Translate this page into:
Prevalence of catheter associated biofilm producing bacteria and their antibiotic sensitivity pattern
⁎Corresponding author. malmalki@kfu.edu.sa (Mohammed A. Almalki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biofilm bacteria and antibiotic sensitivity pattern was studied. Identified different microbial strains. P. mirabilis, and Citrobacter were resistant against spectrum of antibiotics.
Abstract
Nosocomial urinary tract infections account for more than 40% of various hospital-acquired infections. Many uropathogens have been reportedly resistant against many commonly available antibiotics, treating patients with UTI infected with catheter associated biofilm producing MDR microorganisms has been a steep challenging task to clinicians. In the present study, a total of 350 clinical samples were collected from catheterized UTI cases admitted in wards and ICU. Specimens were cultured in defined media, and then isolates were biochemically identified. Antibiotics sensitive tests were performed to detect Multidrug resistant (MDR), extensive drug resistance (XDR) and pan drug resistance (PDR) in the isolated organisms. In this study, out of 585 isolates from 350 samples were subjected to biofilm detection. Among this, 63.9% of them were non-biofilm forming organism and 36% of the isolates were found to form biofilm. Out of 211 isolates, significant biofilm producers were E coli (24%), ESBL E coli (2%), Klebsiella (19%), E. fecalis (8%), S. aureus (3%), P. mirabilis (18%), Pseudomonas aeruginosa (17%) and Citrobacter (9%). In multi drug resistance, E coli was found to have PAN drug resistance followed by Klebsiella and Pseudomonas which exhibited XDR. However all other isolates like E. fecalis, S. aureus, P. mirabilis, and Citrobacter were found to be resistant against a limited spectrum of antibiotics. From this study, we could conclude that highest number of isolates was reported from UTI ward than the ICU. Imipenem showed better activity against all isolates.
Keywords
Biofilm
Bacteria
Antibiotic sensitivity
1 Introduction
Hospital associated urinary tract infection (UTI) accounts more than 40% of nosocominal infections. UTI infection is highly complicated in patients with functional or structural urinary tract abnormalities, or in immunocompromised patients. Moreover, higher number of people is being affected by this infection every year. UTIs are mainly associated with urinary catheters, which is a usual device used in hospitalized patients (Burke, 2003). About 25% of patients admitted in the hospitals have subjected to urinary catheters during their treatment. Also the surface of indwelling device potentially encourages the growth of biofilm producing microorganisms. In spite of measures have been taken to preclude hospital associated UTI, biofilm producers have been a big obstacle in terms of effective treatment and cure. Females are highly vulnerable to have this infection than males. They occur frequently from the ages 16 to 35 years, with more than 10% of females suffering an infection in their life period. Also, recurrence of this infection is common, an estimated 50% of individuals suffer due to re infection within a year (Tambyah et al., 2002). The mortality rate of catheter associated urinary tract infection was approximately 15% (Fortin et al., 2012). Various antibiotics have been used to control this infection; nevertheless various resistant UTI isolates have been reported in upper and lower urinary tract in developed countries. In recent years, patients with UTI are increasing rapidly and the nature of infection differ according to regional and geographical locations (Parameswarappa et al., 2013). Now a days there is an increasing concern on antimicrobial resistant Escherichia coli, which have been frequently reported as the major causative agent of UTI. However, bacteria associated with UTI are reported mostly from Enterobacteriacea group, which covers more than 75% of the strains (Al-Dhabi et al., 2016, 2018a, b, 2019a). In complicated UTI cases, bacteria such as, Enterococcus faecalis and multi drug resistant Gram –negative rod shaped bacteria including, Pseudomonas spp. are very common (Al-Dhabi et al., 2019b; Al-Dhabi et al., 2019c; Arasu et al., 2017). The occurrence of these bacterial pathogens varies widely based on hospitalization, catheterization, sex and age (Tantry and Rahiman, 2012). UTI is one of the common infections found in India, however the research on this ever-challenging field is minimal. Insensible application of