Translate this page into:
Potential mechanism of Jatropha gossypifolia phenolic derivatives in enhancing insulin-signalling cascades GLUT 4, IRβ and GSK-3β in streptozotocin nicotinamide induced type II diabetic in wistar rat model
⁎Corresponding author. jesuaroa@srmist.edu.in (Jesu Arockiaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Diabetes mellitus is a metabolic syndrome that alters the composition of carbohydrates, proteins and fat content in the body due to decreased insulin secretion. This disrupts the insulin-signaling cascade, resulting in failure in the targeted tissues, such as insulin-stimulated glucose absorption muscle and fat. Natural products such as plants, which are rich in nutrient, offer a wide range of treatments for a number of diseases and infections including diabetes mellitus. Hence, this study investigates the anti-hyperglycemic efficacy of the methanolic leaves extract of Jatropha gossypifolia in experimental diabetic rats.
Methods
Phytochemical constitution of J. gossypifolia showed tannin content to be significantly increased in methanolic extract and HPLC analysis also revealed phenolic derivative tannic acid to be main constitution. An intraperitoneal administration of 110 mg/kg body weight (b.w) nicotinamide and 45 mg/kg b.w. streptozotocin was provided to male Wistar albino rats, thus inducing diabetic. Diabetic induced rats treated with methanolic leaves extract of J. gossypifolia at the concentration between 100 and 50 mg/kg for 35 days. Periodic blood glucose level and body weight were measured. Methanolic extract influence was evaluated with q-PCR and western blot analysis on the cellular targets of insulin signaling cascades.
Results
The results showed the decrease in blood glucose levels and increased serum insulin level in treated groups was significant (P < 0.05) in oral administration of methanol extract. Methanolic extract exhibited a profound increase in the gene expression of the cellular targets of insulin signaling cascade including IRTK, IRS1, PI3K, GS, GLUT 4 and a basal level expression of GSK-3β. In addition, methanolic extract increased protein expression of IRβ (2.7-fold), GSK-3β (2.5-fold) and GLUT 4 (2.4-fold) translocation as evidenced from western blot analysis with the plasma membrane fractions.
Conclusion
Conclusively, methanolic leaves extract of J. gossypifolia exhibited a profound anti-hyperglycemic activity by modulating the expression of the key targets of insulin signaling pathway.
Keywords
Jatropha gossypifolia
Wistar albino rats
Streptozotocin–nicotinamide induction
Gene and protein expression
Anti-hyperglycemic activity
- b.w
-
Body weight
- DM
-
Diabetes Mellitus
- GAPDH
-
Glyceraldehyde 3-phosphate dehydrogenase
- GLUT 4
-
Glucose transporter 4
- GS
-
Glycogen synthase
- GSK-3β
-
Glycogen synthase kinase 3β
- HD
-
High dose
- IPGTT
-
Intra peritoneal glucose tolerance test
- IRS-1
-
Insulin receptor substrate 1
- IRTK
-
Insulin receptor tyrosine kinase
- IRβ
-
Insulin receptor
- J. gossypifolia
-
Jatropha gossypifolia
- LD
-
Low dose
- ME
-
Methanolic extract
- PI3K
-
Phosphoinositide 3-kinase
- PKB
-
Protein kinase B
- PM
-
Plasma membrane
- STZ-NIC
-
Streptozotocin nicotinamide
- T2DM
-
type 2 Diabetes Mellitus
Abbreviations
1 Introduction
The metabolic syndrome of diabetes mellitus affects carbohydrates, proteins and fats in the body which lowers the segregation of insulin (Moree et al., 2013; Gaur et al., 2014; Praveen et al., 2020). This entails the disruption of insulin-signalling pathway leads to failure in the targeted tissues including insulin-stimulated glucose absorption muscle and fat. This is due to the failure of insulin that promote Glucose Transporter 4 (GLUT 4) translocation to the plasma membrane in muscle cells (Epstein et al., 1999; Guru et al., 2021). Natural products such as plants, which are rich in nutrient, offer a wide range of treatments for a number of diseases and infections. Current drug development based on the available knowledge is responsible for the safe discovery of drugs and promotes the creation of new chemical entities (Ribnicky et al., 2006; Singh et al., 2021).
The Jatropha family of Euphorbiaceae projected to have about 170 species. The name Jatropha is derived from the Greek word “jatros” that means doctor and “trophe” means food, altogether it points out its medicinal uses (Sabandar et al., 2013; Félix-silva et al., 2014; Purabi et al., 2020; Issac et al., 2021a). Among them, Jatropha gossypifolia (J. gossypifolia) commonly known as ‘bellayche bush’ that grows widely throughout India and is a perennial herb belongs to Euphorbiaceae family. The various parts of this plant including leaves, stem, heart, seed and latex historically used as medicine and this plant possessed several chemical constituents. Seeds of this plant considered as purgative and used to treat constipation. The oil obtained from its seeds are used to treat leprosy and paralysis. The paste developed from the leaves of Jatropha are used to treat skin diseases and conditions like eczema, rashes, measles and even chicken pox (Olowokudejo, 1993).
The studies on pharmacological and biological activities of J. gossypifolia are so limited. Oral administration of ethanolic extract of J. gossypifolia roots and aerial parts at the concentration between 125 and 250 mg/kg have shown antihypertensive and also reduced chronic blood pressure in rats (Abreu et al., 2003; Vijay et al., 2021; Issac et al., 2021b). The methanolic extract (ME) of J. gossypifolia exhibited antimicrobial activity against Staphylococcus aureus. A new diterpenoid isolated and characterized from J. gossypifolia root extract showed potential anti-proliferative activity in A-549 cell line (Kumar et al., 2017). The oral doses of ME of J. gossypifolia leaves at the concentration between 500 and 1000 mg/kg prevented acute carrageen-induced paw edema in rats; at 50–100 mg/kg, it inhibited chronic cotton pellet induced granuloma formation (Anandharajan et al., 2006); moreover at 200–400 mg/kg of oral administration, it displayed anxiolytic and antidiarrheal behaviour (Ravindranath et al., 2003; Manikandan et al., 2021; Velayutham et al., 2021).
In our preliminary study, we have observed the anti-diabetic efficacy of ME in J. gossypifolia leaves established in L6 myotubes cell line (Kumar et al., 2017). Based on the observation, we have evaluated the anti-hyperlipidemic activity which was evinced in streptozotocin–nicotinamide induced diabetic rats treated with ME of J. gossypifolia leaves. The present study evaluates the anti-hyperglycemic effect of J. gossypifolia leaves ME on insulin resistant in vivo model. The mechanism was further analysed using real time PCR and western blotting.
2 Material and methods
2.1 Chemicals and reagents
All the chemicals used in this study was obtained from Himedia, India. Blood glucose level was analysed using Accu-Chek® Active Blood Glucose Meter. Real-time PCR analysis was performed using KAPA SYBR® FAST qPCR Master Mix (2X). Rat IgA ELISA kit was obtained from eBioscience, Germany. Primary and secondary antibodies for western blotting obtained from Calbiochem, Germany and Santa Cruz Biotechnologies, USA.
