Translate this page into:
Potential of Lactobacillus agilis, Lactobacillus plantarum, and Lactobacillus acidophilus to enhance wheat growth under drought and heat stress
⁎Corresponding author. humaira.yasmin@comsats.edu.pk (Humaira Yasmin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Drought and heat stress affect plant functioning and turgor pressure by reducing water content and affecting cell growth, and together, they can cause severe damage. Lactobacillus agilis, Lactobacillus acidophilus, and Lactobacillus plantarum strains were identified and evaluated for growth-promoting properties via Petri plate test and pot experiment. Wheat seeds were exposed to heat and drought separately and in combination with the bacterial isolates primed for the seeds. In drought-stressed plants, after treatment with L. agilis, there was an elevation in carotenoids by 37% and chlorophyll a and b levels by 55% and 10%, respectively. Similarly, heat-stressed plants, after treatment with L. agilis, showed an elevation in carotenoids by 55% and chlorophyll a and b levels by 70% and 82%, respectively. A further 5% increase in the SOD concentration was noted after the treatment of L. agilis in plants with drought stress. A significant increase (24%) in the APX level was also observed after the treatment of L. agilis in plants with heat stress. In plants with drought stress, a rise in CAT concentration was observed after the treatments of L. agilis and L. plantarum with 33% and 11%, respectively. In plants with drought and heat stresses, the level of POD in combined treatments increased by 39% and 41%, respectively. The study reveals that rhizospheres can serve as reservoirs of plant growth-promoting bacteria, potentially replacing chemical fertilizers and promoting plant growth in stressful conditions.
Keywords
Medicinal plants
Rhizosphere
PGPR
Drought stress
Heat stress
1 Introduction
Aside from the increased interest in food, the scarcity of natural resources and high agricultural production are posing significant challenges in both developing and established countries. However, in today’s world, crop yield has not shown significant improvement despite increasing compost input (Pii et al., 2015). By enhancing plant nutrition and shielding them against microbes, beneficial bacteria found in the microbiome of plant roots provide essential benefits to the plant. PGPR, a type of plant growth-promoting rhizobacteria, colonize plant roots, promoting plant growth through the production or modification of growth regulators and a symbiotic N2 fixation process. Plant growth-promoting rhizobacteria additionally produce siderophores, cyanide, and anti-microbials to provide defense against phytopathogenic microorganisms (Bulgarelli et al., 2013). A diverse number of PGPRs activate plant growth and advancement by inhibiting the action and growth of plant pathogens. Different PGPRs restrain infection indirectly by triggering induced systematic resistance (ISR), which is a technique that gets plant-borne protection systems ready to respond quickly and stronger against abiotic stress (Conrath et al., 2015), as well as facilitating the uptake of essential nutrients from the soil. Rhizobium species are restoring the development of root nodules by settling air nitrogen in those nodules and making it accessible to plants.
Important nutrients generated by PGPR are related with soil phosphate, which promotes iron and nitrate uptake by plant systems (Wintermans et al., 2016). Sustainable agriculture has a global challenge due to the water shortage, as around 215 million hectares (MHA) of wheat are farmed worldwide using irrigation water (12 %) available (Aaboud et al., 2018). Global climate change and uneven rainfall patterns resulted in a significant global rise in drought stress conditions. Water scarcity and heat stresses minimize crop yields, ultimately directing farmers' poor earnings (Daryanto et al., 2016; Zhao et al., 2017). The average worldwide temperature is anticipated to rise approximately 2 °C in the following 50 years (Ahmed et al., 2017). High temperatures retard growth, cell division, and cell elongation in plants, in addition to reducing root growth, number, and diameter.
As harsh and extreme environmental conditions prevail in different areas, it is necessary to cope with conditions such as heat and drought. These stressful conditions affect the yield of crops. So, the key goal of the current research remained to examine the potential of L. agilis, L. plantarum, and L. acidophilus for wheat germination and biomass growth. Secondly, individual and collective strains were explored for their involvement in mitigating the impacts of stressors (drought and heat) on wheat plants. The morphological, physiological, and biochemical responses of stressed wheat by applying L. agilis, L. plantarum, and L. acidophilus were also analyzed in the current study.
2 Methodology
The study used three repetitions and a randomized block design to be conducted in a greenhouse conditions with five treatments in thirteen Petri plates and soil-filled Pots. The treatments are given in Table 1.
