks. Furthermore, macro- and micro-climate factors may influence tick prevalence, causing diverse epidemiological patterns in different agro-climatic regions. (Singh and Rath 2013). The outcomes of this research are consistent with those of Rehman et al. (2016), who reported that the total tick prevalence in domesticated animals in Pakistan was 78.3%. (Rehman 2017).
In Pakistan, two reports showed varied tick occurrence as 31% and 85% (Iqbal et al., 2013) in comparison to India and Iran where it was recorded to be 58% and 77%, respectively. (Singh and Rath 2013). Varied tick predominance is primarily due to differences in the study areas' geographical and climatic conditions, target populations, study seasons and husbandry practices. Tick prevalence in ruminants seems to be much higher in Asia and Africa than in any other continent (Ali et al., 2022).
4.2 In vitro efficiency of different plant derived extracts against H. detritum
Amidines, organophosphates, pyrethroids, and macrocyclic lactones are common chemical acaricides that were effective in reducing the number of hard ticks (Aguilar-Tipacamu et al., 2011). Due to the extensive usage of new chemical acaricides over a long period of time, tick populations have developed a variety of resistances (Rodriguez-Vivas et al., 2011), particularly in the usage region (Aguilar-Tipacamu et al., 2011). Hence, it is essential to explore other products to restrict tick population. It has been demonstrated that plants containing toxicological molecules impact ticks in killing or repelling them from animal bodies. Consequently, plant extracts have the potency to be used as alternatives to presently used acaricides (Magadum et al., 2009). Besides this, when compared to synthetic acaricides, plant extracts get the benefits of being used in organic cattle farming because they are linked to fewer food and environmental contaminations, reduced toxicity to humans and animals as well as slower resistance development. Several plant-based extracts have revealed promising outcomes, but most of them have not been examined on animals to verify and confirm their use. Yet, owing to internal and external variables that affect their chemical composition and a dearth of information regarding active acaricide chemicals, it is difficult to produce their standard formulations. Moreover, since crude extracts sometimes include numerous active chemicals with distinct modes of action, using a variety of potent plant-based extracts may result in a gradual emergence of resistance (Borges et al., 2011).
Farmers used the plants to manage ticks with the most widely mentioned plants used by them are Citrullus colocynthis, Azadirachta indica, Calotropis procera, Eruca vesicaria, Brassica rapa, Peganum harmala, Capsicum annuum, Eucalyptus camaldulensis, Nicotiana tabacum, Juglans regia, Melia azedarach, Prunus persica, Quercus berberidifolia, Trigonella foenumgraecum and Pinus gerardiana. Numerous parts of the plant, such as seed rhizome leaves, resin of the plants and fruits are introduced to prepare plant derived solution against tick infestation. Traditionally, different plant parts were utilized to make powders, pastes, or decoctions. The decoctions have been used for spraying, washing, or bathing, powder for sprinkling, and the paste was used for creams or ointments. (Babar et al., 2012). It has been mentioned that ethanol based extracts of Annona squamosal (Sitaphal) and Azadirachta indica (Neem) are efficacious against the developmental phases of Hyalomma and Boophilus ticks. (Rehman 2017).
The present study findings do not agree with the findings of Shyma et al. (2014) where the mortality rate for the adult tick Boophilus was found to be 80% during 2 weeks after their treatment by crude methanolic extract derived from Allium sativum (Shyma et al., 2014). During this experiment, H. detritum displayed 30% and 39% mortality after their treatment with ethanolic and aqueous extracts derived from A. sativum at the concentration of 100 mg/ml, respectively. It was reported that the tick prevalence can be inhibited by utilizing extract derived from Allium sativum (garlic bulb) as it contains potential repellents for ticks including dithiane and thiophene (Nchu et al., 2016). Furthermore, it was reported that A. sativum has the insecticidal, fumigant, larvicidal and the acaricidal features (Niroumand et al., 2016). Aboelhadid et al. (2013) confirmed that A. sativum has acaricidal activity against Boophilus annulatus during whole life cycle. To control Spodoptera littoralis, the extracts derived from the leaves of A. sativum and bulb agglutinins displayed insecticidal properties (Sadeghi et al., 2008).