2.2 Plant material collection, authentication and sequential extraction
Leaves of J. gossypifolia was collected from east coast region of Chennai, Tamil Nadu, India. The collected material was endorsed by Dr P. Jayaraman, Plant Anatomy Research Institute, Chennai, Tamil Nadu, India (Authentication: No: PARC/2015/3142). The leaves of J. gossypifolia were dried and powdered (50 g). To increase the order of polarity (hexane, dichloromethane, ethyl acetate and methanol), the powder was subjected to sequential extraction using Soxhlet devices with different solvents. The obtained filtrate was subjected to rotary evaporation and obtained dried extract (Anandharajan et al., 2006).
2.3 Estimation of phytochemical components
All the four leaves extracts (n-hexane, Dichloromethane, Ethyl acetate and Methanol extracts) of J. gossypifolia were subjected to preliminary phytochemical testing that was performed to analyse the presence of flavonoids using colorimetric aluminium chloride method (Chang et al., 2002). Total phenolic content was determined by Folin-Ciocalteau method (McDonald et al., 2001; Silva et al., 2006) and determination of Tannins was performed according to Porter et al. (1985).
2.4 HPLC analysis of ME
HPLC (Agilent Technologies, 1200 infinity series, Germany) analysis was carried out in crude ME and tannic acid was used as a standard (Beasley et al., 1977; Kanaujia et al., 2010). The following protocol was used for the HPLC analysis (Mohammadi et al., 2014): Mobile phase-Water: Acetonitrile: Acetic acid [89:10:1], Flow rate – 0.8 ml/min, Injection sample – 10 µL, Sample concentration – 1 mg/mL and UV–VIS reading – 280 nm.
2.5 Experimental animals
The in vivo study was conducted with the consent from the animal ethical committee of the Institute (Ethical Clearance No. 090/835/IAEC-2014). Wistar male rats aged between 6 and 8 weeks old and weighed 180–200 g was used in the study. Thirty animals have been recruited from the University of Veterinary Sciences of Tamil Nadu, Chennai, India, transported to the animal house in the Institute and acclimatized for a week. All animals fed with sterilized water and pellet diet ad libitum during the acclimatization period. The rats were kept with sterile paddy husk as bedding in sterile polypropylene cages; the temperature and light and dark condition was maintained as 25 °C and 12L/12D.
2.6 Induction of diabetes
Diabetes was instigated by intraperitoneal administration in male wistar rats with the combination of Nicotinamide (NIC) (110 mg/kg) dissolved in saline and Streptozotocin (STZ) (single dose of 50 mg/kg b.w.) dissolved in 0.01 M freshly prepared citrate buffer (pH 4.5); STZ was injected after 15 min from the injection of Nicotinamide (Masiello et al., 1998).
2.7 Animal grouping and drug administration
Thirty rats (20 diabetic induced rats and 10 normal rats as control) divided into 6 groups; each consists 5 rats. Regular diet was provided to the control groups and high fat diet (commercially available) was supplied to the diabetic induced rats. The diet composition of normal and diabetic induced rats mentioned in Table 1. Composition of high fat (diabetic rat) and regular diet (normal rat). Diet was provided ad libitum to animals; high fat diet was provided to the diabetic animals.
Nutrients and Ingredients
Normal Rats
Diabetic Rats
Nutrients (%/100 g)
Protein
20%
25%
Carbohydrate
55%
48%
Fat
5%
40%
Fiber
5%
–
Calcium
0.8%
–
Ingredients (g/100 g)
Groundnut oil
–
6 g
Ghee
–
6 g
Milk powder
–
20 g
Total Energy (k cal/100 g)
306 k cal
414 k cal
A small cut was made in the tail and blood was drawn to measure the blood glucose level using glucometer, (Accu-check, Germany). On day 3 and 7 of induction, the rats pronounced with hyperglycemia (FBG 250 mg/dl) which further selected and studied as follows:
Group 1: Control rats (0.5% CMC); n = 5.
Group 2: Control rats treated with ME (100 mg/kg b.w.); n = 5.
Group 3: Diabetic rats (STZ + NIC); n = 5.
Group 4: Diabetic rats (STZ + NIC) treated with ME (50 mg/kg b.w.); n = 5.
Group 5: Diabetic rats (STZ + NIC) treated with ME (100 mg/kg b.w.); n = 5.
Group 6: Diabetic rats (STZ + NIC) treated with metformin (500 mg/kg b.w.); n = 5.
Body weight and blood glucose level was periodically checked on day 0, 14, 21, 28 and 35. Blood from the internal canthus of the eye was obtained by retro-orbitally capillary tubes. Glucometer was used to assess the blood glucose levels. The serum was centrifuged 2000 rpm for 2 min and stored at −20 °C for further study.
2.8 Intraperitoneal glucose tolerance test (IPGTT)
At the end of the experimental phase, the rats were fasted overnight and injected with glucose (2 g/kg) intraperitoneally. Then, blood was drawn from the tail vein at 0, 15, 30, 60, 90 and 120 min and the glucose level was measured using Accu-check glucometer (Andrikopoulos et al., 2008).
2.9 Sacrification of animals and organ collection
At the end of the experimental phase (day 35), the animals were deprived of food and sacrificed. For the blood glucose assessment, blood was collected in a tube containing EDTA; and for insulin level measurement, the plasma was isolated from the blood by centrifugation. Pancreas and skeleton muscles are dissected out washed in ice-cold saline and stored in 10% formalin for further studies.
2.10 Estimation of insulin level and HOMA index
The obtained blood samples were used to determine the plasma insulin level using Rat IgA ELISA kit. Further, to quantify the insulin resistance, homeostatic model assessment (HOMA) index was calculated as follows (Mohammadi et al., 2014).
2.11 Histopathological examination
Thin sectioning of the pancreas (10 µm) was sectioned using microtome (Leica Bio system RM 2125 RTS, Germany) and further processed and stained with 0.1% haematoxylin followed by eosin (Abdollahi et al., 2011; Kumar et al., 2013). The section were observed under microscope at 40× magnification.
2.12 Isolation of RNA and real-time RT-PCR analysis
Muscle tissue was homogenized using trizol reagent and obtained the total RNA as explained earlier (Arockiaraj et al., 2011, 2012a; Kumaresan et al., 2014). The isolated RNA was converted to cDNA as reported (Arockiaraj et al., 2012b, 2013; Chaurasia et al., 2016a,b; Chomczynski and Sacchi, 1987). The obtained cDNA was used as template and subjected to qPCR analysis (Bhatt et al., 2014; Kumaresan et al., 2015). Real-time PCR investigation was achieved by KAPA SYBR® FAST qPCR Master Mix (2X) in a total volume of 20 µL with specific primers as mentioned in Table 2. GAPDH served as internal control gene. Initial denaturation carried out at 95 °C for 5 min, trailed at 95 °C for 1 min for denaturation. The annealing temperature maintained at 1 min at 64 °C for IRTK; and 62 °C for IRS-1, PI3K and 63 °C for GSK-3β, GS and GLUT 4. Extension was accomplished at 72 °C for 1 min. This entire process repeated for 40 cycles, trailed by a final extension at 72 °C for 10 min and hold at 4 °C. The ΔΔct value was calculated after normalizing with GAPDH and the fold change was estimated (Arasu et al., 2014; Chaurasia et al., 2016a,b; Palanisamy et al., 2015).