2.1 Isolation and morphological examination of Lactobacillus isolates
Lactobacillus agilis (L. agilis) strain NMCC-15 (MK614016) was used, as already reported in our previous study (Khan et al., 2021). Each (1 g) rhizosphere soil sample collected from Ficus carica and Azadirachta indica was serially diluted to 10-1, 10-2, 10-3, 10-4, and 10-5 according to the protocol of Khan et al. (2021) for the identification of Lactobacillus plantarum (L. plantarum) and Lactobacillus acidophilus (L. acidophilus) strains. To inoculate and spread each dilution on Petri plates, MRS agar media (selective for Lactobacillus isolates) at pH 5.4 was prepared (De Man et al., 1960) and pure cultures were obtained by sub-culturing on MRS agar Petri plates and incubated the cultured plates at 32 °C for 24 h. Bergey's handbook was used to define the morphological characteristics, which include colony color, form, margin, elevation, texture, and size (Cossart, 1987). In order to identify the strains of L. plantarum and L. acidophilus, several conventional and biochemical tests, such as gas generation, estimation of catalase, oxidase, urease, methyl red, citrate, triple sugar iron, and fermentation were applied. Phase-contrast microscope (Phase Contrast 2, Nikon, Tokyo, Japan), and Gram staining of the bacterial isolates was carried out to analyze the specific type of bacteria.The interpretation of the data was based on “Bergey's Manual of Determinative Bacteriology” (Holt et al., 1984).
2.2 Collection of seeds and germination
Johar-16 wheat (Triticum aestivum L.) seeds were provded by the National Agriculture Research Centre (NARC), Islamabad. The seeds were decontaminated for one minute via a 70 % ethanol solution, and then five times washed with distilled water. Before conducting the studies, the seeds were given an overnight period to get vernalized.
2.3 Preparation of the bacterial suspension
The bacterial strains of L. agilis, L. plantarum, and L. acidophilus were used to evaluate their potential as growth promoters under heat and drought stress. Luria broth (LB) media of 25 mL was prepared aseptically and added to sterilized flacon tubes in a culture hood for each bacterial inoculum. After the sterilized medium reached room temperature, it was transferred into falcon tubes within the biosafety cabinet. The isolates of L. agilis, L. plantarum, and L. acidophilus were then individually inoculated in falcon tubes with 100 μL of each and labeled according to the strain names. Tubes were sealed and placed in a shaking incubator at 30 °C for 24 h. After 24 h of incubation, falcon tubes were picked and centrifuged at 8000 rpm for 15 min. The pellet formed on the bottom of the tubes was stored, and the supernatant was removed. Pallets of each L. agilis, L. plantarum, and L. acidophilus were suspended in 10 μL of autoclaved water and shaken properly.
2.4 Bacterial isolates for plant growth enhancement
The bacterial isolates were evaluated in the laboratory of applied microbiology and biotechnology at COMSATS University Islamabad for their potential to promote plant growth. Before being planted in pots, seeds were first cultured in glass Petri plates with aluminum and sterile cotton to retain water. Seeds were primed for three to four hours prior to sprouting with strains of L. agilis, L. plantarum, and L. acidophilus and were then left to grow for two to three days at room temperature and in the dark (Khalid et al., 2004). Each day, distilled water was sprayed on the seedlings. After 15 days, wheat plants were collected, and analyzed for promptness index (PI), seedling vigor index (SVI), germination percentage (GP), and germination index (GI) as part of a biomass study.
2.4.1 Petri plates experiment under drought stress condition
Seeds were primed with solutions containing L. agilis, L. plantarum, and L. acidophilus strains for 3–4 h. A single Petri plate contained about 10–15 seeds for each treatment. Average room temperature and dark conditions were provided for an initial 2–3 days until the seeds germinated. Distilled water was regularly provided for 15 days. The wheat harvesting process was performed according to the protocol of Xiong et al. (2006) after 15 days, and a biomass study was done, which involved the analysis of lateral root (LR), fresh weight (FW), root length (RL), dry weight (DW), shoot length (SL), and leaf area (LA). The best PGPRs were used in the final pot experiment that were selected in the Petri plates experiment on the basis of their potential growth-promoting properties.
2.4.2 Pot assays
Pot assays were carried out at an average temperature of 15 °C and 64 % humidity in the greenhouse at COMSATS University in Islamabad (33.7294 °N, 73.0931 °E). In order to execute the experiment, natural light was ensured. Each 10x10 cm pot included 1.5 kg of autoclaved dried soil (3:1, soil:sand), along with holes for drainage in the bottom. Each pot had a plastic plate underneath it to catch the leachate, which was then watered on the surface of soil. Eight (08) seeds primed in triplets with bacterial solutions were planted in each pot containing autoclaved soil. Using distilled water, the soil's moisture content was kept at about 60 %. Plants at the three-leaf stage were exposed to drought and heat stress and were harvested after 15 days of treatment, in accordance with Zhang et al. (2019) established protocol. Table 1 lists the specifics of the randomized trial design. Biomass analysis of RL, SL, LA, F.W., D.W., and GP was performed.
