Translate this page into:
Pretreatment of microalgal biomass to improve the enzymatic hydrolysis of carbohydrates by ultrasonication: Yield vs energy consumption
⁎Corresponding author. defariassilva@studenti.unipd.it (Carlos Eduardo de Farias Silva)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Microalgal biomass has been considered as a possible alternative source of carbohydrates and lipids in fermentative/reactional processes, called third generation of biofuels. Carbohydrates from microalgae are mostly composed by glucose and some pentose-derived polymers that must be hydrolyzed to be efficiently used. When enzymatic hydrolysis is applied a pretreatment is required. Sonication/ultrasonication is one of the most promising methods, and in this paper the influence of pretreatment time, sonication intensity and biomass concentration was validated, and the energy consumed in the process compared as well. Sonication intensity had the major role on the enzymatic accessibility. Pretreatment time can be used to decrease hydrolysis time. More than 90% of hydrolysis efficiency was reached when higher amplitude (sonication intensity) and pretreatment time were used. The applied energy influenced indirectly the hydrolysis process. The best saccharification/energy relation was reached when 50% of amplitude for 25 min was applied, obtaining 91% of hydrolysis yield and spending 2.4 MJ/kg of dry biomass.
Keywords
Microalgae
Ethanol
Fermentation
Amylase
Cellulase
Sonication
1 Introduction
Microalgal carbohydrates have been showed as an interesting alternative to conventional fermentative processes, thus contributing to third generation of biofuels which has several advantages in comparison to the two first ones: it avoids the food vs fuel competition as well as recalcitrant lignocellulosic problems (Silva and Bertucco, 2016). Ethanol (Silva and Bertucco, 2016), methane (Ding et al., 2016), hydrogen (Kumar et al., 2016) and buthanol (Wang et al., 2014a) can be cited as promising applications. Most sugars present in microalgal biomass are glucose polymers (cellulase, starch or glycogen, the last for cyanobacteria), but to be efficiently fermented this sugar content needs to be hydrolyzed (Chen et al., 2013; Vitova et al., 2015).
Among the algal hydrolysis methods, dilute acidic and enzymatic procedures exhibit better efficiencies. Acidic hydrolysis is a non-specific reaction generally performed with acid concentration between 1 and 10% and temperatures of 110–140 °C (Agbogbo et al., 2006; Logothetis et al., 2007; Nguyen et al., 2009; Nasser and Moghaz, 2010; Miranda et al., 2012; Ho et al., 2013; Lee et al., 2013; Wang et al., 2014b; Ashokumar et al., 2015; Wang et al., 2016; Silva et al., 2018), but has as bottlenecks the need of high amount of chemicals during the hydrolysis, a pH adjustment prior to the fermentation step and a high amount of salt formation which has inhibiting action against yeasts (Agbogbo et al., 2006; Logothetis et al., 2007; Nasser and Moghaz, 2010; Casey et al., 2013; Silva et al., 2018). On the other hand, enzymatic processes, besides of the high cost of enzymes, provides a more specific process at middle temperature and pressure, resulting in lower heating costs, and decrease the possibility for degradation processes to occur. However, to apply this last method, a pretreatment to improve the accessibility of carbohydrates to the enzymatic attack is necessary (Silva and Bertucco, 2016).
Several methods for algal cell disruption have been evaluated so far: ultrasonication, bead beating, microwave, osmotic shock (NaCl) and autoclaving (at 121 °C), and the results are different (Jeon et al., 2013; McMillan et al., 2013; Kurokawa et al., 2016). Sonication has the advantage of being able to disrupt the cells at relatively low temperatures (lower than microwave and autoclaving), faster extraction, suitability for all cell types, and it does not require beads or chemicals thus decreasing production costs (Jeon et al., 2013; McMillan et al., 2013; Byreddy et al., 2015; Kurokawa et al., 2016). Generally, it is divided in two ranges: low frequency non-focused ultrasound (LFNFU), at 20–50 kHz, and high-frequency focused ultrasound (HFFU), at 1–3 MHz; the first range is more suitable to heterogenous and enzymatic catalysis of biomass (Wang et al., 2014c). The high-energy impact and corrosion by high-intensity ultrasound to the biomass system contributes more easily to pretreat, fractionate and react with biomass under mild conditions, resulting in a higher yield of reaction and catalytic activity over thermochemical methods (Luo et al., 2014).