S. No
Primer
Forward primer sequence
Reverse primer sequence
Reference
1
IRTK
5′ATCTGGATCCCCCTGATAACTGTC3′
5′ATGTGGGTGTAGGGGATGTGTTCA3′
GenBank Accession ID: NM_017071
2
IRS-1
5′AAGTGGCGGCACAAG TCGAG3′
5′CGGGTGTAGAGAGCCACCAG3′
GenBank Accession ID: XM_002749924
3
PI3K
5′TGACGCTTTCAAACG CTATC3′
5′CAGAGAGTACTCTTGCATTC3′
Liberman and Eldar-Finkelman, 2005
4
GLUT 4
5′CGGGACGTGGAGCTG GCCGAGGAG3′
5′CCCCCTCAGCAGCGAGTGA3′
Manikandan et al., 2021
5
GSK-3β
5′GGTGAATCGAGAAGA GCCAT3′
5′CTCCTGAGTCACAAAGTTTG3′
Masiello et al., 1998
6
GS
5′CGTGGTGAGAGGAAG GAACTGAGC3′
5′CCGTCAGACCGTGGAGACA3′
McDonald et al., 2001
7
GAPDH
5′CCACCCATGGCAAAT TCCATGGCA3′
5′TCTAGACGGCAGGTCAGGTCCACC3′
Mohammadi et al., 2014
2.13 Preparation of plasma membrane fractions and total cell lysate from skeletal muscle
Plasma membrane fractions (PM) were obtained as described earlier (Sujatha et al., 2010). Skeletal muscle tissues were homogenized with Glass-Teflon potter homogenizer using 2 ml of the ice-cold buffer (pH 7.4) that contained 250 mM/L Sucrose, 5 mM NaN3, 20 mM HEPES, 200 µM/L PMSF, 1 µM/L Pepstatin, 1 µM/L Aprotinin and 2 µM/L EGTA. Cell lysate was obtained using Dounce homogenizer (20 S, 0.5 cycles, 10 pulses; 2 min each and lag time of 1 min for each pulse). The total cell lysate homogenate was centrifuged in12000 RPM at 4 °C for 20 min. The cell homogenates was further centrifuged in 12,000 RPM for 5 min at 4 °C in order to remove cell debris. The resulting supernatant was again centrifuged in 15,000 RPM for 40 min at 4 °C; and the obtained pellets were resuspended in buffer to constitute the Plasma Membrane (PM) fraction.
Total cell lysate was prepared as described earlier (Kanaujia et al., 2010). In a summary, homogenized tissue washed with ice-cold buffer contained 50 mM Hepes, 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 1 mM sodium orthovanate, 50 mM NaF, 10 µg/ml Aprotinin, 10 µg/ml Leupeptin and 1% Triton X 100 (pH: 7.4). Cell lysate obtained was homogenized using Dounce homogenizer (20 S, 0.5 cycles, 10 pulses; 2 min each and lag time of 1 min for each pulse). The Total cell lysate homogenate was centrifuged at 4 °C 12,000 rpm for 20 min.
Bradford method was used to determine the protein contents; the protein was subjected to 10% of SDS-PAGE and transferred onto a membrane with nitrocellulose. The membrane is blocked at 4 °C overnight in 5% skimmed milk powder and immunoblotted with the respective antibody. PM fractions were used to analyse the GLUT 4 translocation, whereas whole cell lysate was used to detect the proteins including IR-β and GSK-3β.
2.14 Western blot analysis
The immunoblot was probed with the respective antibodies (GLUT 4, IRβ and GSK3β) for 2 h. Upon washing, the membrane was incubated with HRP antibody; and the immunosignals were detected using luminol/H2O2 reagent and visualized under chemiDoc XRS+ imaging system (Biorad, USA). Quantification of the protein expression was performed in densitometric scanning using Image Studio Lite (ver. 5.2).
2.15 Statistical analysis
All the data were shown in this study are mean of three replicates ± standard deviation (SD). Data were subjected to one-way variance analysis (ANOVA) followed by Duncan’s multiple range test (DMRT) using statistical package of social science (SPSS, ver. 17.0). The significance level considered at p < 0.05.
3 Results and discussion
3.1 Phytochemical screening
It is imperative to note that the majority of the phytochemical constituent reported in literature are not about compounds isolation, rather on the phytochemical screening of the significant classes through qualitative methods. Phytochemical screening of n-hexane, Dichloromethane, Ethyl acetate and ME of J. gossypifolia showed the presence of phytochemical constitution such as tannin, flavonoid and phenolic compounds. Particularly, ME have shown significant (p < 0.05) increase in these phytochemical constituents than the other extracts as shown in Table 3. Presence of flavonoids, phenols and tannins in plants are found to be possessed antidiabetic activity (Kumari and Jain, 2012; Tiwari and Rao, 2002). Similarly, phytochemical screening of the ME of Melastoma malabathricum indicated the presence of terpenoids, flavonoids, phenolic compound, tannins, Saponins and triterpenes; however, flavonoid and phenolic substances are present in significant amount. Altogether, ME of M. malabathricum showed potential antidiabetic activity as reported by Kumar et al. (2013).