catheters in ICU patients is main reason or MDR bacteria colonization, and increasing the incidence of catheters associated UTIs. The risk was estimated as 5% in a day of catheterization. The other risk factors for catheters associated UTIs, pregnancy, female gender, older age, fecal incontinence, poor nutrition, severity of illness, use of systemic antibiotics, elevated creatinine level and impaired immune system function (Ellen et al., 2009; Arasu et al., 2013; Arasu et al, 2019a,b)). The idea of the antibiotic susceptibility pattern and etiology of UTI causing pathogens is essential in tackling this menace. UTI treatment on patients is mainly based on antimicrobial resistance pattern of the various urinary pathogens. Moreover, uncontrolled antibiotic usage greatly increased antimicrobial resistance patterns among pathogens causing UTI (Kripke, 2005; Arasu et al., 2019c). Despite the use of UTI control, the infection rate is increasing in recent years. In US, more than 93,000 patients have been hospitalized due to UTI infection in a year. These complicated UTIs were seriously associated with serious complications, high rate of treatment failure, especially development or relapse of antibiotic resistance (Arokiyaraj et al., 2015; Boovaragamoorthy et al., 2019; Gurusamy et al., 2019; Roopan et al., 2019; Valsalam et al., 2019). Most of the UTI pathogens spread in hospitals though these patients. Also the growth of the elderly population, patients under severe immunosuppression, and patients undergoing transplant operation, development of relapse and antibiotic resistance are the major issues. In this case, Pseudomonas aeruginosa, is highly associated with UTI infection and poses a serious challenge. Treatment of Pseudomonas aeruginosa infection is a grave risk to the clinicians, because treatment options proposed have serious side effects. Uro-pathogens like, Enterococcus, Enterobacter, Klebsiella, Citrobacter, Pseudomonas aeruginosa, Providencia, Serratia and Staphylococcus epidermidis are the most important multidrug resistant isolates associated biofilm formation (Karlowsky et al., 2002). In 2010, Infectious Disease Society of America and the European Society for Microbiology and infectious diseases, recommended trimethoprim sulfamethoxazole or nitrofurantoin monohydrate/macrocrystals (Gupta et al., 2011; Ilavenil et al., 2015; Balachandran et al., 2015). In this present study, we examine the trends of the antibiotic-resistant uropathogens forming biofilm on the surface of indwelling devices in the hospitalized UTI cases and their susceptibility toward various antibiotics are evaluated. This study will further help in formulating guidelines for establishing a proper empirical therapy for UTIs while awaiting culture sensitivity reports. This will also reflect on changes in the susceptibility pattern of some of the most common uropathogens over the years in Southern India implying the need for periodic monitoring in order to decrease the number of therapeutic failures and to boost an effort to arrest the growing occurrences of antibiotic resistance.
2 Materials and methods
2.1 Samples
In this study, totally 350 samples were collected, out of 350, 288 were collected from wards and 62 were collected from ICU. The samples were collected aspectically and were analysed. The antibiotics such as, nalidic acid, ampicillin, gentamicin, amikacin, norfloxacin, ciprofloxacin, cefuroxime, cefotaxime, nitrofurantoin, cefepime, ofloxacin, vancomycin, penicillin, oxacillin, erythromycin imipenem and piperacillin were used to study the isolates and then classify them into categories such as sensitive, intermediate and resistant.
2.2 Isolation and identification of UTI causing pathogenic organisms
A clean-catch midstream urine sample of all catheterized patients was aseptically collected in a sterile container (50 ml) prior to antibiotic therapy. The samples were cultured in MacConkey agar and Brain heart infusion agar for isolating both Gram positive and Gram negative isolates and subsequently were incubated for 24 h at 35 ± 2 °C. UTI infection was considered as positive when the density of the culture exceeds to 105 colony forming units (CFU/ml). The isolated colony was biochemically identified using standard protocol up to the species level (Bonadio et al., 2001).
2.3 Antibiotic susceptibility testing of UTI pathogens
Antimicrobial susceptibility testing was carried out following the Kirby-Bauer disc diffusion method prescribed by Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2005).
