Translate this page into:
Potential role of Albizia lebbeck and Emblica officinalis on smooth muscle contractions in experimental animal models
⁎Corresponding author. sasdag@mcst.edu.sa (Syed Mohammed Basheeruddin Asdaq), sasdaq@gmail.com (Syed Mohammed Basheeruddin Asdaq),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background and objective
Spasms are involuntary muscular contractions commonly seen frequently. This study used isolated tissue preparations to test the efficacy of Albizia lebbeck (A.L) and Emblica officinalis (E.O) extracts for spasmolytic activity.
Materials and methods
The herbal extracts were tested in isolated guinea pig ileum, rat uterus, rat fundus, and rabbit jejunum. Histamine was used as spasmogen in guinea pig ileum, while 5-hydroxytryptamine (5-HT) was in rat uterus and rat fundus. Spontaneous contractions' amplitude and frequency were recorded in the rabbit jejunum after administering herbal extracts. The influence of the extracts on smooth muscle contraction was calculated and statistically analyzed by one-way ANOVA. The P value was kept at <0.05 for all statistical analyses to consider it significant.
Results
Observation from the present study indicated that A.L significantly (p < 0.05) enhanced the contraction induced by histamine and 5-HT in guinea pig ileum (50 mcg/ml) and rat fundus (150 mcg/ml), respectively. In the rabbit jejunum, the amplitude and frequency of contraction were significantly (p < 0.05) reduced at 500 mcg/ml. E.O. was found to suppress the spasmogenic (histamine and 5-HT) at doses beyond 150 mcg/ml and, in rabbit jejunum, enhanced the amplitude and frequency of contraction at 50 and 150 mcg/ml. The IC50 values for E.O. in guinea pig ileum, rat uterus, and rat fundus were 35.2, 50.3, and 124.7 mcg/ml, respectively.
Conclusion
The observation suggests that A.L enhanced smooth muscle contraction in the presence of known spasmogens and reduced it in the absence. Opposite effects were found for E.O., where it reduced contraction in the presence of spasmogens and increased in the absence. These findings suggest potential spasmogenic/spasmolytic activities of the tested extracts.
Keywords
Albizia lebbeck
Emblica officinalis
Spasmolytics
Isolated tissue preparations
1 Introduction
Smooth muscle spasms are involuntary contractions of a group of muscles and are often associated with a painful condition (Murata et al., 2019). The etiology of spasms ranges from benign causes, such as electrolytic disturbances that might modify muscle physiology, to sometimes more severe neurological and degenerative conditions. In many instances, the spasms get relieved on their own (Achem and Gerson, 2013). However, if the painful condition persists, medical interventions such as non-steroidal anti-inflammatory drugs (NSAIDs) are most preferred (Liu et al., 2016). These medications were known to relieve only the symptoms without affecting the cause of the disease. Further, the chronic use of NSAIDs is known to cause several adverse effects, aggravating the existing patients’ conditions (Bindu et al., 2020).
The use of herbal substances for treating diseases dates back 5000 years. These medicines are popular due to their easy availability, cheap cost, and per se, lower side effects (Ziemska et al., 2019). According to an estimate, still, around 80% of the population living in Asian and African countries use herbal medicines as the primary source for treating diseases. An earlier study reported that approximately 25% of modern drugs used in the United States had been derived from plants (Jafari et al., 2014). Some examples include aspirin from willow bark, morphine from the opium poppy, and quinine from cinchona bark. Being derived from nature, these compounds are reported to act on multiple pathways in treating not only the symptoms but also the origin of the disease (Luo et al., 2020).
Alzibia lebbeck Benth (A.L) (Family: Fabaceae) is a deciduous tree that grows to 18 m. The leaves are bipinnate and are alternately arranged on smooth and green twigs. The plants can be found in Asia, Africa, America, and Australia (Latif et al., 2019). Different parts of the plant are traditionally used for treating several diseases. Some therapeutic activities reported for A.L. are anti-inflammatory, anti-spasmodic, anti-nociceptive, anti-cancer, anti-malarial, anti-allergic, anti-hyperglycemic, nootropic, wound healing, and neurodegenerative diseases (Balkrishna et al., 2022). Several phytoconstituents have been isolated and identified, and important among them are lebbeckosides, 2-pentyfuran, E-geranyl acetone, α-ionone, lebbeckalysin, geraldone and isookanin. Different parts of the plant, such as bark, leaves, flowers, seeds, and roots, have been used for medicinal purposes (Desai and Joshi, 2019).
Emblica officinalis Geartn. (E.O), belonging to the family Euphorbiaceae is a deciduous tree found in several countries. The leaves of the plant are greenish-yellow and small. The fruits are almost globular with conical depression at the longitudinal axis and are pale green or yellowish in color (Hashem-Dabaghian et al., 2018). Fruits are famous for medicinal purposes and are also used as components of culinary items. Fruits are considered a good source of vitamin C. Besides, emblicanin, phyllaemblicin, punigluconin, pedunculagin, phyllantine, and leukodelphinidine have also been isolated and identified. The plant and its constituents have been found to possess several biological activities. They are folklore used as antimicrobial, radioprotective, hypolipidemic, anti-inflammatory, anti-diabetic, anti-cancer, anti-spasmodic, hepatoprotective, and anti-ulcer (Variya et al., 2016).
In earlier studies, both A.L and E.O were reported to have exhibited anti-diarrheal activity suggesting spasmolytic potential (Khan et al., 2019a,b; Perianayagam et al., 2005). The present study was planned to provide more scientific evidence, and a possible mechanism for the spasmolytic activity of A.L and E.O. The extracts of the plants were tested using isolated tissue preparations such as guinea pig ileum, rat uterus, rat fundus, and rabbit jejunum.