J. gossypifolia
ExtractsPhenol estimation (mg/ml)
Flavonoid estimation (mg/ml)
Tannin estimation (mg/ml)
Hexane
1.103 ± 0.001
1.0315 ± 0.0025
0.106 ± 0.002
Dichloromethane
0.0701 ± 0.002
1.1505 ± 0.002
0.0825 ± 0.0015
Ethyl acetate
0.297 ± 0.0015
0.9785 ± 0.0105
0.364 ± 0.003
Methanol
1.732 ± 0.001*
1.312 ± 0.0015*
1.995 ± 0.001*
3.2 HPLC analysis of ME
HPLC analysis of J. gossypifolia crude ME presented in Fig. 1(a and b). Totally 10 phenolic compounds were identified in ME to the retention time using tannic acid as standard (Fig. 1b). Maximum peak (37.77%) in the ME was observed due to the presence of Tannic acid followed by Rutin (11.41%) and Resorcinol (9.78%) as represented in Table 4. Among phenolic compounds present in ME, tannic acid availability is significantly abundant. Souza et al. (2008), investigated the presence of condensed tannins in Brazilian folk medicine Maytenus ilicifolia using HPLC analysis which contains epigallocatechin, epicatechin and epiafzelechin that supports our data.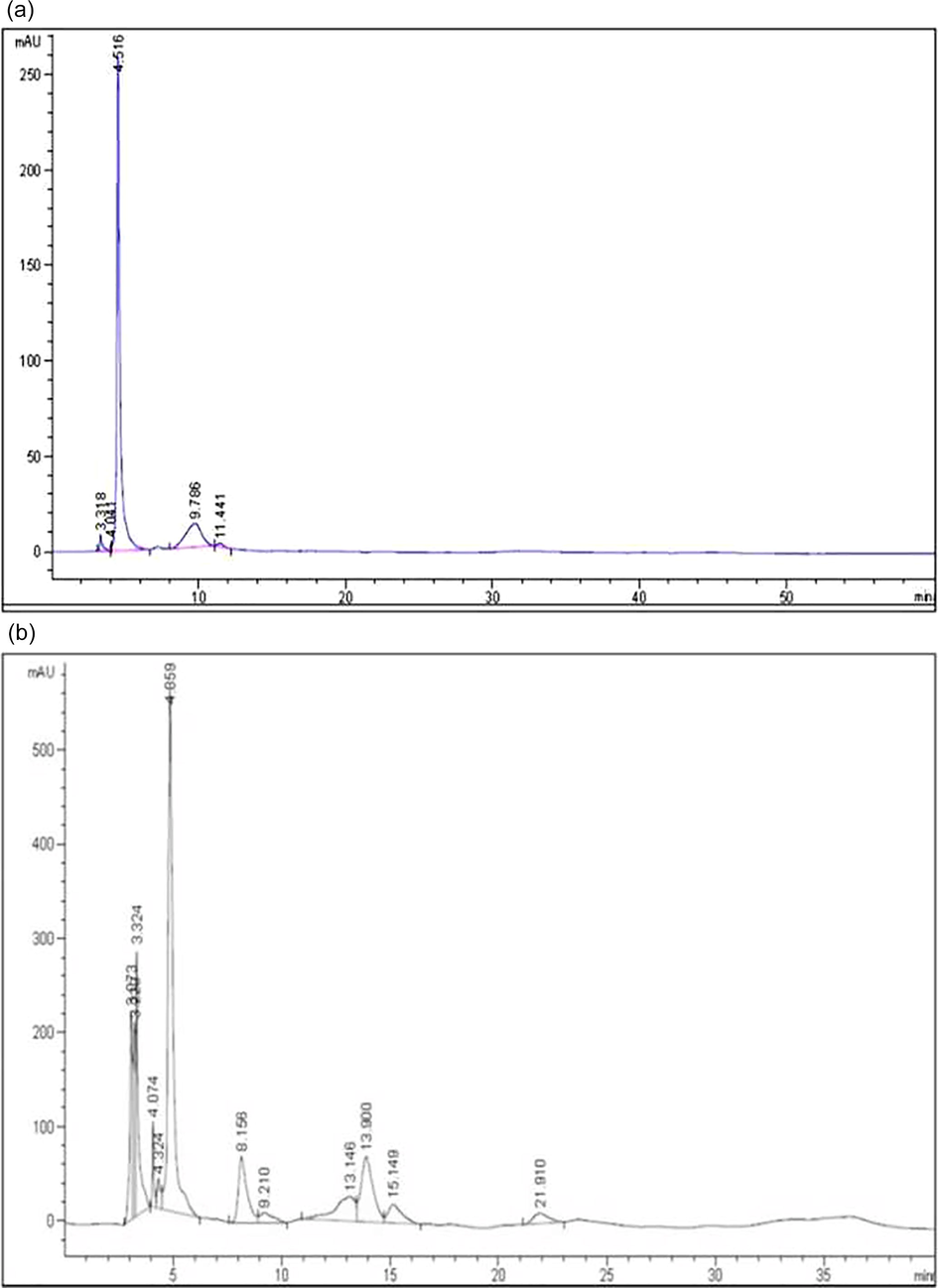
(a) HPLC analysis of standard Tannic acid, (b) HPLC analysis of J. gossypifolia ME.
S. No
Retention time
Peak % area
Name
Chemical name
Mol. wt. (g/mol)
1
3.073
7.69
Hydroquinone
C6H6O2
110.11
2
3.229
6.41
Epicatechin
C15H14O6
290.27
3
3.324
9.78
Resorcinol
C6H6O2
110.1
4
4.324
1.59
Vanillic acid
C8H8O4
168.15
5
4.859
37.77
Tannic acid
C76H52 O46
1701.19
6
8.156
8.1
Gallic acid
C8H8O3
152.13
7
9.21
1.86
Cinnamic acid
C9H8O2
148.16
8
13.14
7.28
Gentisic acid
C7H6O4
154.12
9
13.9
11.41
Rutin
C27H30O16
610.52
10
15.14
3.66
Digalloyl glucose
C20H20O14
484.37
3.3 Effect of ME on body weight in STZ-NIC induced diabetic rats
STZ-NIC induction led to significant (p < 0.05) body weight loss (from 201.6 ± 2.5 g to 124 ± 1.7 g) compared to the controls. This proves that STZ-NIC had induced diabetes in rats, which is characterized by severe loss in the bodyweight. The treated groups (50 and 100 mg/kg b.w.) showed significant improvement in body weight from 199.6 ± 4.7 g (day 0) to 201 ± 3.1 g (day 35) and 199 ± 2.6 g (day 0 to 201.6 ± 2.5 g (day 35 compared to diabetic group, respectively. Metformin (a diabetic drug) treatment also showed weight gain from 198.2 ± 2.6 g to 205.6 ± 2.5 g (Table 5). STZ is transported into β-cells via the glucose transporter GLUT2 and causes DNA damage and the partial protective action of β cells in NA is due to the inhibition of PARP-1 activity. NA inhibits this enzyme, preventing depletion of NAD+ and ATP in cells exposed to STZ. The reduction in body weight noticed in the diabetic group due to the effect of protein squandering, because of inaccessibility of carbohydrate for use as a vital source. Similar findings were observed in type 2 diabetic induced (by streptozotocin-nicotinamide) rats, which was treated by Alangium lamarckii Thwaites leaves (AAL) alcoholic extract. Further, the reports showed a decrease in body weight from 174.16 ± 8.07 g (day 0) to 131.16 ± 7.12 g (day 14 in diabetic group; and in AAL treated group (250 and 500 mg/kg), it was 178.83 ± 4.72 g (day 0) to 142.66 ± 4.92 g (day 14) and 171.50 ± 3.51 g (day 0) to 157.83 ± 3.32 g (day 14) (Kumar et al., 2011). Each value is given as mean ± SD (n = 5) from three independent experiments. Statistical evaluation was analysed by one-way ANOVA followed by Duncan’s multiple range test. * stands for p < 0.05 as compared with control group and # stands for p < 0.05 as compared with diabetic group. ME: Methanolic extract; LD: Low Dose; HD: High Dose; STZ: Streptozotocin; NIC: Nicotinamide; LD 50 mg/kg b.w. and HD 100 mg/kg b.w.