So far, ultrasonication has been used to prepare microalgal biomass, generally often, without verifying the intensity or energy efficiency of the process (for carbohydrate extraction and hydrolysis efficiency). For instance, Chlamydomonas fasciata was submitted to 30 W and 20 kHz for 30 min and extracted practically all intracellular starch (Asada et al., 2012), Chlorococcum using 130 W and 40 kHz for 25 min, reached 62.8% of glucose hydrolysis yield (Harun and Danquah, 2011a); Chlorella vulgaris at 24 kHz and 40% amplitude for 15 min achieved 75% of sugars recovery (Kim et al., 2014), in contrast to Chlorella sp. KR-1, with 27.4% of hydrolyzed sugars (Lee et al., 2015) and Chlorella homosphaera (both unpretreated biomass) with 47% of total glucose in biomass (Rodrigues et al., 2015), showing the importance of the pretreatment. Actually, seeking for the most energy efficient pretreatment for algal materials by parameter optimization is a research need (Luo et al., 2014).
Additionally, the sugars enzymatic hydrolysis process from microalgal biomass has shown lower yield in comparison with acidic treatment, and longer hydrolysis time are required (considering an acceptable value of biomass concentration in view of large scale exploitation), as for example, 27.4 instead of 93.3% for Chlorella sp. (50 gdry biomass/L and 3 h) (Lee et al., 2015), 64 compared to 96% for Chlorella vulgaris FSP-E (40 gdry biomass/L and 2–3 days) (Ho et al., 2013) and 62.8 instead of 100% for Chlorococcum (10–15 gdry biomass/L and 12 h) (Harun and Danquah, 2011a; Harun and Danquah, 2011b). These results emphasize two probable problems of the experiments: ineffective pretreatment and/or specificity/concentration of the enzymes.
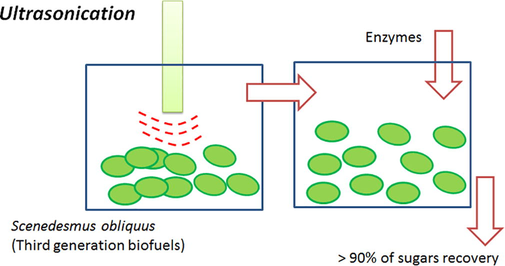
For this reason, this chapter is focused on the pretreatment using ultrasonication changing the time, intensity of ultrasound and biomass concentration, and verifying the relation between them and the energy consumption to saccharify Scenedesmus obliquus biomass.
2 Material and methods
2.1 Microalgal biomass
Scenedesmus Obliquus 276.7 (SAG- Goettingen) biomass was obtained by continuous cultivation in photobioreactor at 23 ± 1 °C, and maintained in a modified BG11 with nitrogen limitation to promote the accumulation of carbohydrates (Rippka et al., 1979; Silva et al., 2017), using 180 mg/L of N-nitrogen and 100 mg/L of P-phosphorous, being the rest of nutrients provided in double concentration.
The continuous cultivation was performed in a vertical flat-plate polycarbonate CSTR (continuous stirred tank reactor) PBR (photobioreactor) with a working volume of 700 mL, a depth of 1.2 cm, and the irradiated surface measuring 30 cm (length) and 19.5 cm (width).
CO2 in excess was provided by a CO2–air mixture (5% v/v) bubbling at the reactor bottom (1 L/h of total gas flow rate), which also provided mixing. A magnetic stirrer was used as well to prevent any deposition of biomass and thus ensuring a good mixing of the reactor. The fresh medium was fed at a constant rate by a peristaltic pump (Watson-Marlow sci400). The flowrate was regulated to obtain a residence time τ = 2.3 ± 0.3 days.
Light was provided by a LED lamp (Photon System Instruments, SN-SL 3500-22) and fixed at 650 µmol photons/(m2 s). Photon Flux Density (PFD) was measured on both the reactor front and back panels using a photoradiometer (HD 2101.1 from Delta OHM), which quantifies the photosynthetically active radiation (PAR). Biochemical characterization included the determination of moisture (method 934.01), ash (method 942.05), protein (method 2001.11), lipid content (method 2003.05), carbohydrates and monomers by HPLC (AOAC, 2002).
2.2 Enzymatic hydrolysis and analytical procedures
Enzymatic hydrolysis was performed using citrate buffer 50 mM, pH 5.0 at 50 °C. Enzymes mix was composed by Viscozyme® L (Novozymes cellulases mixture with ≥100 FBGU/g – betaglucanase units), AMG 300 L (amyloglucosidase from Aspergillus niger with 260 U/mL) and Pectinex Ultra SP-L (pectinase from Aspergillus aculeans with ≥3800 U/mL). All of these were produced by Novozymes® and purchased from Sigma-Aldrich®.