2 Materials and methods
2.1 Chemicals, botanicals, and drugs
The chemicals required for the study were procured from the standard chemical suppliers of the Institution and were of analytical grade meant for the research purpose. Herbal extracts of A.L and E.O in spray-dried powder form were obtained as a ‘gift sample’ (only for research) from Natural Remedies, Bangalore, India. The herbal components were identified and authenticated by the scientist of Natural Remedies, and the samples were stored in their herbarium. Histamine hydrochloride was purchased from the distributor of Acros Organics, Belgium, and 5-hydroxytryptamine (5-HT) from Sigma-Aldrich, Bulgaria. Chlorpheniramine hydrochloride, cyproheptadine hydrochloride, and diethylstilbesterol were purchased from Wockhardt Limited, Mumbai, India.
2.2 Animals
Eighteen Albino rats (Wistar strain) of either sex weighing 150–200 gm and aged 4–5 months were used in the study. The animals were in-bred at the central animal house, Al-Ameen College of Pharmacy (AACP), Bangalore. The animals were maintained with free access to food (standard pellet diet, Amrut feed, Bangalore) and at room temperature. Female rats were used for uterine preparation and treated intraperitoneal for 3 days with diethylstilbesterol before sacrificing. Twelve guinea pigs were procured from National Tuberculosis Institute, Bangalore. Animals of either sex weighing 500–900 gm were used for the study and were maintained in the Central animal house, AACP, Bangalore, one week before the experiment. The Central animal house, AACP, Bangalore, provided rabbits. Rabbits of either sex weighing 1–3 kg were selected. The number of animals used in the study was allocated depending on the number of test compounds, doses, and the number of trials. The study was conducted per the guidelines of CPCSEA and after prior approval from the Institutional Animal Ethics Committee (AACP/M-112). The animal experimentation was conducted as per the guidelines of ARRIVE (https://arriveguidelines.org/).
2.3 Physiological salt solution
Tyrode solution was prepared in the guinea pig ileum, rat fundus, and rabbit jejunum as per the procedure mentioned in the literature (Donnerer et al., 2014). The composition of Tyrode solution in mM is NaCl 137, KCl 2.7, NaHCO3 11.9, NaH2PO4 0.4, MgCl2 0.1–1.0, CaCl2 1.8, and glucose 11.1. De Jalon was used in the rat uterus preparation and prepared per the procedure described in the literature (Ngadjui et al., 2021). The composition of De Jalon solution in mM is NaCl 154, KCl 5.6, NaHCO3 6.0, CaCl2 0.55, and glucose 2.78.
2.4 Extraction and preparation of test compounds
The active Phyto ingredients were extracted by maceration process using methanol: water (70:30%). The crude botanicals were kept in the solvent mixture for 48 h at 25 °C, filtered, and subjected to spray drying to preserve the integrity of the components. The stock solution of A.L and E.O was freshly prepared during the experiment using spray-dried powders. The test compounds were dissolved in distilled water with the help of a vortex, and the supernatant of the stock solution was further diluted as per the required dosage (50 – 5000 mcg/ml) (Latif et al., 2019; Balkrishna et al., 2022; Desai and Joshi, 2019; Hashem-Dabaghian et al., 2018; Variya et al., 2016). Fig. 1 represents the overview of the procedure adopted to test the spasmolytic activity of the herbal extracts.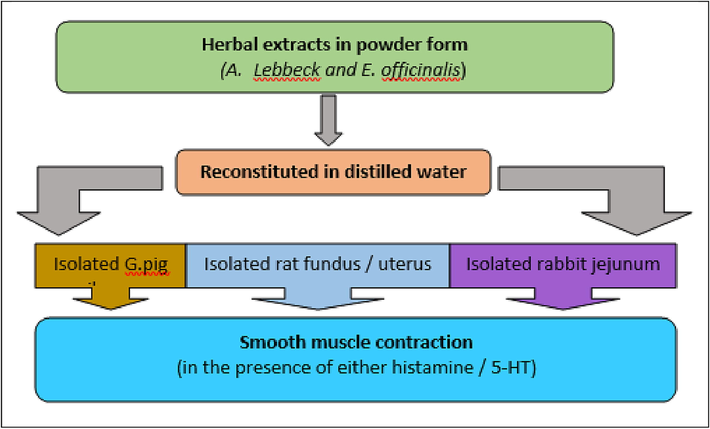
Overview of the procedure adopted to test the spasmolytic activity.
Stock solutions of histamine and 5-HT were prepared by dissolving 20 mg of the agents in 20 ml of distilled water. Further dilutions were made with distilled water to obtain the required concentration. Chlorpheniramine (CPM) and cyproheptadine (CYP) solutions were prepared by dissolving the drugs in distilled water to get the concentration of 1 mg/ml (stock solution), which were later reconstituted to obtain the required doses.
2.5 Isolated guinea pig ileum preparation
The animal was sacrificed by cervical dislocation under ether anesthesia, and the ileum was isolated. The terminal 10 cm near the ileocecal junction was discarded, mesentery was trimmed, and the 2–3 cm long ileum was mounted in a 20 ml organ bath containing Tyrode’s solution. Experiments were conducted at 37 °C, tension 0.5 g, and the medium was aerated with air (Rezania et al., 2010). The tissue was connected to a force transducer, and the contractions were recorded on a polygraph (Recorders and Medicare Systems, Ambala, India). The tissue was given a 30-min equilibration period with medium changes every 5 min before the experimental procedures. A concentration–response curve was initially obtained for histamine, and the sub-maximal concentration was determined. Varying concentrations of test compounds (adjusted in 0.1 ml) were added to the organ bath, and after a 2-min contact period, the agonist (histamine) was added, and contractions were recorded. Similarly, standard antagonist chlorpheniramine (CPM) responses were recorded on the same tissue. Experiments were repeated 3–4 times for each concentration of the test and CP.