2.4 Detection of biofilm
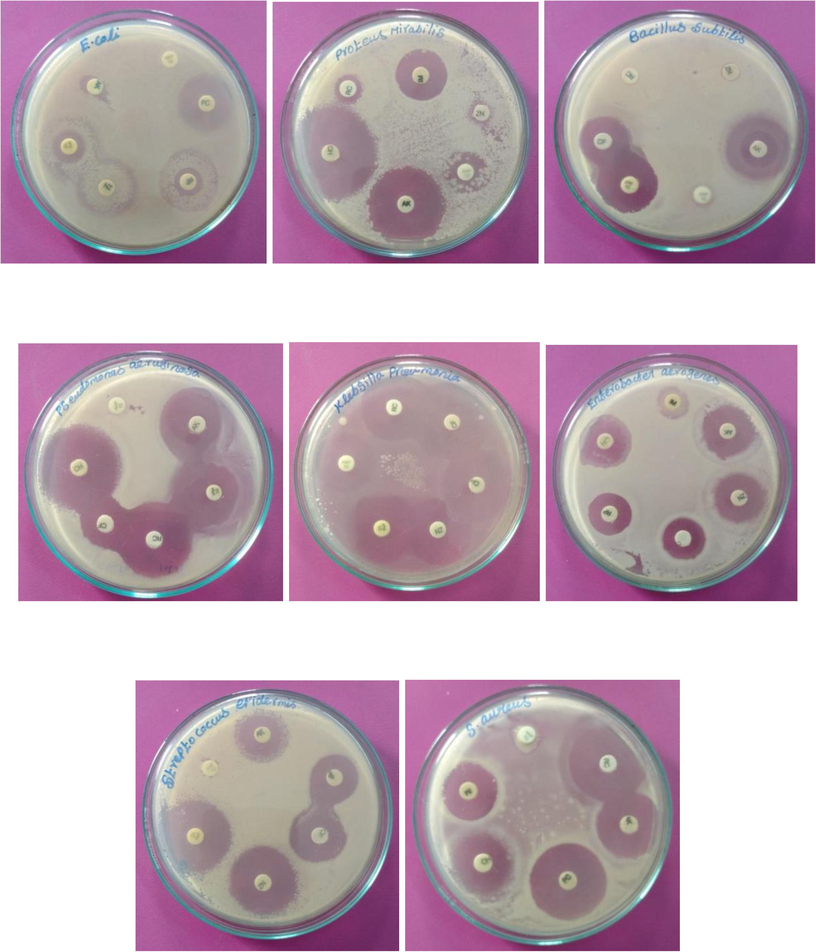
Loopful of an organism was inoculated into series of tubes containing Trypticase soya broth and 1% glucose and then tubes were incubated for 24 h at 37 °C. Culture was then decanted and the tubes were washed with a buffer (pH 7.3) Tubes were dried and after stained using crystal violet and the visible bio-film lined the wall of the tube (Lima et al., 2018).
3 Results
3.1 Distribution of biofilm producer from ward and ICU
In the present study, a total of 350 clinical samples were collected over a period of 10 months and Out of these 350 samples, 288 were collected from wards and from 62 were from the ICU. All the cultures were either monomicrobial or polymicrobial. Out of 585 isolates, 63.9% were non biofilm forming and 36.06% produced abiofilm. So non biofilm forming isolates were excluded from the further studies and the research was concentrated on the microbes which formed biofilms and were prevalent in the catheter associated UTI cases. A total of 211 isolates, E coli 24%, ESBL E. coli 2%, Klebsiella 19%, E. fecalis 8%, S. aureus 3%, P. mirabilis 18%, Pseudomonas aeruginosa 17%, Citrobacter 9% are found to be associated with the biofilm forming group of drug resistant microorganisms. In this group, E. coli was the most significant biofilm producer in catheterized cases followed by Klebsiella spp. Pseudomonas spp. In urine culture studies, Females at 76% had the highest percentage of isolates compared to their male counterparts (Fig. 1)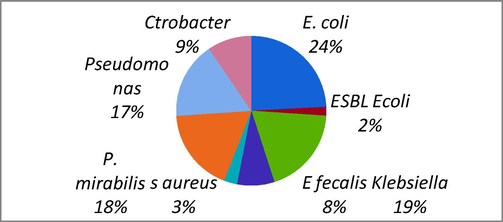
Bacterial strains causing biofilm in patients.