Enzyme concentrations per gram of biomass were fixed since the experiments must validate the effect of ultrasonication on extraction and saccharification of microalgal sugars. The concentrations were: Viscozyme L® – 20 U/gbiomass, AMG 300 L® – 100U/gbiomass and PectineX Ultra SP-L® – 1000U/gbiomass. Operating conditions were based on published papers been the environmental conditions able to permit each enzyme to work with a sufficient activity to perform the hydrolysis adequately (Harun and Danquah, 2011a; Asada et al., 2012; McMillan et al., 2013; Kim et al., 2014; Lee et al., 2015). Sodium azide was used in a concentration of 0.02% (w/v) to prevent contamination.
Saccharification efficiency is given by:
Dry cell weight was measured by gravimetry using cellulose acetate filters of 0.45 μm (Whatman®) at 105 °C and 2 h. Filters were pre-dried for 10 min at 105 °C in order to remove any moisture. Carbohydrate content (CC) in biomass was determined by Anthrone method which performed total sugars saccharification with concentrated sulphuric acid, and then all monomers react with antrone (Trevelyan and Harrison, 1952). Monomers (M) were determined by DNS method which determines only monomers present in the broth (Miller, 1959; Silva et al., 2015).
2.3 Preliminary experiments
Preliminary experiments to confirm the advantages of ultrasonication with respect to a control condition were carried out. The negative control condition used the microalgal biomass without any treatment and the positive control (exploded cells) utilized biomass suspension after autoclaving at 121 °C and 1 atm for 20 min (vapour-lineeco VWR®). Ultrasonication was done by using an Ultrasonic generator (cylindrical 1.5 cm of diameter and 12 cm of height, AA–WG1–800 W – SN 154, Active Arc Sarl, Switzerland) positioned centrally in the reactor (4 cm of diameter and 12 cm of height) with different amplitude/offset and reaction time options. The working volume of the reaction was 20 mL. The parameters were set to 40% of amplitude, 25% of offset for 40 min, which the equipment provided a power of 30 W, value based on previous works (Harun and Danquah, 2011a; Asada et al., 2012; McMillan et al., 2013; Kim et al., 2014; Lee et al., 2015). Sonication was applied in continuous (non-pulsed) mode with constant amplitude. The energy consumed in the process changed depending of the amplitude/offset and reaction time (calculated by the generator from the beginning to the end of process (input energy) and displayed in the equipment panel) and will be discussed in Section 3.3. Biomass concentration in all preliminary experiments was 10 g/L and optical microscopic visualization was verified before and after the pretreatments with a magnification of 75×.
2.4 Experimental design and statistical treatment
Ultrasonication assays were carried out according to a factorial experimental design 23 with three central point (2n where n is the number of independent variables), totalizing 11 experiments performed in independent duplicates. The variables studied were time (min), intensity of sonication (amplitude/offset) and biomass concentration (g/L) and the levels of the experimental design are summarized in Table 1.
Variable
−1
0
+1
Time (min)
5
15
25
Amplitude/Offset (%)
50/−40
60/−10
70/25
Biomass Concentration (g/L)
10
55
100
All statistical analysis were performed by the software Statistica® for factorial design analysis assuming p < 0.05 (95% of significance), considering the variables and their linear interactions.
The efficiency of the process was compared also with respect to the energy consumed per gram of biomass, to verify if it is the intensity and the energy applied by ultrasonication can provide higher accessibility of biomass to the enzymatic hydrolysis.
3 Results and discussion
Scenedesmus obliquus steady-state cultivation achieved a cell dry weight of 3.97 ± 0.09 g L−1. Biochemical characterization (% of dry matter) resulted in 33.63 ± 4.15 of proteins, 44.9 ± 4.5 of carbohydrates, 25.34 ± 0.64 of lipids and 6.83 ± 0.01 of ash. The profile of monosaccharides (% of carbohydrate content) was composed by 79.78 ± 3.96 of glucose, 16.14 ± 0.11 of xylose and 4.08 ± 0.31 of other monomers.
3.1 Preliminary experiments
In Fig. 1, preliminary results with and without pretreatment are shown. It is possible to conclude that sonication improved at least by 30% the saccharification yield in comparison with the negative and positive controls (without pretreatment and autoclaving pretreatment). In fact, these reached around 70% while with sonication all carbohydrate content was practically hydrolyzed in monosaccharides.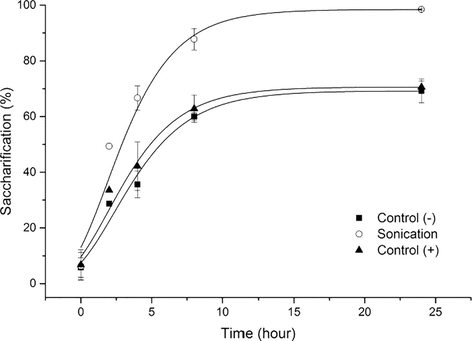
Saccharification of the preliminary experiments. Control (−) – Whitout treatment; Sonication – 40% amplitude, 40 kHz for 40 min and Control (+) – Autoclaving at 121 °C for 20 min.