2.6 Isolated rat uterus preparation
Young female Wistar rats were injected with diethylstilbesterol (0.25 mg/100 g) intraperitoneally for three days (to prime the animals and maintain the estrus phase) before sacrificing the animal. The animal was then sacrificed by cervical dislocation under ether anesthesia, and the abdomen was opened. The uterus’s horns were dissected, separated from the surrounding fatty material, and transferred to a dish containing De Jalon solution. The tissue was mounted and connected to the transducer. Experiments were conducted at 37 °C, tension 0.5 g, and the medium was aerated with air (Bafor et al., 2011). The contractions were recorded on a polygraph (Recorders and Medicare systems, Ambala, India). The tissue was given a 30-min equilibration period with medium changes every 5 min before the experimental procedures. Initially, a concentration–response curve was obtained for 5-hydroxytryptamine, and the sub-maximal concentration was determined. Different concentrations of test compounds were added to the organ bath, and after a 2-min contact period, the agonist (5-HT) was added, and contractions were recorded. The test and standard antagonist cyproheptadine (CYP) responses were recorded on the tissue. The experiment was repeated 3–4 times for each test and CYP concentration.
2.7 Isolated rat fundus preparation
Young Wistar rats of either sex weighing 150–190 were sacrificed by cervical dislocation under ether anesthesia, the abdomen was opened, and the stomach was exposed. Fundus was identified and cut along the pylorus, leaving a thin band of the pyloric tissue attached to the fundus, and the tissue was placed in the dish containing. The incision of the fundus was made from the lesser curvature and opened longitudinally. Alternate zig-zag cuts were made to make a fundal strip preparation. Both ends were tied with the thread and mounted in an organ bath containing Tyrode’s solution at 37 °C, and the tissue was well aerated. 1 gm load was applied to allow the preparation to equilibrate for 30 min (Kumar et al., 2015). Initially, a concentration–response curve was obtained for 5-hydroxytryptamine, and ceiling response was determined. Various concentrations of herbal extracts were added to the organ bath, and after a 2-min contact period, the agonist (5-HT) was added (30-sec contact period), and contractions were recorded. The test and standard antagonist cyproheptadine (CYP) responses were recorded on the same tissue. Experiments were repeated 3–4 times for each test and CYP concentration. Additionally, IC50 values were computed for each test technique. The inhibitory concentration (IC50) value is defined as the concentration of inhibitor (antagonist-CYP) required to inhibit a substance's (agonist-5-HT) action by 50% under test conditions (Das et al., 2023).
2.8 Isolated rabbit jejunum preparation
An experimental selected rabbit was sacrificed by cervical dislocation under ether anesthesia. The lower abdomen region and the intestine was exposed; the jejunum part is located by leaving 10 cm from the stomach and around 15 cm from the caecum. The isolated tissue was transferred to the beaker containing Tyrode solution and cut into 2–3 cm long pieces free from mesenteric attachment were used. The tissue was tied to the force transducer and the lower end to the tissue holder. The preparation was mounted in an organ bath of 25 ml capacity filled with Tyrode’s solution at 37 °C and aerated well. 1gm load was applied, and the tissue was allowed to stabilize for 30 min. Rhythmic contractions were amplified and recorded on two-channel pyrite. The time interval between individual doses was one minute (Hu et al., 2009). The spasmolytic activity of the herbal extracts was recorded at different concentrations and the ability to inhibit the spontaneous contraction of the jejunum was noted. Before subjecting the tissue to the test drug, a normal response was recorded, and the decrease in response with the test drug was compared with the normal.
2.9 Statistical analysis
Data obtained from the study are expressed as means ± standard error (SE). Statistical analysis was done using the statistical program IBM SPSS version 20.0 for Windows (SPSS Corporation, Chicago, IL, USA). The mean percentage of contractions induced by different test /CPM/CYP concentrations was determined, taking the contraction induced by histamine/5-hydroxytryptamine as 100%. One-way analysis of variance (ANOVA) and Dunnett’s posthoc test were used to analyze the statistical significance for multiple groups. P < 0.05 indicates the statistical significance when comparing different groups.
3 Results
3.1 Dose-response curve using isolated guinea pig ileum, rat uterus, and rat fundus
Fig. 2 represents the dose–response curves of different spasmogens tested in the present study. Histamine was used in guinea pig ileum, while 5-HT was tested in rat uterus and fundus preparation. Sigmoid curves were recorded when these agonists were tested. The threshold values for histamine in guinea pig ileum were found to be 4 × 10−7 M, for 5-HT in rat uterus, it was 4 × 10−7 M, and 5-HT in rat fundus was 8 × 10−7 M. At 64 × 10−7 M, all spasmogens exhibited maximum response in their tested isolated tissues.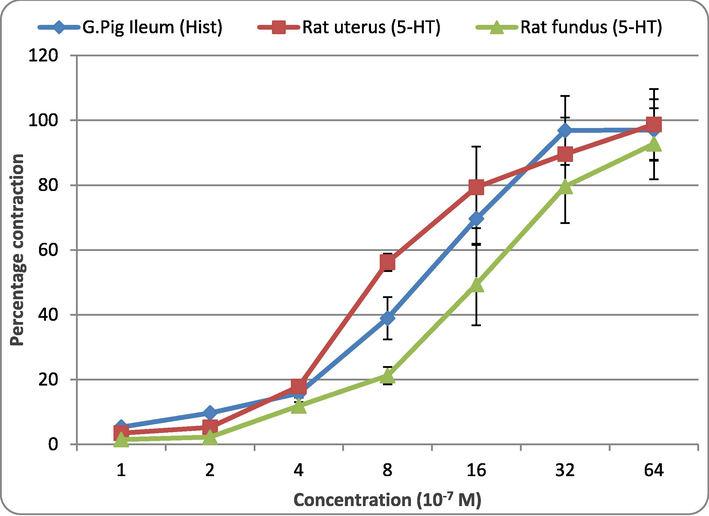
Dose-response curve of spasmogens using isolated tissue preparations.