Abbreviations
Treatments Abbreviations
C
Control
H
Heat Stress
D
Drought Stress
D + H
Drought Stress + Heat Stress
L. ag + D
Lactobacillus agilis + Drought
L. ag + H
Lactobacillus agilis + Heat
L. ag + D + H
Lactobacillus agilis + Drought + Heat
L.pla + D
Lactobacillus plantarum + Drought
L.pla + H
Lactobacillus plantarum + Heat
L.pla + D + H
Lactobacillus plantarum + Drought + Heat
L. ag + L.pla + D
Lactobacillus agilis + Lactobacillus plantarum + Drought
L. ag + L.pla + H
Lactobacillus agilis + Lactobacillus plantarum + Heat
L. ag + L.pla + D + H
Lactobacillus agilis + Lactobacillus plantarum + Drought + Heat
2.5 Biomass study of wheat
A biomass study included the evaluation of characteristics such as root length, fresh weight, shoot length, dry weight, relative water content, germination percentage, germination index, seedling vigor index, and promptness index. A measuring scale and weighing balance were used to measure the harvested plants for RL, SL, LA, F.W, D.W, and GP (Tian et al., 2015). Given below were the formulas used for these measurements:
Germination index (Gt/Tt) = where, Gt is referred to the number of germinated seeds on Tth day and Tt refers to the total number of days.
Promptness index (PI) = nd2 (1.00) + nd4 (0.75) + nd6 (0.5) + nd8 (0.25) where, n refers to the germinated seeds at the respective days like d2 = 2nd day.
2.6 Assessing photosynthetic pigments
Fresh leaves samples of the wheat plants weighing 0.5 g were crushed and placed in test tubes to measure the concentration of carotenoids and chlorophyll a and b. For this purpose, ten (10) milliliters of dimethyl sulfoxide (DMSO) were added to each test tube, and was left at room temperature/ in water bath for 4 h at 65 °C. Following this time, the absorbance of the extracted supernatant was measured at 480, 648, and 665 nm respectively for carotenoids and chlorophyll a and b. Every computation was completed in compliance with the Chappelle et al. (1992) protocol.
2.7 Estimation of antioxidative enzymes
2.7.1 Preparation of crude enzyme extracts
The plant's leaves were homogenized in mortar and pestle using liquid nitrogen in an ice-cold sodium phosphate buffer (50-mM, 7.2 pH) and polyvinylpolypyrrolidone (1 %, w/v), then centrifuged at 6,708 g for 20 min. The supernatant was used for enzymatic assays.
2.7.2 Catalase (CAT) assessment
El-Shabrawi et al. (2010) protocols were modified in order to measure the catalase activity. A reaction mixture consisting of 0.1 mL of 300 mM hydrogen peroxide, 2.8 mL of 100 % diluted 50 mM phosphate buffer, and crude enzyme solution of 0.1 mL was prepared to carry out the catalase test. The absorbance of the combination was measured via spectrophotometry at 240 nm on intervals of 30 s (Kodydková et al., 2014).
2.7.3 Superoxide dismutase (SOD) assessment
Superoxide dismutase activity was assessed after adding 100 μL of crude enzyme extract to 50 mM sodium phosphate, a buffer solution of 7.6 pH, 50 mM sodium carbonate, 0.1 mM EDTA, 50 mM nitro blue tetrazolium, and 10 μM riboflavin to make a total volume of 3.0 ml. This method confirmed the SOD activity after bringing halt in the nitro blue tetrazolium photo-reduction. The reaction mixture was placed at room temperature for 15 min under white light. Following incubation period absorbance was measured at 560 nm via spectrophotometer (Ighodaro and Akinloye, 2018). The values were measured and compared with a crude-extract-free control group.
2.7.4 Peroxidases (POD) assessment
A reaction mixture was prepared that comprised of 100 mM sodium phosphate buffer (pH 7–8), 4-methyl catechol (5 mM), hydrogen peroxide (5 mM), and 500 μL of crude enzyme extract for the determination of POD. The total volume of the mixture was raised to 3.0 mL, and via spectrophotometer, absorbance was measured at 420 nm (Onsa et al., 2004).
2.7.5 Ascorbate-peroxidase (APX) assessment
The oxidation of guaiacol was measured at 470 nm for the confirmation of peroxidase activity (Rao, 1996). A volume of 100 μL of diluted crude enzyme extract, potassium phosphate buffer of 0.9 mL (100 mM), 1 mM EDTA, and 0.1 mL of 10 mM guaiacol were mixed to carry out the experiment. A volume of 100 μL containing 10 mM hydrogen peroxidase was utilized to start the reaction. A 470 m absorbance check was performed using the spectrophotometer at 15-second intervals for up to two minutes. The enzyme's activity was expressed in mg/min.
2.8 Statistical examination
Statistix (version 8.1) software was used to perform statistical study on the data set. ANOVA (analysis of variance) was the method used to examine the variation in the data set in triplicates. Between each treatment's three replicates, the standard error was computed. Using the least significant difference (LSD) at p = 0.05, the treatments were compared.