This result is quite interesting in comparison to heat treatment. As can be seen in Fig. 2, autoclaving microalgal biomass promotes the completely cell explosion/de-structuration, but does not improved hydrolysis efficiency, probably because diffusion effects were limited by biomass aggregation (Fig. 2C). This is also in contrast with some comparisons made in the literature, which mention heating methods as more effective to cell disruption and considered as best method to separate biomass fractions and promote enzymatic hydrolysis (McMillan et al., 2013). On the other hand, after sonication an apparently not significant cell volume reduction as likely to occur (Fig. 2B), and a good volume dispersion (homogenization) is verified. Sonication promotes fissures and cracks on algal cell surface and consequently enhances enzyme accessibility (Jeon et al., 2013), and a reduction of cell volume can or not occur (Kurokawa et al., 2016). In Fig. 2D, an ‘apparently’ cell reduction is observed after enzymatic hydrolysis, a behavior which was already observed for C. homosphaera (Rodrigues et al., 2015).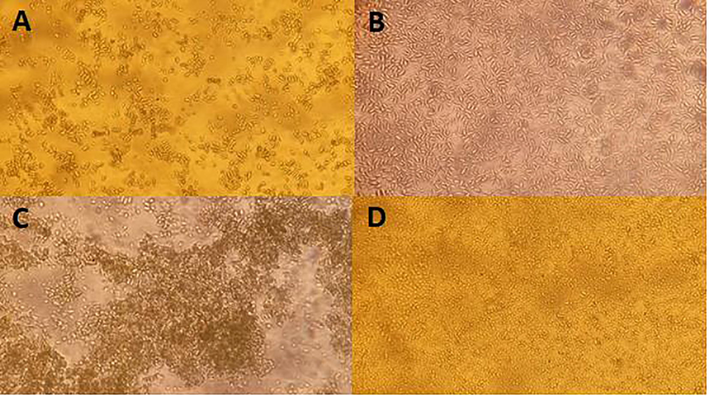
Optical visualization after the pretreatments. A) − Control (without treatment), B) Sonication, C) + Control (Autoclave) and D) Sonication experiment after enzymatic hydrolysis. 10 g/L of biomass concentration and optical magnification of 75×.
3.2 Experimental design and statistical analysis
As aforementioned, pretreatment time, sonication intensity (amplitude) and biomass concentration were validated by a factorial design of the experimental runs. The enzymatic hydrolysis was very successful, with some experiments achieving more than 90% of sugars recovery as monomers. As can be seen in Table 2, the best runs were those with higher sonication intensity, higher pretreatment time and lower biomass concentration (5 and 6), which exhibited values near to 95% of saccharification, reaching almost 90% in 4 h of hydrolysis.
Assay
Time (min)
Sonication parameter amplitude/Offset (%)
Biomass concentration (g.L−1)
Saccharification (%)*
Time (h)
0
2
4
8
24
1
5
50/−40
10
13.00 ± 1.41
32.18 ± 1.19
36.02 ± 2.01
62.26 ± 4.16
79.08 ± 5.06
2
25
50/−40
10
10.50 ± 0.71
37.54 ± 1.19
40.70 ± 0.74
82.24 ± 6.24
83.71 ± 0.89
3
5
50/−40
100
10.97 ± 0.14
66.33 ± 2.74
67.85 ± 1.95
70.00 ± 4.05
79.09 ± 1.35
4
25
50/−40
100
9.00 ± 1.41
65.83 ± 0.08
77.87 ± 0.38
83.06 ± 0.97
90.90 ± 0.08
5
5
70/25
10
11.00 ± 1.41
81.23 ± 6.69
86.66 ± 2.34
92.58 ± 4.46
92.89 ± 5.51
6
25
70/25
10
10.50 ± 0.71
88.50 ± 2.12
89.62 ± 9.29
93.04 ± 0.74
96.38 ± 1.96
7
5
70/25
100
9.89 ± 1.09
65.31 ± 7.20
68.65 ± 2.02
74.21 ± 2.40
79.67 ± 9.52
8
25
70/25
100
12.65 ± 0.04
67.82 ± 4.46
81.26 ± 0.53
85.29 ± 0.23
87.67 ± 8.54
9
15
60/−10
55
8.83 ± 0.45
74.72 ± 4.61
71.55 ± 0.40
74.02 ± 5.89
88.48 ± 7.48
10
15
60/−10
55
9.19 ± 0.05
70.32 ± 2.94
73.06 ± 1.87
75.17 ± 2.14
88.38 ± 5.48
11
15
60/−10
55
8.57 ± 0.19
77.65 ± 0.74
75.12 ± 2.01
74.15 ± 6.42
86.78 ± 5.35