3.2 Effect of A. lebbeck and E. officinalis on spasmogens-induced contraction
3.2.1 Effect of A. lebbeck and E. officinalis on the histamine-induced contractions in guinea pig ileum
The percentage contraction induced by A.L and E. O in the presence of histamine (64 × 10−7 M) indicated a potentiation effect on the contraction. A.L. in the presence of histamine increased the contractile response in a dose-dependent manner. A significant (p < 0.05) enhancement of the histamine-induced contraction was observed at 50 mcg/ml of A.L, and a further increase in the dose of A.L produced a more significant (p < 0.01) augmentation of histamine action. However, a three-fold increase in the dose (from 50 to 150 mcg/ml) has resulted in only a 25% increase in the contraction. Further, a 3.33 times increase (from 150 to 500 mcg/ml) produced only a 20% increase in contraction, indicating a possibility of saturation at high doses.
On the other hand, E.O. had dose-dependent suppression of the histamine-induced responses. A significant (p < 0.05) inhibition on the histamine-induced contraction was observed from E.O 150 mcg/ml dose onwards. The drug at 500 mcg/ml produced total abolition of histamine response. CPM tested as a standard antihistamine also produced a dose-dependent inhibition of the agonist effect. A significant (p < 0.05) suppression of histamine-induced contraction was found from 150 mcg/ml of CPM dose, and none of the tested doses showed complete abolition of the histamine effect (Fig. 3).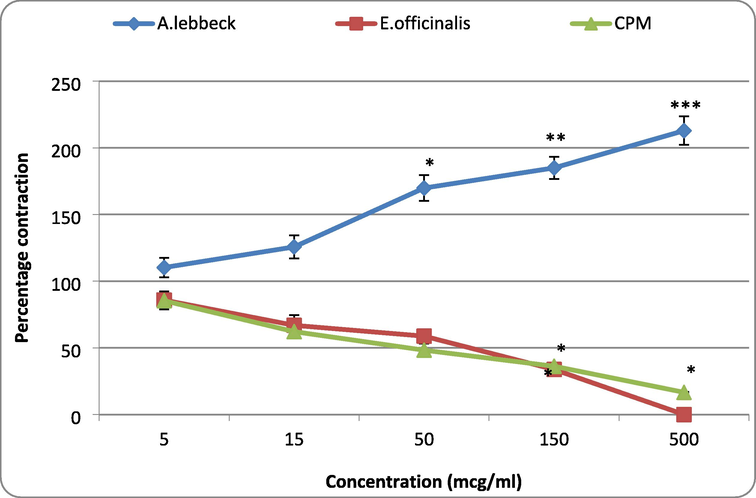
Effect of A. lebbeck and E. officinalis on the histamine-induced contractions in guinea pig ileum. Values are represented as mean ± SE. One-way ANOVA. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control; A. lebbeck: Albizia lebbeck; E. officinalis; Emblica officinalis; CPM: Chlorpheniramine.
3.2.2 Effect of A. lebbeck and E. officinalis on the 5-HT-induced contractions in rat uterus
Fig. 4 indicates the responses of A.L and E.O extracts on the 5-HT-induced contraction in rat uterus. A.L. pre-treatment increased the responses of 5-HT at doses 5, 15, 50, and 150 mcg/ml. However, the highest tested dose (500 mcg/ml) reduced the contractile response of 5-HT. A significant (p < 0.05) enhancement of 5-HT response was observed when A.L was tested at 150 mcg/ml. However, both E.O. and CYP (standard anti-serotonergic) produced a dose-dependent reduction in the responses of 5-HT. A significant (p < 0.05) inhibitory effect was observed when E.O. was tested at 500 mcg/ml; for CYP, it was found to be at 150 and 500 mcg/ml.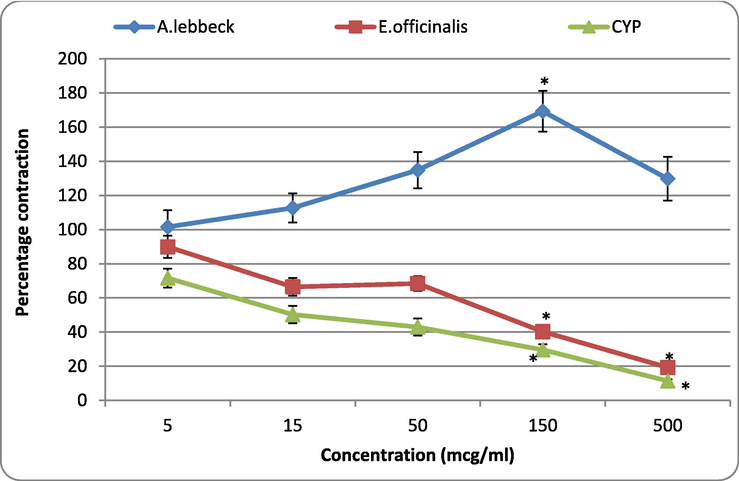
Effect of A. lebbeck and E. officinalis on the 5-HT-induced contractions in rat uterus. Values are represented as mean ± SE. One-way ANOVA. *P < 0.05 compared with control; A. lebbeck: Albizia lebbeck; E. officinalis; Emblica officinalis; CYP: cyproheptadine.