Groups
Body weight (g)
Before treatment
After treatment
Control
201.6 ± 2.244
203.6 ± 2.678
Control + J. gossypifolia
200.2 ± 2.489
201.2 ± 2.489
Diabetic (STZ + NIC)
201.6 ± 2.509
124 ± 1.702*
Diabetic + J. gossypifolia ME (LD)
199.6 ± 2.728
201 ± 3.314#
Diabetic + J. gossypifolia ME (HD)
199 ± 2.632
201.6 ± 2.678#
Diabetic + Metformin
198.2 ± 2.663
205.6 ± 2.509#
3.4 Effect of ME on blood glucose level in STZ-NIC induced diabetic rats
During the entire study, fasting blood glucose level was validated; oral administration of 50 and 100 mg/kg ME showed a significant anti-hyperglycemic effect. Reduction of blood glucose level from before treatment 416.8 ± 3.9 mg/dL (day 0 to after treatment 142.6 ± 3.5 mg/dL (day 35); and 413.4 ± 3.8 mg/dL (day 0) to 120.8 ± 3.3 mg/dL (day 35) was observed upon treatment with J. gossypifolia ME at the concentration of 50 and 100 mg/kg b.w., respectively; however, maximum reduction was observed after treatment. Metformin exhibited 108.8 ± 3.4 mg/dL reduction in blood glucose level at the end of the study as compared to the diabetic group. J. gossypifolia ME treated groups (100 mg/kg) displayed a maximum lowering effect of glucose in diabetic rats compared to low dose of ME (50 mg/kg). The data substantiates the anti-hyperglycemic effect of ME as comparable with the positive control (Table 6). The hyperglycemia was elevated in the blood glucose because of the decreased secretion of insulin. The prolonged blood glucose level after intraperitoneal administration of STZ infers the hyperglycemic condition; and oral organization of 50 and 100 mg/kg of ME for 35 days essentially decreased the fasting blood glucose level in the diabetic rats, which demonstrated the antidiabetic activity of J. gossypifolia ME. Similarly, maximum reduction of blood glucose level was observed in oral administration of ethanolic extract of Helianthus annuus L., seed on day 21 i.e. 175.24 ± 4.12 mg/dL with 250 mg/kg and 124.65 ± 2.64 mg/dL with 500 mg/kg (Saini and Sharma, 2013).
Groups
Blood glucose (mg/dL)
Day 0 (initial)
Day 35 (final)
Control
109.8 ± 3.663
109.6 ± 3.927
Control + J. gossypifolia
111.6 ± 3.812
113.8 ± 3.663
Diabetic (STZ + NIC)
420.2 ± 4.743*
527.4 ± 3.856*
Diabetic + J. gossypifolia ME (LD)
416.8 ± 3.969
142.6 ± 3.583#
Diabetic + J. gossypifolia ME (HD)
413.4 ± 3.812
120.8 ± 3.374#
Diabetic + Metformin
415.0 ± 3.930
108.8 ± 3.476#
3.5 Effect of ME in IPGTT
At the conclusion of the treatment cycle with vehicle and insulin, glucose tolerance was tested by IPGTT (2 g/kg. i.p). Fig. 2 shows that nearly all animals are hyperglycemic at 30 min. This hyperglycemia exacerbated by the i.p glucose load; and after 150 min it did not return to the baseline, which is suggesting the glucose intolerance in diabetic rats. Blood glucose reduction was observed in rats treated with ME (50 and 100 mg/kg) it is revealed a decrease in blood glucose levels of 121.5 ± 2.5 mg/dL (50 mg/kg b.w.) and 118.8 ± 2.1 mg/dL (100 mg/kg b.w.). This is due to the interference of intestinal absorption or by the potentiation of insulin secretion and in addition, by increasing the use of glucose levels in muscles as demonstrated by Fuhlendorff et al. (1998). Equally, oral administration of Codariocalyx motorius treatment (200 mg/kg b.w.) was also significantly increased the glucose tolerance after glucose administration from 30 to 120 min (Uma et al., 2014).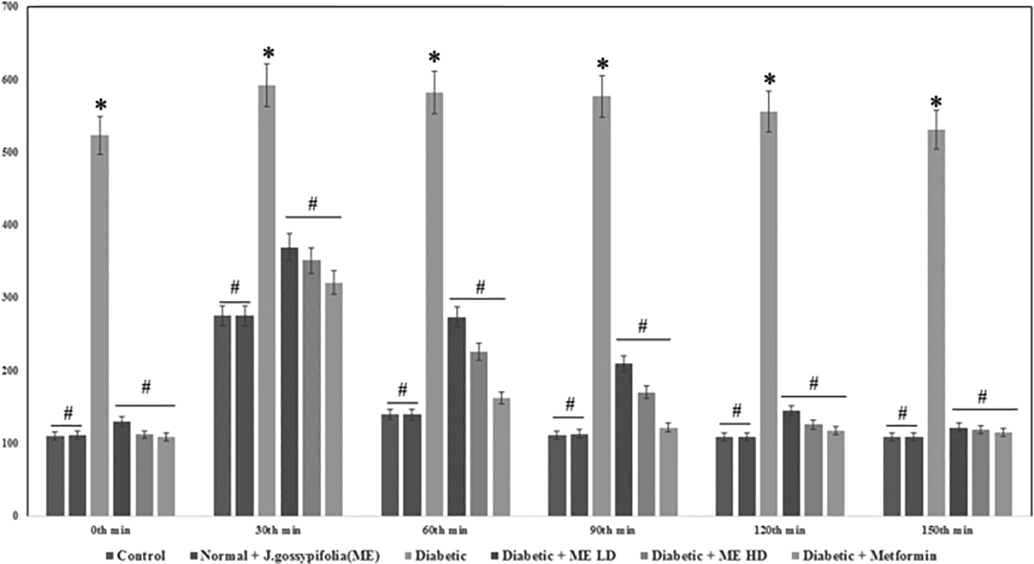
Effect of J. gossypifolia ME dose 50 (LD) and 100 mg/kg b.w (HD) on intra peritoneal glucose tolerance test. Values are given as means ± SD (n = 5) from three independent experiments in triplicate. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *p < 0.05 as compared with control group, #p < 0.05 as compared with Diabetic group.