In fact, in the Pareto charts represented in Fig. 3 for each hydrolysis time considered in this paper, it is possible to see that at the beginning (3A) no influence of the variables in the pretreatment was observed, i.e., the sugars concentration starts with approximately the same value. This is important, because the temperature in some experiments remained with a maximum value of 40 °C while other reached 90 °C (details are reported in Section 3.3). After, all experiments were influenced positively by sonication intensity (amplitude) and negatively for the linear interaction of sonication intensity and biomass concentration (2 L by 3 L), i.e., higher sonication intensity promoted more enzyme accessibility and, consequently, hydrolysis, and lower biomass concentration was better pretreated. However, with respect to biomass concentration, it is unfeasible to maintain an industrial process with 10 g/L of biomass, which is generally accounted to be at least 100 g/L. Another detail is the energy consumption of the process and how much the hydrolysis yield is influenced by the energy used in the pretreatment process, a point later discussed in Section 3.3.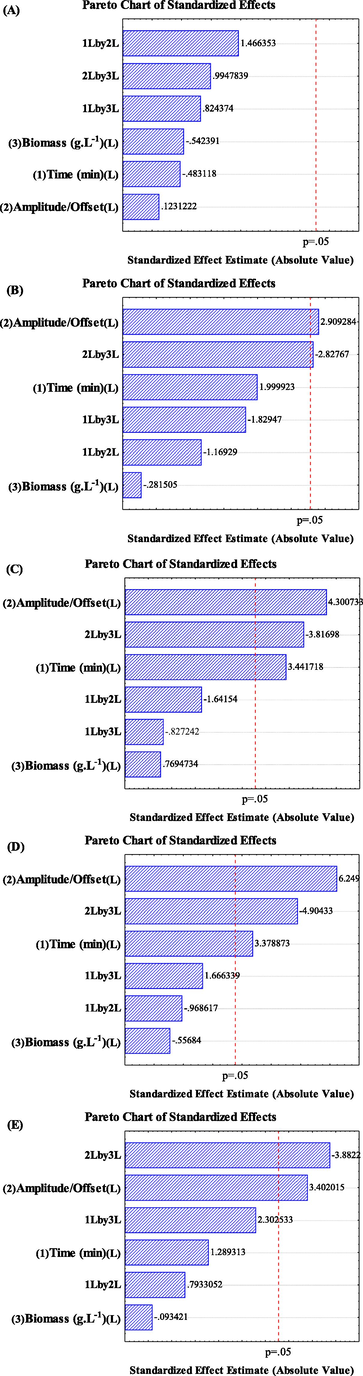
Effect of the variables on enzymatic yield for each hydrolysis time. A) 0, B) 2, C) 4, D) 8 and E) 24 h.
Additionally, an interesting consideration was found in the experiments with 4 and 8 h of hydrolysis: the pretreatment time influenced positively, i.e., if a faster hydrolysis time is required, higher pretreatment time can be used.
We are not aware any sonication intensity study (amplitude) applied to algal pretreatment for sugars hydrolysis in the literature. But some information is provided by sonication frequency sensitivity to disrupt algal cells: Chaetoceros gracilis, Chaetoceros calcitrans and Nannochloropsis sp., treated with frequencies between 0.02 and 4.3 MHz, demonstrated the values of 2.2–4.3 MHz as efficient in cell reduction (%) – a parameter used to evaluate the process efficiency (Kurokawa et al., 2016). Scendesmus dimorphus and Nannochloropsis oculata treated with 20 kHz and 3.2 MHz (low and high frequency) to evaluate chlorophyll and lipid fluorescence and consequently extraction, demonstrated no differences in lipid recovery, even though the combination of high and low frequencies could decrease the pretreatment time (Wang et al., 2014c).
Wang et al. (2014c) also verified that the pretreatment time influenced significantly the lipid extraction, proportionally between 1 and 5 min, reaching value from 50 to 100% of lipid extraction. In dark fermentation of ethanol, volatile fatty acids (VFA) and hydrogen, the pretreatment time was a key-point to promote bioaccessibility/bioavailability of microalgal biomass (Scenedesmus obliquus YSW15) in microbial fermentation reducing the cell surface hydrophobicity and increase ethanol and VFA production (Jeon et al., 2013). Thus, the additional positive influence of the pretreatment time was expected and confirmed.