3.2.3 Effect of A. lebbeck and E. officinalis on the 5-HT-induced contractions in rat fundus
The observations recorded from isolated rat fundus indicated that A.L at 5 and 15 mcg/ml showed a mild increase in the contractile response of 5-HT. Also, when higher doses (50, 150, and 500 mcg/ml) were tested, the extract showed either a placid decrease in the actions of 5-HT or no effect. None of the tested doses of A.L. significantly affected the responses of 5-HT. On the other hand, E.O. and CYP (standard anti-serotonergic) showed a dose-dependent reduction in the response of 5-HT. E.O produced significant (p < 0.05) suppression at 150 and 500 mcg/ml, while CYP exhibited significant (p < 0.05) reduction of 5-HT responses at 50, 150, and 500 mcg/ml (Fig. 5).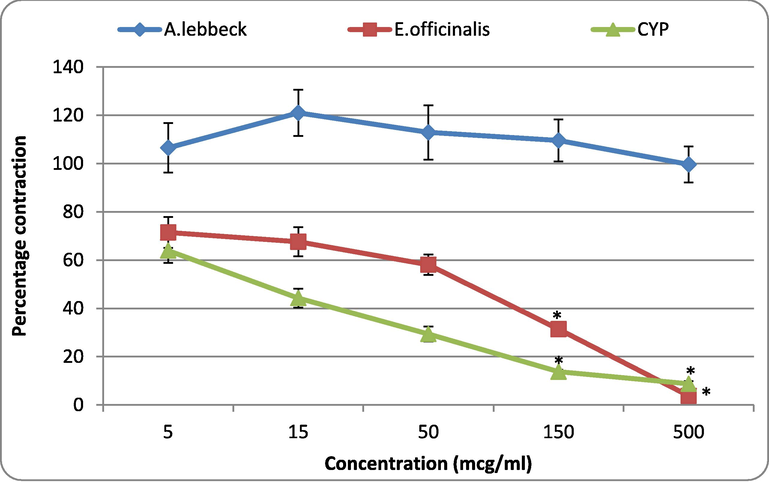
Effect of A. lebbeck and E. officinalis on the 5-HT-induced contractions in rat fundus. Values are represented as mean ± SE. One-way ANOVA. *P < 0.05 compared with control; A. lebbeck: Albizia lebbeck; E. officinalis; Emblica officinalis; CYP: cyproheptadine.
3.2.4 Percentage change induced by A. lebbeck and E. officinalis on the spasmogenic responses in isolated tissues
The observation recorded in isolated guinea pig ileum suggests that administration of A.L at different doses and histamine produced a progressive enhancement in the contractile responses. A 10% increase in contraction was observed at the lowest tested dose (5 mcg/ml), and a 112.8% increase was recorded at the highest dose (500 mcg/ml). In rat uterus, A.L enhanced the 5-HT induced contraction from 5 to 150 mcg/ml in a dose-dependent manner. Maximum potentiation of 5-HT response was found at 150 mcg/ml, and at the highest dose (500 mcg/ml), the enhanced contraction decreased to 29.7%. A.L in rat fundus preparation increased the 5-HT induced contraction at 5 and 15 mcg/ml, and a further increase in the dose (50 and 150 mcg/ml) produced a feeble increase. At 500 mcg/ml, the response of 5-HT was slightly lowered (−0.36%) (Table 1). Note: A.L – Albizia lebbeck, E.O – Emblica officinalis, CPM – Chlorpheniramine, CYP – Cyproheptadine.
Doses of the extract
G. pig ileum
Rat uterus
Rat fundus
A.L
E.O
CPM
A.L
E.O
CYP
A.L
E.O
CYP
5 mcg/ml
10.25
−14.19
−14.68
1.56
−10.06
−28.38
6.52
−28.55
−36.07
15 mcg/ml
25.69
−33.11
−37.835
12.68
−33.52
−49.74
20.98
−32.39
−55.74
50 mcg/ml
69.84
−41.22
−51.74
34.85
−31.55
−57.04
12.9
−41.91
−70.6
150 mcg/ml
84.96
−66.22
−63.79
69.28
−59.72
−70.36
9.54
−68.58
−86.22
500 mcg/ml
112.87
−100
−83.36
29.74
−80.74
−88.63
−0.36
−96.2
−91.233
E.O. and CPM in guinea pig ileum were found to decrease the histamine-induced contraction progressively. At the highest tested dose (500 mcg/ml), E.O. produced total abolition (−100%), and CPM exhibited an 83.4% reduction of 5-HT response at the same dose. In both rat uterus and rat fundus isolated preparations, E.O. and CYP produced a dose-dependent reduction in the responses of the 5-HT. Around 80% and 90% reductions were observed in the rat uterus and rat fundus, respectively, when the agents were tested at the highest dose (500 mcg/ml) (Table 1).
3.2.5 IC50 values of E. officinalis, chlorpheniramine and cyproheptadine
Fig. 6 indicates the IC50 values of E.O, CPM/CYP. The IC50 values recorded in isolated guinea pig ileum were 35.2 mcg/ml for E.O, 114.9 mcg/ml for CPM, 50.6 mcg/ml for E.O, and 42.1 mcg/ml for CYP in isolated rat uterus and 124.6 mcg/ml for E.O and 26.1 mcg/ml for CYP in isolated rat fundus preparation. The highest and lowest IC50 values were observed in the rat fundus preparation.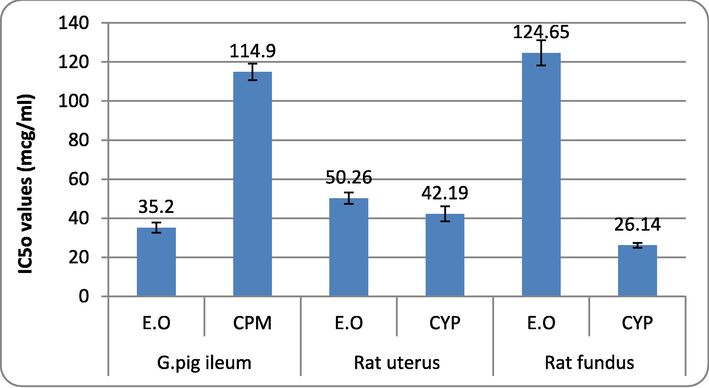
IC50 values of E. officinalis, chlorpheniramine and cyproheptadine against spasmogens-induced contractions in isolated tissue preparations. Values are represented as mean ± SE; A.L – Albizia lebbeck, E.O – Emblica officinalis, CPM – Chlorpheniramine, CYP – Cyproheptadine.