The current study yielded the results which are partly supporting the findings of Shyma et al. (2014) who found mortality rate of adult ticks as 33.33% for 2 weeks when crude methanolic extract derived from A. indica was used to treat them. But, during this study, when H. detritum, the adult ticks, were given treatment with the solutions ethanolic and aqueous that contain concentration of 100 mg/ml of extract derived from the leaves of Azadirachta indica, 46.66% and 71.11% mortality rate was acheived, respectively. This mortality rate was attained by immersing for 120 h at time points of 0 and 48 h while incubating at 28 °C with relative humidity of 85%.
The present study produced the results which are closely related to the findings of Shyma et al. (2014) who found the mortality rate of adult ticks as 66.67% during 2 weeks of their treatment using crude methanolic extract derived from Datura stramonium. However, the current study findings are different from Al-Hasnawi and Wathah (2019) where phenolic, alkaloids and terpenoids extracts obtained from Datura metel leaves showed no effects in terms of the mortality of adult female and male Hyalomma schulzei ticks (Al-Hasnawi and Wathah 2019). Later on, after performing bioassay, it was demonstrated that 90% mortality of the eggs and larvae, whereas 74.21% and 64.41% mortality of starved and fed nymphs, respectively, was caused by crude phenolic compounds, alkaloids and terpenoids derived from leaves of Datura metel. That alkaloid extracts derived from Datura metel leaves displayed no effect on adult female and male ticks but may have a pivotal role in controlling different stages of Hyalomma schulzei life cycle. This study revealed 56.66% and 76.67% in vitro efficacy against H. detritum, for ethanolic and aqueous extracts derived from the leaves of Datura alba, respectively. The results variation can be attributed to differences in plant varieties. Lower efficacy was reported for methanolic or aqueous extracts derived from Citrullus colocynthis mortality of adult females of hyalomma dromedarii (Mahran et al., 2020). Whereas this study revealed 90% efficacy of both ethanolic and aqeuous extract of Citrullus colocynthis whole fruit (Peal and seed) for H. detritum. Such findings are exactly in line with the results reported by Ullah et al. (2015) where it was demonstrated that ethanolic and aqeuous extracts derived from Citrullus colocynthis fruit, seeds of Peganum harmala, and Curcuma longa in combination found to be 100% efficient in terms of mortality of Rhipicephalus microplus larvae. In addition, minimum mortality was shown for the extract derived from P. harmala against larvae. To control ectoparasites of animals, the utilization of plant-derived crude extracts has been recommended. The fruit and seed of Citrullus colocynthis plant produce secondary metabolites having activities against aphids (Soam et al., 2013).
The essential oils derived from several botanicals such as Cymbopogon martinii (palmarosa), Coriandrum sativum (coriander, Chinese parsley, cilantro), Laurus nobilis (laurel), Geranium spp (cranesbills, several species), Thymus vulgaris (thyme) and Rosa spp (Roses several species), contains geraniol which is a natural alcohol and a monoterpenoid. The palmarosa oil derived from Cymbopogon martini contains upto 70–85% geraniol and being used in several cosmetics, perfumes, industrial production of citronella, the tobacco industry, citronellol and food products. It is also found to act as a repellent and insecticide for mosquitoes, fleas, ticks and lice (Marsin et al., 2020).
Available literature demonstrates that evaluation of essential oils and various plant-derived extracts for acaricidal activity have been perfomed (Borges et al., 2011, Rodriguez-Vivas et al., 2018). The acaricide activity is attributed to the compounds identified such as acids, alcohols, aldehydes, sulfurated compounds, terpenes coumarins, geraniol, and stilbenes, especially to counter genera such as Dermacentor, Ixodes, Argas, Amblyomma, Rhipicephalus and Hyalomma. Ethanolic extract derived from A. indica leaves found to be less efficient (30%) as compared to the extract derived from A. indica seeds (80%) against adult R. microplus (Srivastava et al., 2008).