3 Results
3.1 Isolation
Bacterial isolates were obtained after subculturing the samples on MRS agar media, that were all biochemically and morphologically distinct. The color (creamy/white), form (circular/rods), and surface texture (smooth/rough) of all the isolates varied considerably. Bacteria with Gram-positive, catalase-negative, oxidase negative and positive for citrate utilization were found in all the samples which are the typical properties of lactic acid bacteria. L. plantarum and L. acidophilus strains were identified by using Bergey’s manual and Bergey’s Manual of Determinative Bacteriology.
3.2 Bacterial isolates for plant growth enhancement
Wheat showed a 40–65 % increase in rate of germination with PGPR priming. The maximum germination rate (65 %) was observed in the L. agilis treatment by comparison with the control (without PGPR treatment). In comparison with the control, L. plantarum and L. acidophilus, also showed a prominent increase in seedling vigor index (SVI), germination index (GI), promptness index (PI), and germination percentage (GP). Maximum effectiveness was seen in the treatment of L. agilis such as GP 20 %, GI 21 %, PI 20 %, and SVI 60 % when compared to the controls seedling (Table 2). Alpha 0.05. Between the means, there is significant statistical difference.
Treatments
GP
GI
PI
SVI
Control
83.33C ± 0.0.12
2.08B ± 0.11
6.25B ± 0.13
7.12C ± 0.14
L. agilis
99A ± 0.12
2.5A ± 0.11
7.5A ± 0.13
11.46A ± 0.14
L. acido
96.67B ± 0.12
2.41A ± 0.11
7.25A ± 0.13
9.99B ± 0.14
L. pla
99A ± 0.12
2.5A ± 0.11
7.5A ± 0.13
10.97B ± 0.14
3.3 Screening of PGPR against drought and heat stress
Comparing the stressed plants to the control, the stressed plants displayed substantial reductions in leaf area (LA), shoot length (SL), lateral root (LR), dry weight (DW), fresh weight (FW), and shoot length (RL). In the absence of PGPR priming, only 35 % of the wheat seeds were germinated. In contrast to the control (without PGPR treatment), plants treated with L. agilis showed 50 % and 60 % higher germination rates in stressed and non-stressed plants, respectively. While L. acidophilus showed decreased activity in stressful conditions, the other strain of bacteria, L. plantarum, demonstrated a significant growth rate with better seed germination potential. Based on the plant growth promotion abilities, L. agilis and L. plantarum were selected for the pot experiment conducted on the plants (Table 3; Fig. 1). Alpha 0.05. Between the means, there is significant statistical difference.
Treatments
SL (cm)
RL (cm)
FW (g)
DW (g)
LR (cm)
LA (cm2)
Control
8.63B ± 0.12
3.40E ± 0.11
0.37C ± 0.07
0.10C ± 0.07
4A ± 0.14
8.23C ± 0.13
C (drought)
2.10E ± 0.12
5.50CD ± 0.11
0.12E ± 0.07
0.03D ± 0.01
2.3B ± 0.14
1.66G ± 0.13
L. agilis
11.46A ± 0.12
6.83BC ± 0.11
0.50A ± 0.07
0.15A ± 0.13
4A ± 0.14
11.1A ± 0.13
L.agilis (drought)
8.50B ± 0.12
9.83A ± 0.11
0.24D ± 0.07
0.09E ± 0.04
2B ± 0.14
6.12D ± 0.13
L. acido
6.13A ± 0.12
4.63D ± 0.11
0.15E ± 0.07
0.09E ± 0.04
3AB ± 0.14
5.5E ± 0.13
L. acido (drought)
4.06D ± 0.12
5.8CD ± 0.11
0.09F ± 0.073
0.02D ± 0.01
2B ± 0.14
3.73F ± 0.13
L. pla
10.96A ± 0.12
6.76C ± 0.11
0.42B ± 0.07
0.12B ± 0.11
2.6AB ± 0.14
10.3B ± 0.13
L. pla (drought)
7.33C ± 0.12
7.56B ± 0.11
0.2D ± 0.07
0.06E ± 0.03
2.3B ± 0.14
4.86E ± 0.13

Shows Petri plate assays of stress induce wheat plant and L. agilis and L. plantarum C = Control, D = drought.
3.4 Pot experiment
All treatments including primed wheat seeds containing strains of L. agilis and L. plantarum demonstrated a significant increase in the germination rate when compared to unprimed seeds. Drought and heat stress showed significant decrease in RL, SL, LA, FW, DW in comparison with the unstressed controlled plants. In stressed plants, L. plantarum treatment revealed a significant growth in RL, SL, LA, FW, DW ranges from 7 to 20 %. Whereas, in case of L. agilis treated plants the values ranged from 14 to 25 %. A combined treatment of L. agilis and L. plantarum exhibited a maximum rise range from 15 to 70 % (Table 4). Alpha 0.05. Between the means, there is significant statistical difference.