3.3 Rabbit jejunum preparation
The influence of the two extracts on the amplitude and frequency of spontaneous contraction in the isolated rabbit jejunum was tested. A.L. at the lower doses (5–150 mcg/ml) did not induce significant change. Still, a higher dose (500 mcg/ml) reduced both the amplitude and frequency of contraction significantly (p < 0.05) compared to the control. The reduction was observed to be lower than the normal spontaneous contraction. E.O. showed a dose-dependent increase in both the amplitude and frequency of smooth muscle contraction. A significant (p < 0.05) amplitude and contraction frequency increase was observed at 50 and 150 mcg/ml compared to the control. Further, when the extract dose was increased to 500 mcg/ml, both the amplitude and, the contraction frequency decreased non-significantly (Table 2). Note: mm – millimeter, cpm – contractions per minute; A. lebbeck:Albizia lebbeck; E. officinalis;Emblica officinalis. Values are represented as mean ± SE. Statistics: One-way ANOVA.
Doses of the extract
A. lebbeck
E. officinalis
Amplitude (mm)
Frequency (cpm)
Amplitude (mm)
Frequency (cpm)
5 mcg/ml
8.12 ± 1.09
12.99 ± 2.02
7.68 ± 1.64
11.28 ± 2.11
15 mcg/ml
8.25 ± 0.88
11.85 ± 1.98
10.68 ± 0.87
12.69 ± 1.26
50 mcg/ml
7.95 ± 1.11
12.05 ± 1.38
15.68 ± 1.99*
16.18 ± 3.39*
150 mcg/ml
8.66 ± 0.66
12.65 ± 0.99
17.21 ± 2.36*
18.27 ± 4.08*
500 mcg/ml
4.12 ± 0.25*
6.84 ± 0.28*
9.04 ± 1.69
7.12 ± 0.65
4 Discussion
The present study evaluated the spasmolytic activity of A.L and E.O using isolated tissues such as guinea pig ileum, rat uterus, rat fundus, and rabbit jejunum. The role of herbal extracts on the spasmogens-induced contractions was tested in guinea pig ileum, rat uterus, and rat fundus. In contrast, the influence of the extract on spontaneous contraction was studied in rabbit jejunum. The known spasmogens (histamine and 5-HT) produced a dose-dependent contraction in the isolated tissue preparation. Peak contractile response was observed at 64 × 10−7 M of the spasmogens (Fig. 3).
Histamine is reported to induce smooth muscle contraction by binding to H1 receptors. The H1 receptors are membrane-bound and are coupled with G-protein, especially Gq/11. The activation increases the phospholipase A2 and D phospholipase A2 and D level inside the cell, increasing diacylglycerol and intracellular calcium levels, resulting in muscle contraction (Naganuma et al., 2018). On the other hand, 5-HT-induced smooth muscle contraction involves binding to 5HT2A that is coupled with G-protein Gα. The interaction activates the phospholipase C that, causes the release of inositol triphosphate and diacylglycerol. The inositol triphosphate triggers calcium release from the sarcoplasmic reticulum, whereas diacylglycerol starts the phosphokinase C and ultimately leads to smooth muscle contraction (Bhaskaran et al., 2014).
In the present study, the administration of A.L produced a dose-dependent enhancement in the contractions induced by histamine in guinea pig ileum. The augmentation of the contractile responses was found to be significant (p < 0.05) from 50 mcg/ml doses onwards (Fig. 3). Similarly, A.L. increased the contractions of 5-HT in the rat uterus, and a significant effect (p < 0.05) was recorded at 150 mcg/ml. However, A.L. increased 5-HT-induced contraction in isolated rat fundus, the response was mild and non-significant (Fig. 6). The observations suggest that A.L might have potentiated the contractile action of spasmogens on the smooth muscles in isolated tissues. The observation contradicts earlier findings that the extract of A.L minimized the carbachol-induced smooth muscle contraction in isolated tissue (Khan et al., 2019a,b).
The observation from the present study also suggested that E.O. produced dose-dependent suppression of histamine-induced contraction in guinea pig ileum, and a significant (p < 0.05) effect was observed at 150 and 500 mcg/ml (Fig. 4). Similarly, E.O. in the other two isolated tissue preparations (rat uterus and rat fundus) inhibited the contractile responses of 5-HT. Both preparations observed significant suppression at 150 and 500 mcg/ml (Figs. 5 and 6). The known anti-spasmodic drugs (CPM for histamine and CYP for 5-HT) produced a dose-dependent reduction in the actions of spasmogens. The data in table-1 also suggests that A.L potentiated the responses of spasmogens while E.O and CPM/CYP inhibited the action of spasmogens. Being a known antagonist, CPM in this study might have blocked H1 receptors from the actions of histamine (Díaz Nebreda et al., 2019). Similarly, CYP reduced the 5-HT responses by blocking the 5HT2A receptors on the smooth muscles (Kim et al., 2019).
E.O. in the earlier study is reported to possess anti-diarrheal and anti-spasmodic activities (Perianayagam et al., 2005). Although a detailed mechanism is not described in the literature, the components present in E.O. might have acted as an antagonist to block the H1 receptors in guinea pig ileum and 5-HT2A in isolated rat tissue preparations. The IC50 values also suggested that E.O and CPM/CYP produced antagonistic effects against the spasmogens when tested in isolated smooth muscle preparations (Fig. 6). The literature review suggests that the anti-spasm activity exhibited by the crude herbal compounds is due to active phytoconstituents (Borgi and Chouchane, 2009).