5 Conclusion
It is evident from history that the key factor for passing down ethnoveterinary knowledge was inheritance and for the treatment of animal diseases, various locally available botanicals were utilized. With the advent of high-tech medicine and modern facilities, trend to treat sick animal treatment is changed. Among advanced nations, excessive use of modern medicine in humans and animals resulted in the resistance development against the drugs used. Therefore, to escape or minimize the resistance development, plant-derived martials can be employed for better health and fitness practices as well as producing medicine. Further research and evaluation can be expedited by conserving the ethnoveterinary knowledge in the shape of national documents. To prepare standard extracts for the control of ticks, more investigations are required to better understand pharmacokinetics of plant-derived extracts examined in this experiment. It is pivotal to maintain the proportions of active compounds in the extracts and more efforts are needed to keep homogenous plants by gaining better understanding about the climate, soil and cultivation-related protocols. In addition, for the identification of the risk factors involved for animal and human health, toxicological studies are required.
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R745), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet. Parasitol.. 2014;203(1–2):6-20.
- [Google Scholar]
- Phenotype changes inherited by crossing pyrethroid susceptible and resistant genotypes from the cattle tick Riphicephalus (Boophilus) microplus. Exp. Appl. Acarol.. 2011;54(3):301-311.
- [Google Scholar]
- Al-Hasnawi, M. R. A. and E. F. Wathah, 2019. Effect of temperature and different extracts of Datura metel leaves on some biological aspects of Hyalomma schulzei (Acari: Ixodidae). Journal of Physics: Conference Series, IOP Publishing.
- Ali, A., S. Shehla, H. Zahid, et al., 2022. Molecular Survey and Spatial Distribution of Rickettsia spp. in Ticks Infesting Free-Ranging Wild Animals in Pakistan (2017–2021). Pathogens. 11 (2) 162.
- An Inventory of the Plants Used for Parasitic Ailments of Animals. Pakistan Vet. J.. 2012;32(2)
- [Google Scholar]
- Tick repellents and acaricides of botanical origin: a green roadmap to control tick-borne diseases? Parasitol. Res.. 2016;115(7):2545-2560.
- [Google Scholar]
- Borges, L. M. F., Sousa, L. A. D. d. and Barbosa, C. d. S. 2011. Perspectives for the use of plant extracts to control the cattle tick Rhipicephalus (Boophilus) microplus. Revista Brasileira de Parasitologia Veterinária. 20, 89-96.
- Distribution of Ixodid Tick Species and Associated Risk Factors in Temporal Zones of Khyber Pakhtunkhwa Province. Pakistan. Pakistan Journal of Zoology.. 2017;49(6)
- [Google Scholar]
- In vitro acaricidal effect of tannin-rich plants against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Vet. Parasitol.. 2011;175(1–2):113-118.
- [Google Scholar]
- First report of a Rhipicephalus microplus tick population multi-resistant to acaricides and ivermectin in the Mexican tropics. Vet. Parasitol.. 2012;183(3–4):338-342.
- [Google Scholar]
- Resistance of Rhipicephalus microplus to amitraz and cypermethrin in tropical cattle farms in Veracruz. Mexico. Journal of Parasitology.. 2012;98(5):1010-1014.
- [Google Scholar]
- Ticks and tick-borne diseases of bovines in a smallholder livestock context: The Pakistani example. Adv. Parasitol.. 2021;114:167-244.
- [Google Scholar]
- A social approach to the validation of traditional veterinary remedies–The Anthra project. Trop. Anim. Health Prod.. 2002;34(2):121-143.
- [Google Scholar]
- Tick-borne diseases of bovines in Pakistan: major scope for future research and improved control. Parasit. Vectors. 2015;8(1):1-13.
- [Google Scholar]
- Epidemiology, distribution and identification of ticks on livestock in Pakistan. Int. J. Environ. Res. Public Health. 2022;19(5):3024.
- [Google Scholar]
- Livestock Keepers' perception of indigenous goat breeds and their contribution to livelihoods in Pakistan. Egyptian Journal of Sheep and Goat Sciences.. 2013;65(1242):1-23.