Treatments
RL (cm)
SL (cm)
LA (cm2)
F.W (g)
D.W (g)
GP
Control
13BC ± 0.1
18.6FG ± 0.2
12.6CD ± 0.13
0.37A ± 0.2
0.25B ± 0.11
100A ± 0.3
Heat
12C ± 0.1
21.6BCD ± 0.2
15.6BC ± 0.13
0.18C ± 0.2
0.07D ± 0.11
96.6B ± 0.3
Drought
11C ± 0.1
16G ± 0.2
10D ± 0.13
0.28AB ± 0.2
0.183C ± 0.11
100A ± 0.3
D + H
10C ± 0.1
16.6FG ± 0.2
10D ± 0.13
0.15DE ± 0.2
0.11C ± 0.11
100A ± 0.3
L. agilis + D
16A ± 0.1
24.6AB ± 0.2
18.A3 ± 0.13
0.31AB ± 0.2
0.22B ± 0.11
100A ± 0.3
L. agilis + H
13BC ± 0.1
20CDE ± 0.2
12.6CD ± 0.13
0.16DE ± 0.2
0.12C ± 0.11
100A ± 0.3
L. agilis + D + H
17A ± 0.1
26.3A ± 0.2
16.6AB ± 0.13
0.07EF ± 0.2
0.043D ± 0.11
100A ± 0.3
L. pla + H
12C ± 0.1
19G ± 0.2
13CD ± 0.13
0.02F ± 0.2
0.01CD ± 0.11
96.6B ± 0.3
L. pla + D
11C ± 0.1
17EFG ± 0.2
10.6D ± 0.13
0.16CD ± 0.2
0.123C ± 0.11
96.6B ± 0.3
L. pla + D + H
11C ± 0.1
19.6DEF ± 0.2
12CD ± 0.13
0.073DEF ± 0.2
0.043D ± 0.11
96.6B ± 0.3
L. agilis + L. pla + H
15A ± 0.1
22.3BC ± 0.2
15.6ABC ± 0.13
0.27B ± 0.2
0.20BD ± 0.11
100A ± 0.3
L. agilis + L. pla + D
15AB ± 0.1
22BC ± 0.2
15BC ± 0.13
0.16C ± 0.2
0.08D ± 0.11
100A ± 0.3
3.5 L. agilis and L. plantarum treatments impact on photosynthetic pigments in stressed plants
Chlorophyll a, b and carotenoid concentrations in wheat plants were decreased after heat and drought stress. Compared with the non-stressed control, drought stress decreased carotenoid to 6 %, chlorophyll a to 35 %, and chlorophyll b to 14 %. In contrast to the non-stressed control, heat stress reduced the levels of carotenoid by 33 %, chlorophyll a by 63 %, and chlorophyll b by 81 % (Fig. 2). All of the treatments notably increased the amounts of carotenoid, chlorophyll a, and chlorophyll b in the unstressed plants. When plants under drought stress were treated with L. agilis, levels of carotenoids increased by 37 %, and levels of chlorophyll a and chlorophyll b also increased by 55 % and 10 %, respectively, in comparison to plants not under drought stress. After being treated with L. agilis, heat-stressed plants displayed increased levels of carotenoids by 55 % and chlorophyll a and chlorophyll b by 70 % and 82 %, respectively, in