3.2 Susceptibility of antibiotics of UTI pathogens
In our study, 15 antibiotics were used to evaluate antibiotic sensitivity of catheter associated biofilm forming bacteria. Out of 211, 43.3% isolates were found to be sensitive against Nalidixic acid whereas 56.6% were not significantly resistant. Number of patients with each biofilm producer towards antibiotics pattern was recorded in Table 1. The antibiotics such as norfloxacin and ciprofloxacin were highly active against biofilm producers. In multi drug resistance, E. coli were reported to have PAN drug resistance followed by Klebsiella and Pseudomonas which exhibited XDR, however all other isolates like E. fecalis, S. aureus, P. mirabilis, Citrobacter were found to be resistant against limited antimicrobial spectrum. Among 211 isolates, 33 were found to be resistant against ampicillin. However amikacin and cephalosporin group effectively acted upon Gram positive and Gram negative isolates. Among all antibiotics, Imipenem was found to be effective against all tested isolates. The antibiotic sensitivity pattern of UTI pathogens among patients are described in Table 2. The clinical isolates showed varying degree of resistance against tested antibiotics (Fig. 2). The percentage composition of antibiotic susceptibility pattern of biofilm producing organisms is demonstrated in Table 2.
Biofilm producing organism
Antibiotic susceptibility pattern
Nal
Amp
tetr
Ami
Nor
Cipro
Cefuro
Nitro
Cefe
Olf
Van
Pip
Oxa
Imi
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
S
R
E. coli (n = 55)
16
39
43
12
14
41
39
16
49
6
45
10
44
11
13
42
39
16
48
7
11
44
33
22
11
44
53
2
Klebsiella pneumoniae (n = 40)
11
29
38
2
35
5
37
3
39
1
40
0
36
4
6
34
40
0
7
33
0
40
31
9
1
39
40
0
E .fecalis (m = 17)
9
8
17
0
15
2
17
0
0
17
0
17
17
0
10
7
17
0
17
0
3
14
14
2
17
0
17
0
S. aureus (n = 6)
2
4
5
1
5
1
0
6
3
3
0
6
0
6
2
4
0
6
2
4
0
6
5
1
1
5
6
0
P. mirabilis (n = 38)
35
3
34
4
38
0
35
3
38
0
38
0
38
0
5
33
35
3
38
0
33
5
6
32
3
35
38
0
Pseudomonas (n = 35)
10
25
33
2
31
4
34
1
33
2
35
0
34
1
3
32
35
0
5
30
0
35
29
6
1
34
35
0
Citrobacter (n = 20)
9
11
8
12
20
0
20
0
20
0
20
0
20
0
20
0
19
1
20
0
2
18
1
19
3
17
20
0
Total
92
119
178
33
158
53
182
29
182
29
178
33
189
22
59
153
185
16
137
74
49
162
119
91
37
174
209
2
Antibiotics
No. of isolates(n = 211)
Sensitive
%
Resistant
%
Nalidic acid
92
43.88
119
56.39
Ampicilin
178
84.36
33
15.63
Tetracycline
158
74.89
53
25.21
Amikacine
182
86.25
29
13.74
Norfloxacin
182
86.25
29
13.74
Ciprofloxacin
178
84.36
33
15.63
Cefuroxime
189
89.57
22
10.42
Nitrofurantoin
59
27.48
153
72.51
Cefepine
185
87.6
16
12.3
Ofloxacin
137
65
74
35
Vancomycin
49
23.22
162
76.77
Piperacillin
119
56.87
91
43.12
Oxacillin
37
17.5
174
82.5
Imipenem
209
99.99
2
0.01
Erythromycin
138
65.4
73
34.6
Gentamycin
154
72.98
57
27.02
Antibiotic resistance patterns of isolated uropathogens from the urine sample of admitted patients in hospital.