The regression curves and coefficients of the variables effects on saccharification yield are presented in Eqs. (2)–(5), and their surface graphs are in Fig. 4, showing more specifically the visual representation of these effects.
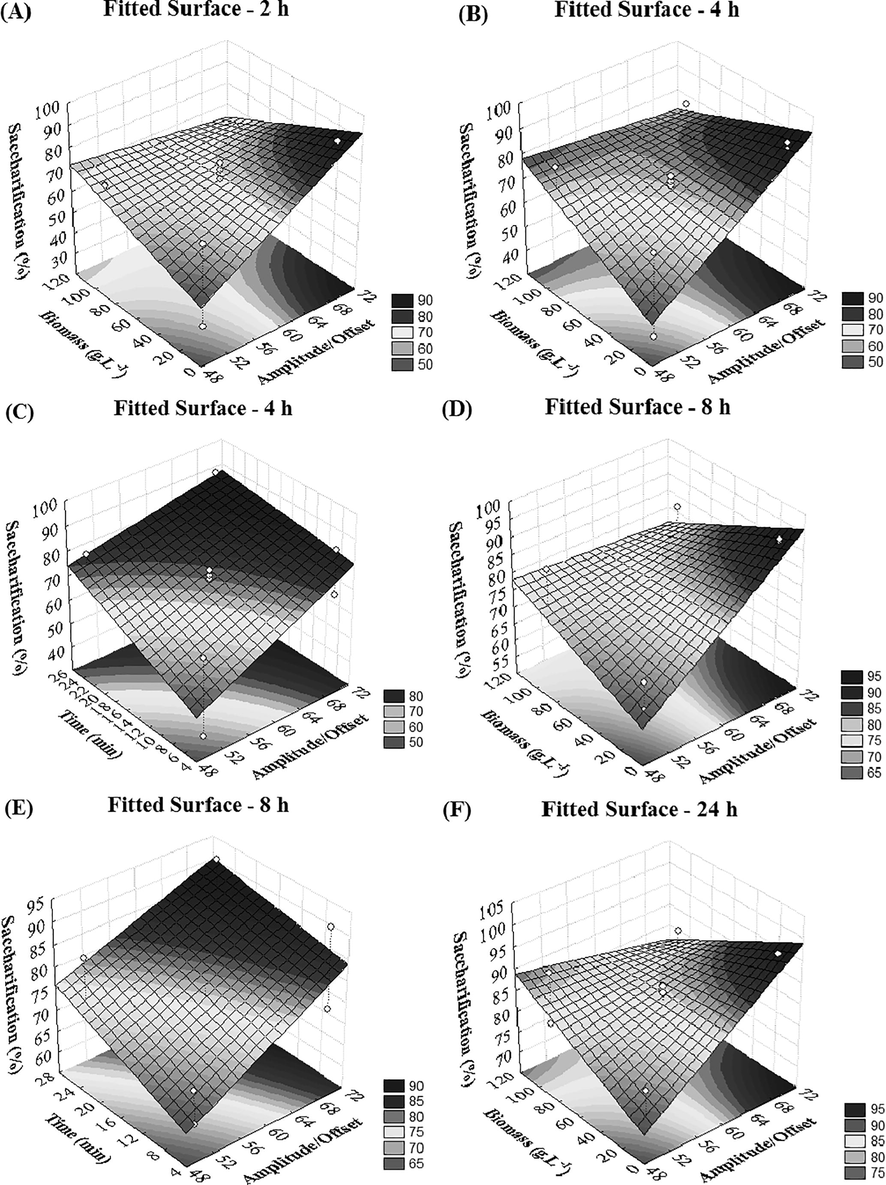
Surface graphs of the models obtained by the experimental design.
R2 = 0.8632
R2 = 0.9243
R2 = 0.9515
where: SY – Saccharification yield (%), Time – pretreatment time (min), Amp – Amplitude/Offset (%) and Cbiomass – biomass concentration (g/L).
3.3 Energy analysis
The energy analysis of the process is important towards an industrial application. Energy duty is often a bottleneck of sonication treatment, thus, optimizing the energy required to provide an efficient saccharification is a must. As reported in Table 3, the ratio energy/biomass changed a lot. Experiments 5 and 6, which reached around 95% of hydrolysis yield spent a considerable amount of energy and make them an unfeasible choice. On the other hand, experiment 4, reached 90% of hydrolysis (24 hrs) and 83% after 8 h but using between 30 and 100 times less energy, i.e., 2.4 MJ/kgbiomass in comparison with the experiments 5 and 6, respectively.