Studies conducted in the past have identified and isolated several vital phytochemical components from E.O., such as tannins, alkaloids, flavonoids, polyphenols, vitamins, and minerals. Certain flavonoids, such as quercetin and kaempferol, have been reported to possess spasmolytic activity (Fig. 7) (Ventura-Martínez et al., 2018; Wahid et al., 2022). Quercetin is a plant pigment in several fruits, vegetables, leaves, seeds, and grains. Chemically, it is 3,3′,4′,5,7-pentahydroxyflavone and possesses antioxidant properties against several reactive free radicals (Xu et al., 2019). Kaempferol is 3,4′,5,7-tetrahydroxyflavone found in different parts of the plant. This compound was also reported to exhibit antioxidant activity besides antimicrobial, anti-inflammatory, and neuroprotection (Alam et al., 2020).![Important spasmolytic phytochemicals isolated from E.O [28,31].](/content/185/2023/35/7/img/10.1016_j.jksus.2023.102818-fig7.png)
Important spasmolytic phytochemicals isolated from E.O [28,31].
In the isolated rabbit jejunum preparation, A.L at 500 mcg/ml reduced significantly (p < 0.05) both the amplitude and frequency of spontaneous contraction. Administration of E.O. showed a significant (p < 0.05) increase in amplitude and frequency of contraction at 50 and 150 mcg/ml (Table 2). According to the literature, the spontaneous contraction in rabbit jejunum is mediated by intracellular calcium ions (Khan et al., 2022). The responses showed by higher doses of A.L and E.O suggests that the herbal extracts might have reduced the contraction of calcium ions intracellularly in intestinal smooth muscles to reduce the amplitude and frequency of spontaneous contractions. Based on our findings and other reported information, a possible E.O. action mechanism is proposed in Fig. 8. Also, when higher doses (50, 150, and 500 mcg/ml) were tested, the extract showed either a placid decrease in the actions of 5-HT or no effect; however, the fall in the contraction was not significant as we can find with E.O. The spasmolytic activity observed in this study could mostly be mediated by the phytochemicals present in E.O., including quercetin and kaempferol. However, the spasmogenic effect produced by A.L in isolated guinea pig ileum, rat uterus, rat fundus, and E.O. in rabbit jejunum needs further research.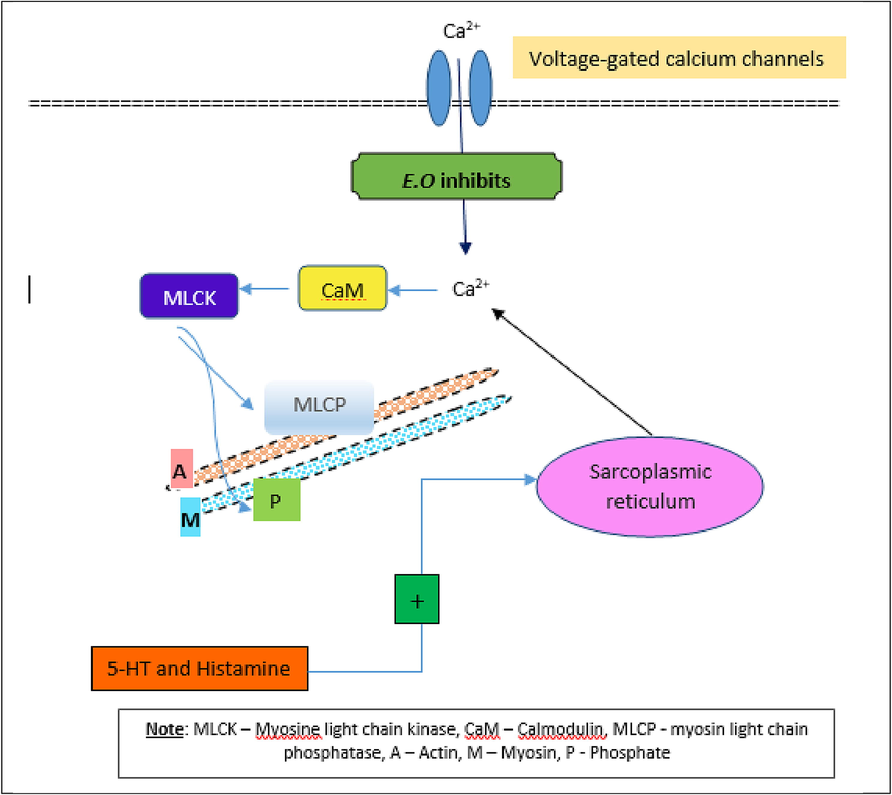
Proposed spasmolytic mechanism of action of E.O.
5 Conclusion
The study’s findings suggest that Albizia lebbeck, in the presence of known spasmogens, potentiated their actions, but when tested alone, such as in rabbit jejunum, reduced the smooth muscle contractions. Emblica officinalis on the other hand, reduced spasmogen-induced contraction but, when tested alone, increased the spontaneous contraction in rabbit jejunum. Multiple pathways apart from modulating intracellular calcium levels seem to be involved in these actions. The data is inconclusive to suggest the spasmogenic/spasmolytic potential of the tested herbal extracts. More research involving different experimental models is essential for understanding the precise role of this herbal medicine in spasmogenic disorders.
Acknowledgment
This research was supported by the Researchers Supporting Project number (RSP2023R431) at King Saud University, Riyadh, Saudi Arabia.
Funding
The authors are thankful to AlMaarefa University for their financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules. 2020;25(18):4073.