3.3 Distribution of biofilm producer in UTI among patients at various ages
Biofilm associated catheterized UTI is caused by either Gram-negative or Gram-positive bacteria. Most of the uropathogen positive cases were found in female individuals than in males. Females in the age group between 31 and 35 were more susceptible to urinary tract infection. Also, another study suggests that the age group between 11 and 15 were also highly susceptible than the age group between 16 and 20. The occurrence of infection in females is very high and this is mainly associated with pathogenic or anatomical factors such as, short length of the urethra, hence smaller distance for the uropathogens to move up the urinary tract. Maximum number of cases was reported in age-group 31–35 years followed by in age group 36–40. The important factor for high occurrence of UTI is mainly due to sexual activity and pregnancy. Also, majority of culture growth positive cases were in the age group of less than five years in case of females. This is mainly because children are not well trained to use toilets and due to fecal flora infection (Fig. 3).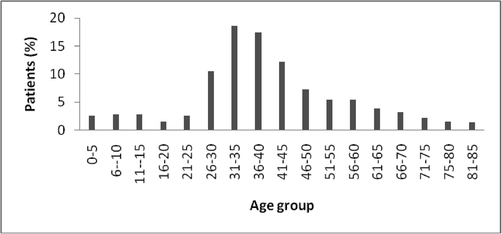
Distribution of UTI among patients at various ages.
3.4 Biofilm pathogens cause urinary tract infection
In the present study, the occurrence of biofilm producers among UTI causing pathogens was evaluated. Among the isolates, E. coli was found to be the important pathogenic bacteria that caused UTI and has been found to be one of the major virulent bacterial species in this study. Enterococcus infection was found to be high in ward UTI cases, however the percentage of infection due to Pseudomonas was found to be high in ICU patients. UTI due to the occurrence of pathogenic Proteus mirabilis and Citrobacter sp. have also been identified in this study, however the percentage of occurrence was found to be very less. The present study revealed that E. coli is the primary etiological agent among UTI patients (Table 3).
Organism
Biofilm groups
Non biofilm producer
Biofilm producer category
Total biofilm producer
Obstinate
Moderate
Weak
E. coli (n = 189)
161
7
18
23
55
Klebsiella pneumoniae (n = 93)
63
6
19
15
40
E. fecalis (n = 45)
79
4
6
7
17
S. aureus (n = 32)
36
1
3
2
6
P. mirabilis (n = 61)
27
8
22
15
38
Pseudomonas (n = 46)
23
9
12
14
35
Citrobacter n = 34)
25
5
9
6
20
Total
374
40
89
82
211
3.5 Bacterial biofilm pathogens from the male and female individuals
It was observed that UTI most commonly occurred in female individuals and more than 30% of female population experience with UTI during their lifetimes. The prevalence of UTI due to E. coli infection has been very high in females (40.3%) than males (20%). Also, the occurrence of Enterococcus spp., S. aureus, Proteus, Klebsiella spp., Pseudomonas spp. was predominant in females than males.
3.6 Antibiotic resistance of uropathogen in hospitalized patients
In this study, activity of yielded biofilm forming pathogens was categorized into three groups, MDR, XDR, and PDR. Out of 211 clinical isolates, 72% of them were reported to be resistant against Nitrofurantoin followed by oxacillin (82%) and vancomycin (76%) whereas most of them were sensitive to the antibiotic such as, Imipenem (99%) cefipine (87%), Amikacin (86%), Ampicillin (84%) and cefuroxime (89). E. coli is a most common pathogen that causes UT infection in all age groups especially 31–35. Analysis of resistance on various drugs may provide effective solution to control E. coli infection and subsequent treatment of the UTI. E. coli was treated against Penicillin, Quinolones, Sulfonamides, Tetracycline and Cephalosporin. Among these groups of antibiotics, tetracycline was found to be the most effective against E. coli, followed by Penicillin and Sulfonamides. The distribution of resistance pattern of E. coil to different in antibiotics is demonstrated in (Fig. 4).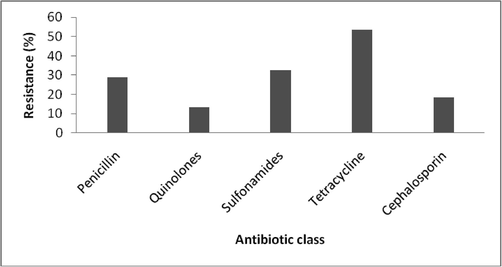
Antibiotic resistance pattern of E. coli from UTI.