comparison to the control group. Plants exposed to drought stress exhibited increased levels of carotenoids (46 %) and chlorophyll a and chlorophyll b (54 and 19 %) respectively, following treatment with L. plantarum, as compared to the control group under drought stress. Fig. 3 illustrates the increase in carotenoids (59 %), chlorophyll a (73 %), and chlorophyll b (85 %), in heat-stressed plants treated with L. plantarum as opposed to drought-stressed control. Plants under drought and heat stress exhibited increased levels of carotenoids (58 %), as well as chlorophyll a (68 %) and chlorophyll b (52 %), following treatment with L. agilis. Drought and heat-stressed plants, when treated with L. plantarum, showed elevations in the carotenoids by 31 % and chlorophyll a and chlorophyll b levels by 22 % and 28 %, respectively when compared with drought- and heat-stressed control. Drought-stressed plants, after combined treatment with L. agilis and L. plantarum, showed 37 % elevation in the carotenoids and 44 % chlorophyll a levels when compared to the drought-stressed control group. Heat-stressed plants, after being treated together with L. agilis and L. plantarum, showed 60 % elevations in the carotenoids concentration and 62 %, 72 % increase in chlorophyll a and chlorophyll b levels, respectively, when compared with the heat-stress control group. In drought- and heat-stressed plants, after combined treatment with L. agilis and L. plantarum, the concentration of carotenoids increased to 71 % and chlorophyll a and b levels to raised to 78 % and 69 % respectively, after being compared with control of drought and heat-stress (Fig. 2).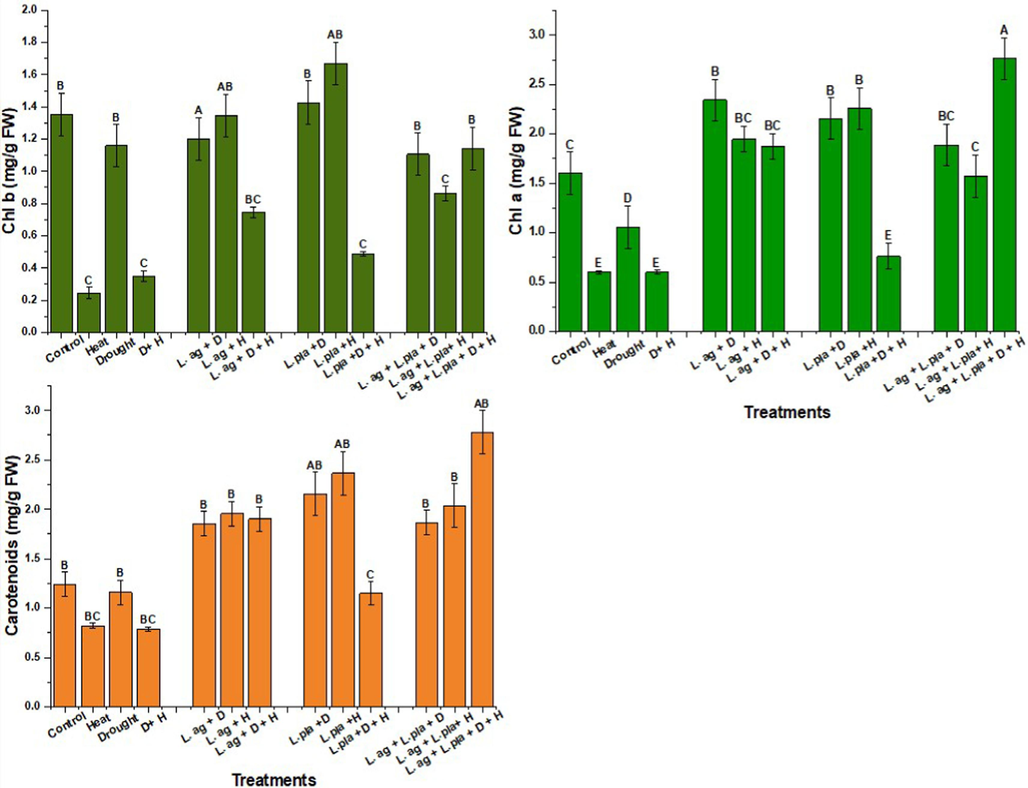
Shows contents of carotenoid, chlorophyll a and b after stress, L. agilis and L. plantarum treatments Alpha 0.05. Between the means, there is significant statistical difference.
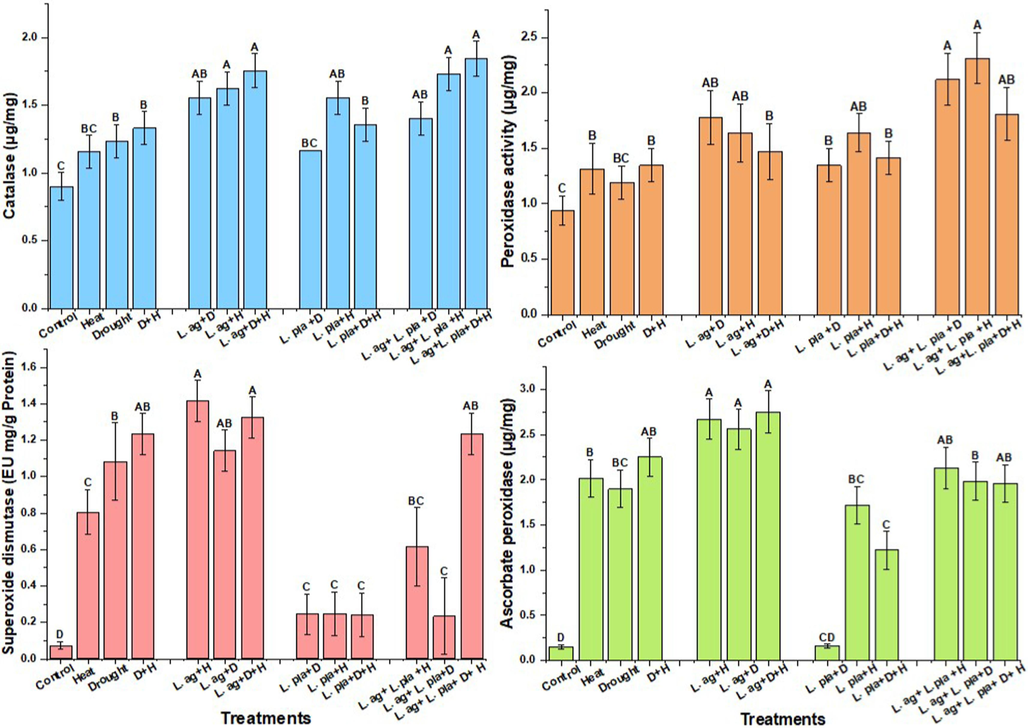
Activities of peroxidase, catalase, super oxide dismutase and ascorbate peroxidase in stress, L. agilis, L. plantarum treatments Alpha 0.05. Between the means, there is significant statistical difference.