- [Google Scholar]
- Oxytocin inhibiting effect of the aqueous leaf extract of Ficus exasperata (Moraceae) on the isolated rat uterus. Acta. Pol. Pharm.. 2011;68(4):541-547.
- [Google Scholar]
- A Comprehensive Insight into the Phytochemical, Pharmacological Potential, and Traditional Medicinal Uses of Albizia lebbeck (L.) Benth. Evid. Based Complement. Alternat. Med.. 2022;2022:5359669
- [Google Scholar]
- Molecular interactions of serotonin (5-HT) and endothelin-1 in vascular smooth muscle cells: in vitro and ex vivo analyses. Am. J. Physiol. Cell. Physiol.. 2014;306(2):C143-C151.
- [Google Scholar]
- Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol.. 2020;180:114147
- [Google Scholar]
- Anti-spasmodic effects of Zizyphus lotus (L.) Desf. extracts on isolated rat duodenum. J. Ethnopharmacol.. 2009;126(3):571-573.
- [Google Scholar]
- In silico studies and evaluation of in vitro antidiabetic activity of berberine from ethanol seed extract of Coscinium fenestratum (Gaertn.) Colebr. J. King Saud Univ.-Sci.. 2023;35(5):102666.
- [Google Scholar]
- Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol.. 2019;231:494-502.
- [Google Scholar]
- Involvement of histamine H1 and H2 receptor inverse agonists in receptor's crossregulation. Eur. J. Pharmacol.. 2019;847:42-52.
- [Google Scholar]
- Hexamethonium-induced augmentation of the electrical twitch response in the guinea-pig ileum longitudinal muscle-myenteric plexus strip. Neurosci. Lett.. 2014;577:34-37.
- [Google Scholar]
- A systematic review on the cardiovascular pharmacology of Emblica officinalis Gaertn. J. Cardiovasc. Thorac. Res.. 2018;10(3):118-128.
- [Google Scholar]
- Antidiarrhoeal and intestinal modulatory activities of Wei-Chang-An-Wan extract. J. Ethnopharmacol.. 2009;125(3):450-455.
- [Google Scholar]
- Personalized medicine: a confluence of traditional and contemporary medicine. Altern. Ther. Health Med.. 2014;20(5):31-40.
- [Google Scholar]
- Potential biomedical applications of Araucaria araucana as an antispasmodic, bronchodilator, vasodilator, and antiemetic: Involvement of calcium channels. J. Ethnopharmacol.. 2022;298:115651
- [Google Scholar]
- Possible Mechanism(s) Underlying the Antidiarrheal, Antispasmodic and Bronchodilatory Activities of the Pericarp of Albizia lebbeck. Int. J. Pharmacol.. 2019;15:56-65.
- [Google Scholar]
- Possible Mechanism(s) Underlying the Antidiarrheal, Antispasmodic and Bronchodilatory Activities of the Pericarp of Albizia lebbeck. Int. J. Pharmacol.. 2019;15:56-65.
- [Google Scholar]
- Histamine receptor antagonists, loratadine and azelastine, sensitize P-gp-overexpressing antimitotic drug-resistant KBV20C cells through different molecular mechanisms. Anticancer Res.. 2019;39(7):3767-3775.
- [Google Scholar]
- Interaction of aqueous leaf extract of Aegle marmelos (L.) Corr. with cholinergic, serotonergic and adrenergic receptors: an ex vivo study. Indian J. Pharmacol.. 2015;47(1):109-113.
- [Google Scholar]
- Pollination biology of Albizia lebbeck (L.) Benth. (Fabaceae: Mimosoideae) with reference to insect floral visitors. Saudi J. Biol. Sci.. 2019;26(7):1548-1552.
- [Google Scholar]
- Study on the synthesis and biological activities of α-substituted arylacetates derivatives. Bioorg. Med. Chem. Lett.. 2016;26(7):1715-1719.
- [Google Scholar]
- Effects of herbal medicines on pain management. Am. J. Chin. Med.. 2020;48(1):1-16.
- [Google Scholar]
- Overlooked muscle cramps in patients with chronic liver disease: in relation to the prevalence of muscle cramps. Eur. J. Gastroenterol. Hepatol.. 2019;31(3):375-381.
- [Google Scholar]
- Histamine-enhanced contractile responses of gastric smooth muscle via interstitial cells of Cajal in the Syrian hamster. Neurogastroenterol. Motil.. 2018;30(4):e13255
- [Google Scholar]
- Uterotonic effects of aqueous and methanolic extracts of lannea acida in wistar rats: An in vitro study. Reprod. Sci.. 2021;28(9):2448-2457.
- [Google Scholar]
- Evaluation of antidiarrheal potential of emblica officinalis. Pharm. Biol.. 2005;43(4):373-377.
- [Google Scholar]
- The effect of lithium chloride on WIN 55,212-2-induced tolerance in isolated guinea pig ileum. Eur. J. Pharmacol.. 2010;627(1–3):265-268.
- [Google Scholar]
- Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res.. 2016;111:180-200.
- [Google Scholar]
- Spasmolytic effect of aqueous extract of Tagetes erecta L. flowers is mediated through calcium channel blockade on the guinea-pig ileum. Biomed. Pharmacother.. 2018;103:1552-1556.
- [Google Scholar]
- Antispasmodic activity of the ethanol extract of Citrullus lanatus seeds: Justifying ethnomedicinal use in Pakistan to treat asthma and diarrhea. J. Ethnopharmacol.. 2022;295:115314
- [Google Scholar]
- Antioxidant activities of quercetin and its complexes for medicinal application. Molecules.. 2019;24(6):1123.
- [Google Scholar]
- Natural medicinal resources and their therapeutic applications. Rocz. Panstw Zakl. Hig.. 2019;70(4):407-413.
- [Google Scholar]