4 Discussion
This study determined the pathogenic profile and various other factors associated with UTI infections caused by biofilm forming microorganisms in catheterised cases. UTI is one of the most important causes of nosocomial infections. This infection is treated by selective potent antibiotics. In most of the cases, the pattern of antimicrobial resistance of UTI pathogens varies in different regions. Hence, the continuous monitoring of antimicrobial resistance can help physicians for prescribing suitable antibiotics to treat UTI and in prevention of the development of drug resistance among uropathogens (Ilavenil et al., 2015). UTI is most prevalent in the age group of 30–40 years may be because of high frequency of sexual intercourse. Our findings are in accordance with the well studied observations and it was also confirmed that females have high frequency of UTI than males. This difference in frequency could mainly due to various clinical factors including, hormonal effects, anatomical variations and different behavioural patterns (Foxman, 2002).
The present study revealed that the prevalence of bacterial UTI in Kanyakumari District is about 30%. Out of this UTI infected patients, asymptomatic and symptomatic patients contributed to 59% and 41%, respectively. About 40% of the patients having bacteriuria were asymptomatic type, and this situation is of great concern since asymptomatic bacteriuria patter is a good predictor ensuing symptomatic type of UTIs (Hooton et al., 2000). The prevalence of UTIs in the present study was found to be little higher than the previous reports. The prevalence was 22.33%, 24.2%, 10%, 38.8% (Kabugo et al., 2016) in previous studies. Higher prevalence of UTIs could have been due to the various risk groups like elderly, infants, pregnant women and diabetes. The present finding revealed E. coli as the prevalent biofilm producing pathogens with 24%, and Klebsiella pneumoniae 19% Pseudomonas aeruginosa, 17%. It is reportedly less, when these results are compared with previous studies (McLaughlin and Carson, 2004). However, the reports on MDR Proteus spp. (18%) are found to be high. E. coli infection is most prevalent in female groups than males. The high prevalence of E. coli in the female individuals could mainly be due to the close proximity of the vagina to the anus. The possibility of UTIs in female individuals is mainly due to the virulence of E. coli for the colonization in urinary tract which moved from the perineum areas highly contaminated with fecal coliforms. Enterococcus sp. was also detected as biofilm producing pathogens with 12.7% of frequency. In recent study, in catheterised UTI cases, Enterococcus sp. such as, Enterococcus faecium, Enterococcus faecalis, Enterococcus pseudoavium have been identified. The hospital isolated E. faecium has displayed a high level gentamicin resistance than the normal E. Faecalis (Iregbu and Nwajiobi-Princewill, 2013).
Staphylococcus aureus also causes biofilm with frequency of 7% in catheterised UTI cases, the frequency rate varies widely. Earlier studies have associated the increasing Staphylococcal UTIs due to increased application of bladder catheters (Lo et al., 2013). Also, the isolation of Klebsiella sp. and Proteus mirabilis is in good agreement with previous studies carried out in Sao Paulo Brazil and South Western Uganda (Kauffman et al., 2000). The present finding reveals that the age group above 20 years, married individuals, female individuals, genitourinary tract abnormalities, diabetes, catheter, hospitalization and increase in use of bladder catheters are significant risk factors. The high prevalence in patients associated with these kinds of factors has been reported previously. Various changes in the causative pathogens of UTI, with an increase in the percentage of UTIs caused by Pseudomonas aeruginosa and Acinetobacter spp. is reported but decrease in the percentage of UTIs caused by Enterobacter sp. is also noted. Candida albicans is the most frequent cause of UTIs, and the involvement of Candida tropicalis, Candida krusei, Candida glabrata and Candida parapsilosis is also reported (Francesco De et al., 2007)
Earlier findings from various regions is that E. coli is the most prevalent biofilm producer, followed by Klebsiella spp. There has been addition to the extended spectrum β-lactamase (ESBL) producing organisms which have been resistant against antibiotic groups such as, fluoroquinolones, and aminoglycoside. In the present study, E. coli with rating 2% were reported to have ESBL (Mandal et al., 2012). The incidence of these enzyme producing organisms among Klebsiella spp. has been increasing rapidly in recent years and accounts for 6–17% of nosocominal UTIs. It was also further reported that K. pneumoniae was resistant against gentamicin and aminoglycoside, followed by third generation ceftriaxone, cephalosporin and ceftazidime. In K. pneumoniae, penicillin resistance can be validated by amoxicillin and ampicillin, mainly by the production of beta-lactamase, SHV-1 encoded on a transferable plasmid or on the chromosome (Kebira et al., 2009).