3.6 Effects of L. agilis and L. plantarum treatments on antioxidant enzyme activities in stressed plants
After the wheat plants exposed to drought and heat stress exhibited 21 % and 29 % elevation in the POD, respectively, in comparison to non-stressed plants (Fig. 3). In plants with drought stress, a further rise in the CAT concentration was noted after the treatments of L. agilis and L. plantarum with 33 % and 11 %, respectively, as compared with the drought-stressed control group. In plants with heat stress, a notable increase in the CAT concentration was observed after the treatments of L. agilis and L. plantarum with 19 % and 20 %, respectively, as compared with the heat-stressed control group. In plants with drought and heat stress, the level of POD in combined treatments increased by 39 % and 41 %, respectively. After comparison to non-stressed plants, wheat plants exposed to heat and drought conditions showed elevations in the CAT of 26 % and 21 %, respectively (Fig. 3). In plants subjected to drought stress, an increase in the CAT level was noted after treatments of L. agilis and L. plantarum with 40 % and 41 %, respectively, as compared to drought-stressed control group. Similarly, plants under heat stress showed a significant raise in the CAT level after treatments with L. agilis at 29 % and L. plantarum at 5 % as compared with the heat-stressed control group. Exposed wheat plants to drought and heat stress exhibited 93 % and 91 % elevation in the SOD, respectively, compared with the non-stressed plants (Fig. 3). When L. agilis was applied to drought-stressed plants, there was an additional 5 % rise in SOD content as compared to the control group. After comparison to the heat-stressed control group, there was a notable rise (42 %) in the SOD level in heat-stressed plants following the application with L. agilis. Exposed wheat plants with drought and heat stress exhibited 91 % and 93 % elevation in the APX, respectively, compared with the non-stressed plants. After L. agilis treatment, there was a further 25 % increase in the APX level in drought-stressed plants after being compared with the drought-stressed control group. After comparison to the heat-stressed control, there was a notable rise (24 %) in the APX level in plants that had received L. agilis treatment (Fig. 3).
4 Discussion
The selected Ficus carica, Azadirachta indica, and Ocimum tenuiflorum plants have been used for medicinal purposes a long time ago, and most of the researchers have concluded the role of extracellular metabolites in the rhizosphere region of the plants to promote plant growth and other potent characteristics. Technologies based on microbe are becoming common, producing nutrients and plant-growth promoting constituents in the rhizospheric plants region (Naamala, 2020). As revealed from the current study, inoculation of seeds with Rhizospheric bacterial isolates significantly enhanced the growth and yield of the wheat plant when compared to non-treatment controls. Results of the current study are correlated to a study that analysed PGPRs assistance in maintaining plant survival under stress conditions by producing various secondary metabolites, phytohormones and enzymes (Mishra et al., 2018). The study found that L. agilis and L. plantarum significantly affected wheat growth and yield, with direct exposure to drought and heat shocks increasing antioxidant (POD, SOD, CAT, APX) levels.
In the current study, drought and heat exposure significantly reduced the germination, shoot length (SL), root length (RL), leaf area (LA), fresh weight (FW), and dry weight (DW) in untreated plants, which is related to the study of Saadaoui et al. (2023), in which it is reported that abiotic stresses severely affected the germination and biomass of wheat. Current findings illustrated the momentous rise in the contents of chlorophyll after PGPR treatments, that also correlated to the study of Neshat et al. (2022), which depicted the linkage between the PGPR and chlorophyll content, enhanced antioxidant levels, and better plant growth under the stress condition. In this study, stresses of drought and heat to the treatments, reduced the photosynthetic pigments significantly in leaves, as revealed by the lower values of chlorophyll a, b, and carotenoids, which related to a study in which chlorophyll synthesis was directly affected by drought and heat stresses through defective electron transport chain mechanisms (Abbas et al., 2018). All the L. agilis and L. plantarum inolucolation significantly enhanced the photosynthetic pigments in both stressed and non-stressed plants, which suggested that plant and bacterial enzymes are responsible for the synthesis of chlorophyll pigments along with essential plant nutrients. The current finding correlated with the research led by Vejan et al. (2016), who found that chlorophyll a and b contents increased after the application of B. subtilis S4 in stressed plants. In this study, the treatments of L. agilis and L. plantarum showed significant enhancements in the chlorophyll a, b, carotenoid contents, and antioxidant enzymes in wheat plants.