- [Google Scholar]
- Selection of an ivermectin-resistant strain of Rhipicephalus microplus (Acari: Ixodidae) in Brazil. Vet. Parasitol.. 2010;168(1–2):97-104.
- [Google Scholar]
- Comparative efficacy of Annona squamosa and Azadirachta indica extracts against Boophilus microplus Izatnagar isolate. Parasitol. Res.. 2009;105(4):1085-1091.
- [Google Scholar]
- In Vitro acaricidal effect of Neem leaves (Azadirachta Indica) and Citrullus Colocynthis extracts against the camel ticks, Hyalomma Dromedarii (Acari: Ixodidae) Journal of Ecosystem and Ecography.. 2020;10(1):264-300.
- [Google Scholar]
- Essential oils as insect repellent agents in food packaging: a review. Eur. Food Res. Technol.. 2020;246(8):1519-1532.
- [Google Scholar]
- Miranpuri, G.S. 1979. Tick taxonomy in India-a review (including notes on their biology, ecology, geographical distribution, hostrelationship, ticks and tick-borne diseases and keys for species identification). Workshop on Advances in Insect Taxonomy in India and the Orient, Association for the Study of Oriental Insects, Himachal, India.
- Repellent activities of dichloromethane extract of Allium sativum (garlic)(Liliaceae) against Hyalomma rufipes (Acari) J. S. Afr. Vet. Assoc.. 2016;87(1):1-5.
- [Google Scholar]
- Alternative methods used by small-holder farmers to control ticks and bovine dermatophilosis and the impact of a changing interface of Amblyomma ticks on dermatophilosis in Zimbabwe. University of Fort Hare; 2014.
- An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red Crescent Med. J.. 2016;18(2)
- [Google Scholar]
- The extent of acaricide resistance in 1-, 2-and 3-host ticks on communally grazed cattle in the eastern region of the Eastern Cape Province, South Africa. J. S. Afr. Vet. Assoc.. 2008;79(3):130-135.
- [Google Scholar]
- GOP. Pakistan economic survey, 2020‐21, 2021. Finance Division, Government of Pakistan Islamabad.
- Rehman, A., 2017. Epidemiology of Ticks and Tick-borne Pathogens in the Semi-arid and the Arid Agro-ecological Zones in Pakistan.
- Synthetic Acaricides as A Promising-ol in Tick Control Program-The Present Scenario. Entomol. Appl. Sci. Lett.. 2020;7:58-69.
- [Google Scholar]
- Acaricidal properties of the essential oil and precocene II obtained from Calea serrata (Asteraceae) on the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Vet. Parasitol.. 2011;179(1–3):195-198.
- [Google Scholar]
- Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res.. 2018;117(1):3-29.
- [Google Scholar]
- Plant products and secondary metabolites with acaricide activity against ticks. Vet. Parasitol.. 2017;238:66-76.
- [Google Scholar]
- Ectopically expressed leaf and bulb lectins from garlic (Allium sativum L.) protect transgenic tobacco plants against cotton leafworm (Spodoptera littoralis) Transgenic Res.. 2008;17(1):9-18.
- [Google Scholar]
- Distribution and abundance of ticks infesting livestock population along Karakorum highway from Mansehra to Gilgit, Pakistan. Journal of the Hellenic Veterinary Medical Society.. 2017;68(1):51-58.
- [Google Scholar]
- Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol. Res.. 2014;113(5):1919-1926.
- [Google Scholar]
- Epidemiology of ixodid ticks in cattle population of various agro-climatic zones of Punjab, India. Asian Pac. J. Trop. Med.. 2013;6(12):947-951.
- [Google Scholar]
- Citrullus colocynthis (LINN.) and Luffa acutangula (L.) roxb, schrad. source of bioinsecticides and their contribution in managing climate change. IJABPT.. 2013;4(4):7-9.
- [Google Scholar]
- Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol. Res.. 2008;104(1):149-153.
- [Google Scholar]