In this study, resistance of biofilm producers was categorized into three groups, MDR, XDR, and PDR. Out of 211 biofilm producers, highest percentage of isolates were reported to have been resistant against Nitrofurantoin (72%), oxacillin (82%) and vancomycin (76%) whereas sensitive to the antibiotic such as, Imipenem (99%) cefipine (87%), Amikacin (86%) and Ampicillin (84%) cefuroxime (89%). The tested samples showed drug resistance against first generation antibiotics. The antibiotic group from the second generations incurred lesser resistance than the first generation antibiotics. Patients of the age group 31–40 years were found highly susceptible to UTI followed by 41–45 years. These results correlated with earlier reports which revealed that females are mostly prone to UTIs than males during adulthood and adolescence (Ehinmidu et al., 2004; Al-Dhabi et al., 2015). The significant factors accounting to this incidence in infections at very young age are male and females are associated with frequent sexual activity, history of recurrent UTIs and recent use of a diaphragm with spermicide. Females are most susceptible due to Enterobacteriaceae and Enterococci infection, however, Proteus mirabilis was found to responsible for UTI in younger females and the cases of K. pneumonia infection were more frequent in elderly patients (Aboderin et al., 2009).
In this study, E. coli was mainly found in the urine samples of wards catheterized UTI infected patients. This finding was in consistent with that of previous report, however, the present finding was differs from the reports of as P. aeruginosa and in Klebsiella sp. where the organisms that have been reported as the major UTI causing uropathogens. The studies on uropathogens showed that E. coli and Klebsiella spp. are the most common uropathogens compared to other clinical isolates. It was previously reported that the higher incidence of Gram-negative bacteria, were mainly related to Enterobacteriaceae. Also, they are able to colonize in the urogenital mucosa with pili, adhesions, fimbriae and other receptors (Khameneh and Afshar, 2009).
The pathogens such as, Acinetobacter spp. and Pseudomonas sp. have been isolated from hospitalized patients. It could be noted that the uropathogen, Pseudomonas spp. can survive and thrive in presence of disinfectants and soaps used for urethral catheterization. Also, Gram-negative bacteria are more prevalent than Gram-positive bacteria. Drug resistance was found to be low in outpatients; however, more number of cases with antibiotic resistance was reported in hospitalized patients (Nickel, 2007).
In some studies E. coli from UTI showed resistance against cotrimoxazole, ampicillin, and cefuroxime and therefore should not be recommended for empiric therapy. In Antibiotic sensitivity tests, a low percentage of E. coli showed pan-drug resistance, whereas Klebsiella pneumoniae and Pseudomonas displayed XDR resistance. It was comparable with previous studies by Hitzenbichler et al. (2018).
5 Conclusion
Organisms growing on surface of indwelling devices like urinary catheter forms biofilm and are resistant to antibiotics and antiseptics. These organisms are a threat and pose a serious challenge to the clinicians in treatment and cure of the hospitalized patients. As Imipenem is not frequently used to treat both Gram positive and Gram negative isolates, many organisms is have failed to develop resistance against this antibiotic. From this study, we can conclude that Imipenem can be the drug of choice to treat biofilm causing uropathogens in catheterized UTI cases.
Acknowledgement
The author acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Nasher Track (Grant No. 186018).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial resistance in Escherichia coli strains from urinary tract infections. J. Nat. Med. Assoc.. 2009;101(12):1268-1273.
- [Google Scholar]
- In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evid. Based Complement Alternat. Med.. 2015;2015:164261
- [Google Scholar]
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 2018
- [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol. B: Biol.. 2018;189:176-184.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Valan Arasu M, Duraipandiyan V, Ponmurugan K. Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health. 2019;12:549-556.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol. B: Biol.. 2017;172:50-60.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B: Biol.. 2019;190:154-162.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng. C. 2019;102:536-540.
- [Google Scholar]
- Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Industrial Crops Prod.. 2019;133:54-62.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from SouthernWestern Ghats. South Indian. J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- Current microbiological and clinical aspects of urinary tract infections. Eur. J. Urol.. 2001;40:439-445.