3.6 Histopathology analysis of pancreas
Control and drug control rats (Fig. 3a and b) showed the presence of normal pancreatic β cells in islets of Langerhans on endocrine portion. Diabetic rats (STZ + NIC) as shown in Fig. 3c displayed a dilated and degranulated islet cells with damage and reduction in size of pancreatic β cells. Diabetic rats treated with ME (Fig. 3d and e) 50 and 100 mg/kg, re-established the necrosis and fibrotic changes; and increased the pancreatic β cells in islets of Langerhans. In other hands, Metformin-treated diabetic rats (500 mg/kg b.w.) showed a normal pancreatic islet cells (Fig. 3f). J. gossypifolia ME increased the number of insulin positive cells in the pancreas and either prevented or improved the β-cells of the islets of Langerhans with accords reported in C. motorius (Uma et al., 2014).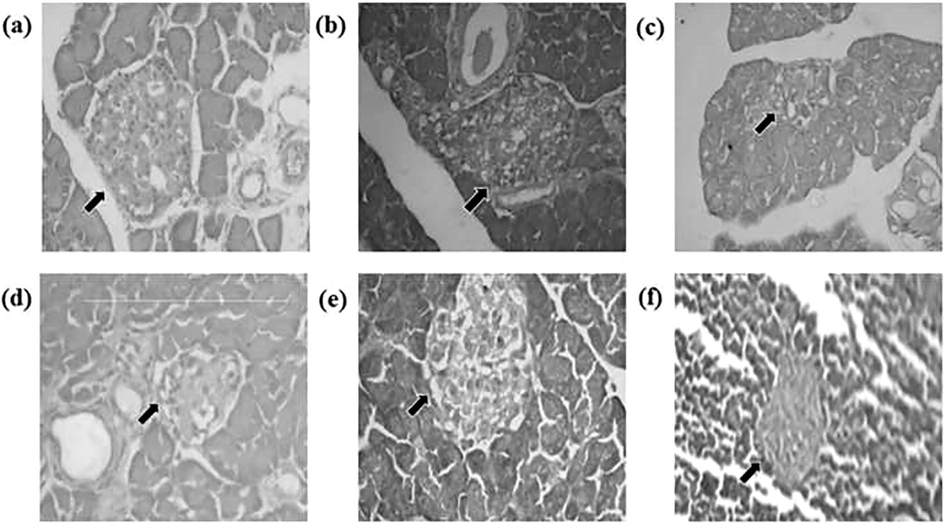
Histopathology of pancreas in STZ-NIC induced diabetic rats after 35 days of treatment. (a and b) Control and drug control (ME 100 mg/kg b.w.) rat indicates the normal pancreas, (C) Diabetic control-degranulated, (d) Diabetic + ME (LD – 50 mg/kg b.w.) and (e) Diabetic + ME (HD – 100 mg/kg b.w.) – showing granulated pancreatic islets, (f) Diabetic + Metformin – granulated and normal pancreas appearance.
3.7 Effect of ME on serum insulin level
The control and the experimental rats displayed serum insulin levels (Fig. 4). A significant increase (p < 0.05) in serum insulin level was observed in the oral administration of 50 and 100 mg/kg b.w. up to 13.01 ± 0.41 µU/ml and 14.13 ± 0.52 µU/ml, respectively as compared to the insulin level dropped in diabetic rats (5.20 ± 0.14 ml). The decrease in the blood glucose level due to the oral administration of ME for 35 days increased the plasma insulin level, this may be due to the phyto-constituents present in the plant extract, which influenced the insulin secretion and protected the active β-cells that yielded insulin and reduced the glucotoxicity. Similarly, Ficus amplissimaIn smith. bark extract in STZ induced diabetic rats showed a decrease in serum insulin level; and administration of extract in three different doses (50, 100 and 150 mg/kg b.w.) caused significant increase in the serum insulin level by 11.81 ± 0.38 µU/ml, 15.77 ± 0.42 µU/ml and 15.87 ± 0.52 µU/ml, respectively (Arunachalam and Parimelazhagan, 2013).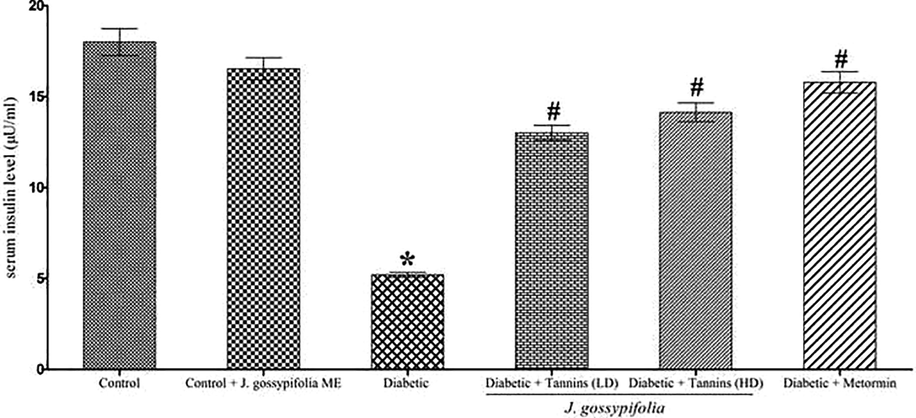
Effect of ME on level of serum insulin on STZ-NIC induced diabetic rats. Metformin used as a standard drug. Data are given as means ± SD (n = 5) from three independent experiments in triplicates. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *p < 0.05 as compared with control group, #p < 0.05 as compared with Diabetic group.
3.8 HOMA-IR index
The HOMA-IR index in ME treated groups (50 and 100 mg/kg b.w) displayed a decreased peripheral resistance to insulin of 4.5 ± 1.5 (50 mg/kg b.w.) and 4.2 ± 1.2 (100 mg/kg b.w.), respectively as compared to the diabetic group (6.7 ± 2.1) (Fig. 5). Insulin plays an important role in maintaining the normal level of blood glucose, which was confirmed by the HOMA-IR results that the insulin resistance developed in the experimental groups. Metformin treated rats showed an index of 4.3 ± 1.6, which is indicating that, the oral treatment of ME improved insulin resistance on par with the standard drug. Previous studies by Wang et al. (2013), demonstrated that the HOMA-IR value of Swertia macrosperma extracts treated diabetic rats were extremely less (ethanolic extract 250 and 500 mg/kg b.w., showed 5.72 ± 0.59 and 5.61 ± 0.78 and n-butanol 250 and 500 mg/kg b.w., showed 6.64 ± 1.07 and 5.25 ± 0.57, respectively) than the diabetic rats 10.19 ± 0.72.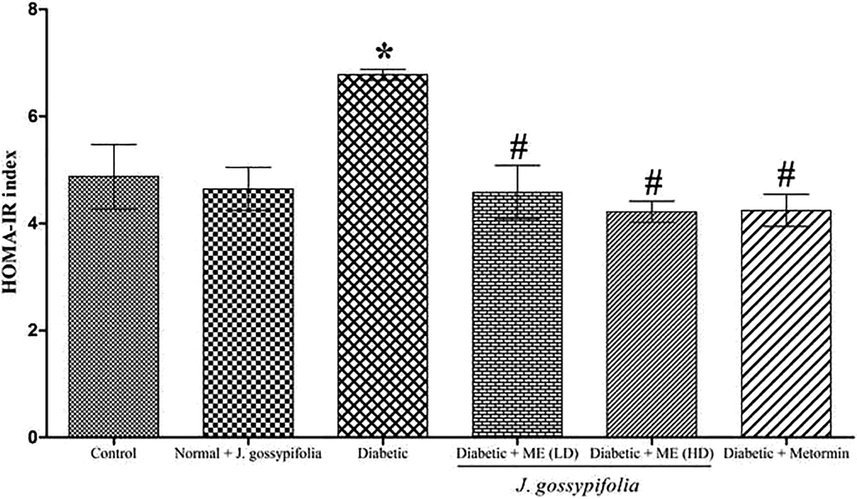
HOMA-IR index on STZ-NIC induced diabetic rats. Metformin used as a standard drug. Data are given as means ± SD (n = 5) from three independent experiments. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *p < 0.05 as compared with control group, #p < 0.05 as compared with Diabetic group.