Assay
Power (W)
Total energy consumption (kJ)
Energy/Volume (kJ/mL)
Energy/Biomass (MJ/kgbiomass)
Final temperature (°C)
Maximum yield of sugars (%)**
1
2–4
0.98
0.049
4.90
30
79.08
2
2–4
6.02
0.301
30.10
42
83.71
3
2–4
0.92
0.046
0.46
32.1
79.09
4*
2–4
4.79
0.240
2.40
37.9
90.90
5
34–55
13.00
0.650
65.00
90.2
92.90
6
29–58
43.20
2.160
216.00
89.5
96.38
7
36–50
11.30
0.565
5.65
90.2
79.67
8
37–59
46.90
2.345
23.45
93.2
87.67
9
13–21
12.60
0.630
11.45
85
88.48
10
9–20
11.60
0.580
10.54
83.9
88.38
11
9–19
11.60
0.580
10.54
83.2
86.78
Some literature values of energy consumption for microalgal pretreatment using sonication are: 70.6 MJ/kg for Scenedesmus obliquus YSW15 (Jeon et al., 2013); 1200 MJ/kg for Thraustochytrid strains (Byreddy et al., 2015) and 44–132 kJ/kg (extrapolated) for Nannochloropsis oculata, but this reduced value demonstrated much lower efficiency in comparison with microwave oven, blender and laser treatments (McMillan et al., 2013). Thus, the value of 2.4 MJ/kgbiomass represents a promising value, mainly considering the energy content of microalgal biomass which is generally between 20 and 22 MJ/kg (Sforza et al., 2014).
In Fig. 5, a plot between the energy consumed and % of saccharification for each hydrolysis time is displayed. It can be concluded that the hydrolysis efficiency does not depend directly on the energy input, even though a trend be observed, but on the intensity of amplitude mainly, emphasizing the message of our study.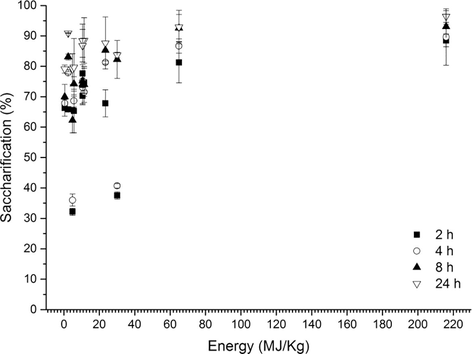
Energy consumption versus % saccharification of the experiments.
Ultrasound is a mechanical acoustic wave with the frequency range from roughly 10 kHz–20 MHz. It imparts high energy to reaction medium by cavitation and secondary effects (physical and chemical) (Luo et al., 2014). When the ultrasonication is used to break cells, it is important to determine the energy intensity (experimentally represented by a combination of amplitude-power generated and time) and population of active cavitation to promote the specific reactivity with cells and increase the accessibility to substrate (Kurokawa et al., 2016). In this paper, the validation was based on enzymatic hydrolysis of microalgal biomass, and, in fact, the intensity of sonication showed to be important, but not directly linked to the energy consumed in the pretreatment process. Specifically, this indicates that physical and chemical changes can be achieved by ultrasound which were sufficient to perform enzymatic hydrolysis.
4 Conclusions
The profitability of performing enzymatic hydrolysis of the sugars contained in Scenedesmus obliquus by ultrasonication was addressed with respect to wave intensity, treatment time and biomass concentration. Ultrasonication was effective to treat microalgal biomass guarantying an excellent enzymatic hydrolysis performance which can compete with chemical methods, as for example, dilute acid treatment. Sonication intensity and pretreatment time had positive importance to enzyme accessibility to reach more than 90% of hydrolysis yield with a limited and acceptable energy duty. The sugars obtained by this way are available to several fermentative possibilities.
Acknowledgement
The authors thank CNPq – Brazil (National Research Council of Brazil) – Process number 249182/2013-0, for resources and fellowship.
References
- Effect of pretreatment chemicals on xylose fermentation by Pichia stipitis. Biotechnol. Lett.. 2006;28:2065-2069.
- [Google Scholar]
- AOAC – Association of Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed., Gaithersburg: Ed. William Horwitz, 2002.
- Efficient extraction of starch from microalgae using ultrasonic homogenizer and its conversion into ethanol by simultaneous saccharification and fermentation. Nat. Resour.. 2012;3:175-179.
- [Google Scholar]
- An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers. Manage.. 2015;101:778-786.