5 Conclusion
Current findings suggest that L. agilis, L. plantarum, and L. acidophilus enhanced the wheat plant's vigor and biomass. However, L. agilis and L. plantarum showed significant impact individually and in consortium to mitigate the adversative effects of drought and heat stresses on wheat plants. Moreover, L. agilis and L. plantarum-mediated stress alleviation was attributed to the modulation of the morphological, physiological, and biochemical responses of stressed wheat. These strains can be tested in field conditions to validate their potential as biofertilizers.
CRediT authorship contribution statement
Allah Nawaz Khan: Writing – original draft, Methodology, Formal analysis. Muhammad Nadeem Hassan: Resources. Rumana Keyani: Data curation. Hafiza Zaineb Amir: Formal analysis. Mohammad Raish: Writing – review & editing, Validation. Rattandeep Singh: Writing – review & editing, Validation. Humaira Yasmin: Supervision, Formal analysis, Conceptualization.
Acknowledgements
The authors are grateful to the Researchers Supporting Project Number (RSPD2024R957) at King Saud University, Riyadh, Saudi Arabia.
References
- Search for dark matter and other new phenomena in events with an energetic jet and large missing transverse momentum using the ATLAS detector. J. High Energy Phys.. 2018;2018(1):1-53.
- [Google Scholar]
- Abbas, S. K., Hassan, H. A., Asif, J., Ahmed, B., Haider, S. S., & others. (2018). Integration of TTF, UTAUT, and ITM for mobile Banking Adoption. International Journal of Advanced Engineering, Management and Science (IJAEMS) Vol-4, Issue-5.
- HER2-specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol.. 2017;3(8):1094-1101.
- [Google Scholar]
- Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol.. 2013;64:807-838.
- [Google Scholar]
- Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ.. 1992;39(3):239-247.
- [Google Scholar]
- Cossart Y. (1987). Bergey’s Manual of Systematic Bacteriology Volume 2. Pathology, 19:324. doi: 10.1016/S0031-3025(16)36764-2.
- Global synthesis of drought effects on maize and wheat production. PLoS One. 2016;11(5):e0156362.
- [Google Scholar]
- A medium for the cultivation of lactobacilli. J. Appl. Bacteriol.. 1960;23(1):130-135.
- [Google Scholar]
- Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245(1):85-96.
- [Google Scholar]
- Holt J.G., Krieg N.R., Sneath P.H.A. (1994). Bergey’s Manual of Determinative Bacterology. The Williams and Wilkins Co.; Baltimore, MD, USA.
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54(4):287-293.
- [Google Scholar]
- Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol.. 2004;96(3):473-480.
- [Google Scholar]
- Antagonistic, anti-oxidant, anti-inflammatory and anti-diabetic probiotic potential of Lactobacillus agilis isolated from the rhizosphere of the medicinal plants. Saudi J. Biol. Sci. 2021;28(11):6069-6076.
- [Google Scholar]
- Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biologica. 2014;60(4):153.
- [Google Scholar]
- Mishra, J., Fatima, T., & Arora, N. K. (2018). Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress. In Plant microbiome: stress response (pp. 127–163). Springer.
- Relevance of plant growth promoting microorganisms and their derived compounds, in the face of climate change. Agronomy. 2020;10(8):1179.
- [Google Scholar]
- Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants. 2022;28(2):347-361.
- [Google Scholar]
- Onsa, G. H., bin Saari, N., Selamat, J., & Bakar, J. (2004). Purification and characterization of membrane-bound peroxidases from Metroxylon sagu. Food Chemistry, 85(3), 365–376.
- Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils. 2015;51(4):403-415.
- [Google Scholar]
- New diet (NTP-2000) for rats in the National Toxicology Program toxicity and carcinogenicity studies. Fundam. Appl. Toxicol.. 1996;32(1):102-108.
- [Google Scholar]
- Effects of drought stress induced by D-Mannitol on the germination and early seedling growth traits, physiological parameters and phytochemicals content of Tunisian squash (Cucurbita maxima Duch.) landraces. Front. Plant Sci.. 2023;14:1215394.
- [Google Scholar]
- A biomass combustion chamber: Design, evaluation, and a case study of wheat straw combustion emission tests. Aerosol Air Qual. Res.. 2015;15(5):2104-2114.
- [Google Scholar]
- Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules. 2016;21(5):573.
- [Google Scholar]
- Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol. Biol.. 2016;90(6):623-634.
- [Google Scholar]
- Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol.. 2006;142(3):1065-1074.
- [Google Scholar]
- Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ.. 2019;670:1-7.
- [Google Scholar]
- Approximating discrete probability distribution of image emotions by multi-modal features fusion. Transfer. 2017;1000(1):4669-4675.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103334.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