3.9 Effect of ME treated diabetic rats on the gene expression in muscle tissues
Administration of STZ-NIC induced experimental diabetes in the rats was causing damages in the pancreatic β-cells and leads to a reduction in the cellular response to insulin resistance. Insulin-signalling pathway impairment leads to insulin-stimulated glucose uptake failure in targeted tissues such as muscle and fat. This is because of the insulin is incapable of stimulating a GLUT 4 translocation from intracellular pool to PM in muscle cells (Epstein et al., 1999). To examine the mechanism underlying the effects of ME on insulin signalling pathway, IRTK, IRS-1, PI3K, GLUT-4, GSK-3β and GS gene expression were analysed. The results showed that 50 and 100 mg/kg b.w ME treatment to the experimental rats significantly (p < 0.05) increased the expression of IRTK (2.3 and 2.9 fold), IRS1 (2.4 and 2.7 fold) PI3K (2.2 and 2.4 fold), GSK-3β (0.4 and 0.3 fold), GS (2.3 and 2.5 fold) and GLUT 4 (2.5 and 2.9 fold) (Fig. 6a–f).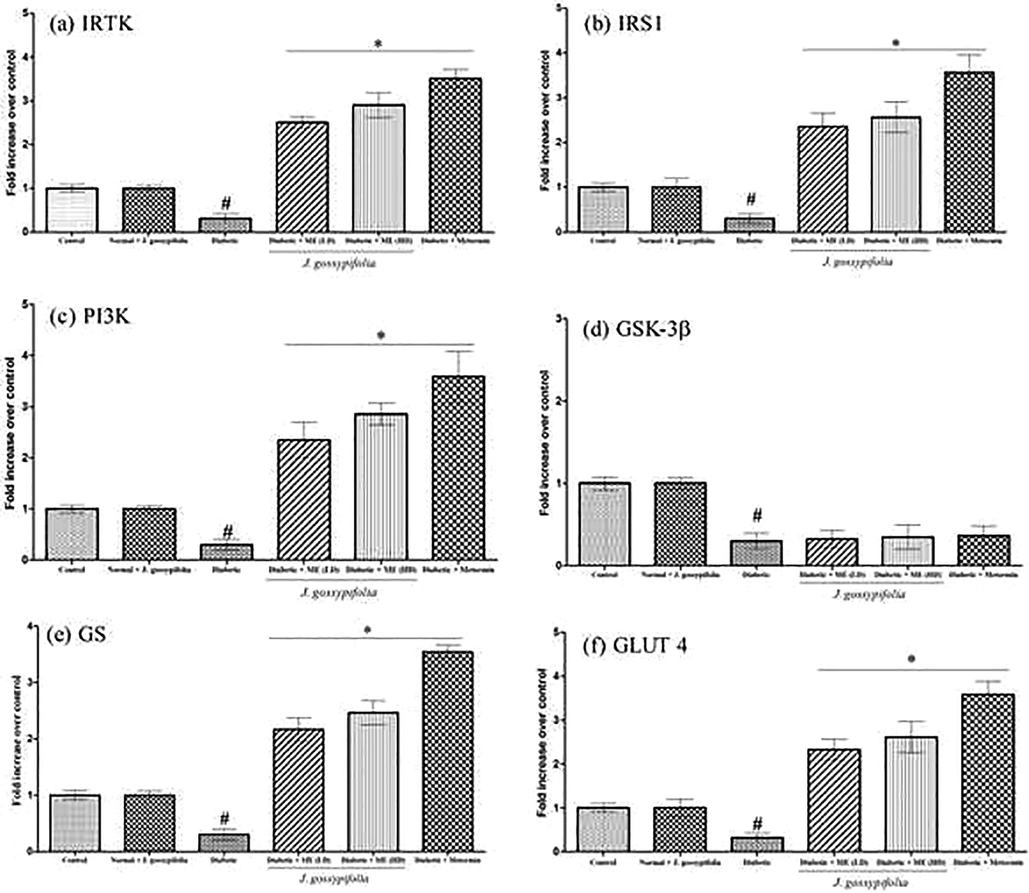
Effect of ME treated diabetic rats on the gene expression in muscle tissues on IRTK (a), IRS1 (b), PI3K (c), GSK-3β (d), GS (e) and GLUT 4 (f) mRNA expression level using quantitative (real time) PCR. Data expressed as mean ± S.D of three independent experiments in triplicates. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *, P < 0.05 as compared with control, #p < 0.05 as compared with Diabetic group.
These results suggested that ME induced insulin to bind to its receptor of tyrosine kinase; the insulin receptor substrate (IRS-1) is leading to tyrosine phosphorylation and the activation of phosphatidyl inositide 3-kinase (PI3 K). AKT/PKB (Protein Kinase B) and PKC (Protein Kinase C) are the downstream targets of PI3K; the activation/phosphorylation of which causes GLUT 4 translocation to the plasma membrane and improves glycogen synthase enzyme by inhibiting the glycogen synthase kinase-3β (GSK-3β). Sujatha et al. (2010), stated that both crude and (3β)-STIGMAST-5-EN-3-OL can increase the expression of mRNA in IRTK, IRS1and PI3K; and eventually the downstream kinases are also activated. Our results are corroborate with the earlier findings in activating the expression of the key targets of insulin signalling pathway.
3.10 Effect of ME on IRβ and GSK-3β protein expression
Insulin receptor originates from the member of receptor tyrosine kinase family, which comprises the receptors for insulin, insulin like growth factors and several other growth factors (Petruzzelli et al., 1982). Effect of ME on insulin receptor β protein in muscle samples are analysed using blot (Fig. 7a). Diabetic rats showed 0.4 fold decrease in IRβ protein, whereas ME treated group (50 and 100 mg/kg b.w.) showed 2.4 and 2.7 fold increase in IRβ protein levels, respectively (Fig. 7b). The oral treatment of ME remarkably reduced the level of lipids in the diabetic induced rats, henceforth increased level of insulin receptor was observed. (Satyanarayana et al., 2015), reported that Azadirachta indica, being a effective antioxidant and anti-hyperlipidemic agent was able to increase the protein expression of IR by reducing the lipid level in type 2 diabetic rats equivalent to that of metformin.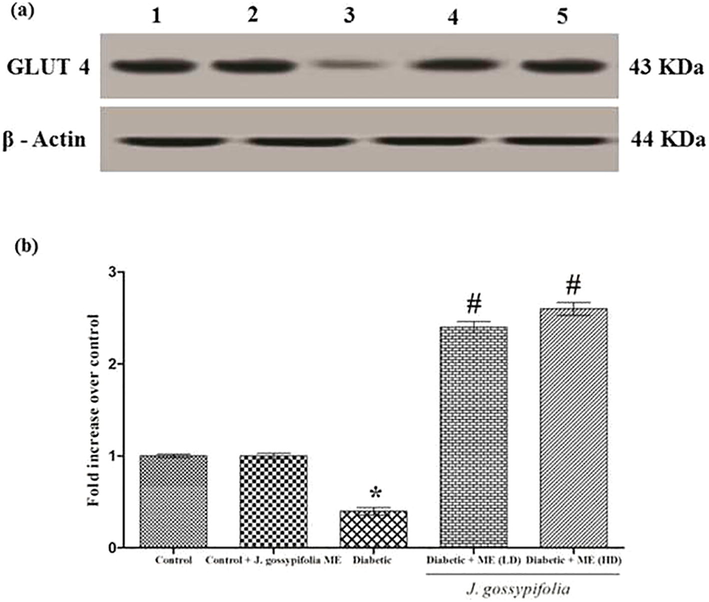
(a) Effect of ME treated diabetic rats on IRβ and GSK-3β protein expression. Lane 1 denotes control, Lane 2 drug control (ME 100 mg/kg b.w.), Lane 3 indicates diabetic, Lane 4 and 5 indicates ME (LD 50 and HD 100 mg/kg b.w.) respectively. IRβ and GSK-3β protein expressions shown. β-Actin served as loading control. (b) Densitometric analysis of IRβ and GSK3β protein expression upon treatment with ME (50 and 100 mg/kg b.w.). Data expressed as mean ± S.D of three independent experiments in triplicates. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *P < 0.05 as compared with control, #p < 0.05 as compared with Diabetic group.