- [Google Scholar]
- Clinically important microbial diversity and its antibiotic resistance pattern towards various drugs. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Clinical and Laboratory Standards Institute, author. Supplemental tables. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement. CLSI Publication M100-S15, M2-A8 and M7-A6. Pennsylvania: CLSI; 2005.
- Isolation and antibiotic susceptibility profile of Neisseria gonorrhoeae isolated from urine samples in Zara, Northern Nigeria. J. Phytomed. Therap.. 2004;9(1):20-24.
- [Google Scholar]
- Reducing use of indwelling urinary catheters and associated urinary tract infections. Am. J. Crit. Care. 2009;18(6):535-541.
- [Google Scholar]
- Healthcare-associated bloodstream infections secondary to a urinary focus: the Quebec Provincial Surveillance results. Infect ion Control Hospital Epidemiol.. 2012;33:456-462.
- [Google Scholar]
- Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med.. 2002;113(1):5-13.
- [Google Scholar]
- Urinary tract infections in Brescia, Italy: etiology of uropathogens and antimicrobial resistance of common uropathogens. Med. Sci. Monit.. 2007;13(6):136-144.
- [Google Scholar]
- International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis.. 2011;52(5):103-120.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B: Biol.. 2019;193:118-130.
- [Google Scholar]
- Antibiotic resistance in E. coli isolates from patients with urinary tract infections presenting to the emergency department. Infection. 2018;46(3):325-331.
- [Google Scholar]
- A prospective study of asymptomatic bacteriuria in sexually active young women. N. Engl. J. Med.. 2000;343(14):992-997.
- [Google Scholar]
- Growth and metabolite profile of Pediococcus pentosaceus and Lactobacillus plantarum in different juice. South Indian J. Biol. Sci.. 2015;1:1-6.
- [Google Scholar]
- Urinary tract infections in a tertiary hospital in Abuja, Nigeria. Afr. J. Clin. Exp. Microbiol.. 2013;14(3):169-173.
- [Google Scholar]
- Factors associated with community-acquired urinary tract infections among adults attending assessment centre, Mulago Hospital Uganda. Afr. Health Sci.. 2016;16(4):1131-1142.
- [Google Scholar]
- Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob. Agents Chemotherapy. 2002;46(8):2540-2545.
- [Google Scholar]
- Prospective multicenter surveillance study of funguria in hospitalized patients. Clin. Infect. Dis.. 2000;30(1):14-18.
- [Google Scholar]
- Isolation and antimicrobial susceptibility testing of Escherichia coli causing urinary tract infections. J. Appl. Biosci.. 2009;22:1320-1325.
- [Google Scholar]
- Antimicrobial susceptibility pattern of urinary tract pathogens. Saudi J. Kidney Dis. Transplant.. 2009;20(2):251.
- [Google Scholar]
- Duration of therapy for women with uncomplicated UTI. Am. Fam. Phys.. 2005;72(11):2219.
- [Google Scholar]
- Biofilm production by clinical isolates of Pseudomonas aeruginosa and structural changes in LasR protein of isolates non biofilm-producing. Braz. J. Infect. Dis.. 2018;22(2):129-136.
- [Google Scholar]
- Community-acquired urinary tract infection: age and gender-dependent etiology. Braz. J. Nephrol. (Jornal Brasileiro de Nefrologia).. 2013;35(2):93-98.
- [Google Scholar]
- Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. Indian J. Med. Res.. 2012;136(5):842.
- [Google Scholar]
- Urinary Tract Infections and Resistant Bacteria: Highlights of a Symposium at the Combined Meeting of the 25th International Congress of Chemotherapy (ICC) and the 17th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Munich, Germany. Rev. Urol.. 2007;9(2):78-88.
- [Google Scholar]
- Isolation, identification, and antibiogram of enterococci isolated from patients with urinary tract infection. Ann Afr. Med.. 2013;12(3):176.
- [Google Scholar]
- CuO/C nanocomposite: Synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng. C. 2019;101:404-414.
- [Google Scholar]
- The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect. Control Hos Epidemiol.. 2002;23(1):27-31.
- [Google Scholar]
- Antibacterial resistance and trend of urinary tract pathogens to commonly used antibiotics in Kashmir Valley. West Ind. Med. J.. 2012;61(7):703-707.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B: Biol.. 2019;191:65-74.
- [Google Scholar]