- [Google Scholar]
- Comparison of cell disruption methods for improving lipid extraction from Thraustochytrid strains. Mar. Drugs. 2015;13:5111-5127.
- [Google Scholar]
- Effect of salts on the co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnol. Biofuels. 2013;6(83):1-10.
- [Google Scholar]
- Microalgae-based carbohydrates for biofuels production. Biochem. Eng. J.. 2013;78:1-10.
- [Google Scholar]
- Co-generation of biohydrogen and biomethane through two-stage batch co-fermentation of macro- micro-algal biomass. Bioresour. Technol.. 2016;218:224-231.
- [Google Scholar]
- Enzymatic hydrolysis of microalgal biomass for bioethanol production. Chem. Eng. J.. 2011;168:1079-1084.
- [Google Scholar]
- Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem.. 2011;46(1):304-309.
- [Google Scholar]
- Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol.. 2013;135:191-198.
- [Google Scholar]
- Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol. Biofuels. 2013;6(37):1-9.
- [Google Scholar]
- Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol.. 2014;153:47-54.
- [Google Scholar]
- Evaluation of different pretreatments on organic matter solubilization and hydrogen fermentation of mixed microalgae consortia. Int. J. Hydr. Energy. 2016;41:21628-21640.
- [Google Scholar]
- Effect of sonication frequency on the disruption of algae. Ultrason. Sonochem.. 2016;31:157-162.
- [Google Scholar]
- Chemeo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga Dunaliella tertiolecta. Bioresour. Technol.. 2013;132(2013):197-201.
- [Google Scholar]
- Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour. Technol.. 2015;196:22-27.
- [Google Scholar]
- Effect of salt hyperosmotic stress on yeast cell viability. Proc. Nat. Sci. U.S.A.. 2007;113:271-284.
- [Google Scholar]
- Ultrasound-enhanced conversion of biomasss to biofuels. Prog. Energy Combust. Sci.. 2014;41:56-93.
- [Google Scholar]
- Evaluation and comparison of algal cell disruption methods: microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy. 2013;103:128-134.
- [Google Scholar]
- Use of dinitrosalicyclic acid reagent for determination of reducing sugars. Anal. Chem.. 1959;31(3):426-428.
- [Google Scholar]
- Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol.. 2012;104:343-348.
- [Google Scholar]
- Comparative study of salt tolerance in Saccharomyces cerevisiae and Pichia pastoris yeast strains. Advances in Bioresearch. 2010;1(1):169-176.
- [Google Scholar]
- Hydrothermal acid pretreatment of Chlamydomonas reinhardtii for ethanol production. J. Microbiol. Biotechnol.. 2009;19(2):161-166.
- [Google Scholar]
- Untreated Chlorella homosphaera biomass allows for high rates of cell wall glucan enzymatic hydrolysis when using exoglucanase-free cellulases. Biotechnol. Biofuels. 2015;8(25):1-16.
- [Google Scholar]
- Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microb.. 1979;111:1-61.
- [Google Scholar]
- Effects of light on cultivation of Scenedesmus obliquus in batch and continuous flat plate photobioreactor. Chem. Eng. Trans.. 2014;38:211-216.
- [Google Scholar]
- Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem.. 2016;51:1833-1842.
- [Google Scholar]
- Stability of carbohydrate production in continuous microalgal cultivation under nitrogen limitation: effect of irradiation regime and intensity on Tetradesmus obliquus. J. Appl. Phycol. 2017 in Press
- [Google Scholar]
- Citric waste saccharification under different chemical treatments. Acta Scientiarum Technology. 2015;37(4):387-395.
- [Google Scholar]
- A systematic study regarding hydrolysis and fermentation from microalgal biomass. Biocatal. Agric. Biotechnol.. 2018;14:172-182.
- [Google Scholar]
- Studies on yeast metabolism. 1. Fractionation and microdetermination of cell carbohydrates. Biochem. J.. 1952;50(3):298-303.
- [Google Scholar]
- Accumulation of energy reserves in algae: from cell cycles to biotechnological applications. Biotechnol. Adv.. 2015;33:1204-1218.
- [Google Scholar]
- Charactherization and kinetics of bio-butanol production with Clostridium acetobutylicum ATCC 824 using mixed sugar medium simulating microalgae-based carbohydrates. Biochem. Eng. J.. 2014;91:220-230.
- [Google Scholar]
- Joint production of biodiesel and bioethanol from filamentous oleaginous microalgae Tribonema sp. Bioresour. Technol.. 2014;172:169-173.
- [Google Scholar]
- Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol.. 2014;153:315-321.
- [Google Scholar]
- Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresour. Technol.. 2016;200:557-564.
- [Google Scholar]