GSK-3β inhibition stimulate an increase in glycogen synthesis and insulin sensitivity. Activation of GSK-3β contributes to a reduction in glycogen synthesis and decreased insulin sensitivity (Coghlan et al., 2000; Liberman and Eldar-Finkelman, 2005). Western blot analysis revealed that the ME groups (50 and 100 mg/kg b.w.) showed 2.2 and 2.4 fold increase in GSK-3β (serine 9 phosphorylation) protein level, respectively as compared with diabetic group (0.4 fold decrease in the expression level) (Fig. 8a and b). Previous studies have shown that the inhibition of GSK3, oral glucose disposal could be improved by increasing liver glycogen synthesis in a Zucker diabetic fatty (falfa) rats (Cline et al., 2002). A selective GSK3 inhibitor upregulated the intracellular glycogen level and increased GLUT 4 expression in the skeletal muscle of ob/ob diabetic mice which was well correlated with our data (Kaidanovich-beilin and Eldar-finkelman, 2006).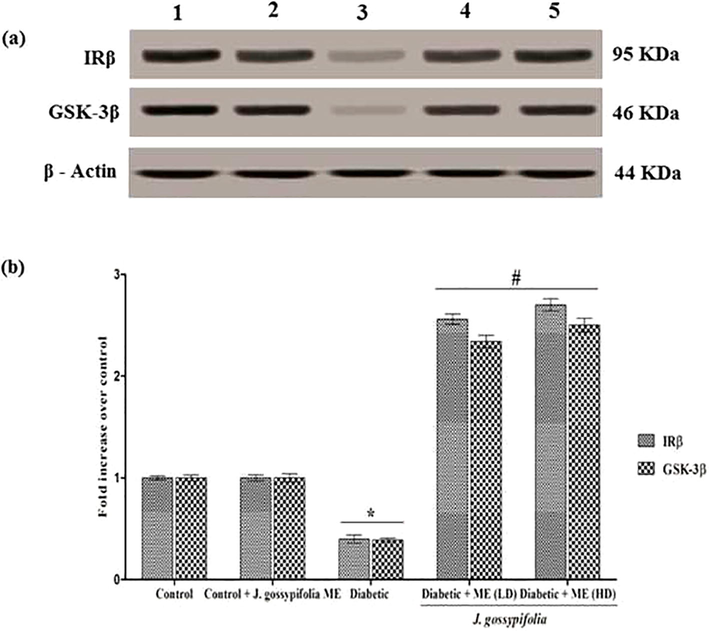
(a) GLUT 4 protein expression by ME treated diabetic rats. Lane 1 denotes control, Lane 2 drug control (ME 100 mg/kg b.w.), Lane 3 indicates diabetic, Lane 4 and 5 indicates ME LD 50 and HD 100 mg/kg b.w. respectively. GLUT 4 protein expressions were shown. β-Actin served as a loading control. (b) Densitometric analysis of translocation of GLUT 4 using PM fractions. The fold increase of GLUT 4 upon treatment with ME quantified arbitrarily. Data expressed as mean ± S.D of three independent experiments in triplicates. Statistical evaluation done by one-way ANOVA followed by Duncan’s multiple range test (DMRT). *P < 0.05 as compared with control, #p < 0.05 as compared with Diabetic group.
3.11 Effect of ME on GLUT 4 protein expression in PM fractions
Skeletal muscles highly express GLUT 4, which is responsible for the insulin stimulated glucose uptake. Effect of GLUT 4 protein expression in plasma membrane fraction detected in the muscle of Albino wistar rats. Western blot revealed that there was no significance difference in the GLUT 4 protein expression in the control group and the extract treated non-diabetic control group. Diabetic rats, showed a decreased GLUT 4 protein expression level (0.4 fold). The 50 and 100 mg/kg b.w. of ME treated rats showed 2.2 and 2.4 fold increase in GLUT 4 protein levels, respectively (Fig. 8a and b)). The oral administration of ME to the experimental groups improved the insulin sensitivity by activating the insulin signalling cascades, which reduced the lipid accumulation and controlled obesity contributing to the restoration of GLUT 4 expression. Similarly, GLUT 4 expression in plasma membrane measured in soleus muscle of STZ-diabetic mice. Treatment with Gardeniae Fructus significantly increased GLUT 4 expression, 1.4 time-greater than that of H2O- treated control group (Yu et al., 2013).
4 Conclusion
In conclusion, from these findings, oral administration of J. gossypifolia ME exhibited anti-hyperglycemic activity in STZ-NIC induced albino wistar rats. The results also revealed that the extract have beneficial effect in improving body weight and reduced blood glucose levels in rats. From the gene and protein expression studies, it is evident that the ME enhanced the insulin-signalling cascade. Overall J. gossypifolia plant which is been used as a food nutrient that can be considered as alternate in pharmacotherapy for Type II Diabetes Mellitus.
Funding
Deanship of Scientific Research (22UQU4320141DSR31), Umm Al-Qura University, Saudi Arabia.
Authors contribution
IPK, PSP, RM – Conceptualization, design, methodology, performed the experiments, wrote the original draft; AAAO, OMA, SASA, RAP, AH, MAA – Methodology, data curation, data analysis, resources, contributed reagents/materials, reviewed and edited the draft; JA – Conceptualization, design, methodology, resources, contributed reagents/materials, reviewed and edited the draft, research supervision
Acknowledgement
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR31).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol. Histopathol.. 2011;26:13-21.
- [Google Scholar]
- Hypotensive and vasorelaxant effects of ethanolic extract from Jatropha gossypiifolia L. in rats. Fitoterapia. 2003;74(7–8):650-657.
- [CrossRef] [Google Scholar]
- In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARγ in L6 myotubes. Phytomedicine. 2006;13(6):434-441.
- [CrossRef] [Google Scholar]
- Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab.. 2008;295:1323-1332.
- [CrossRef] [Google Scholar]
- Molecular characterization of a novel proto-type antimicrobial protein galectin-1 from striped murrel. Microbiol. Res.. 2014;169(11):824-834.
- [CrossRef] [Google Scholar]
- Gene profiling and characterization of arginine kinase-1 (MrAK-1) from freshwater giant prawn (Macrobrachium rosenbergii) Fish Shellfish Immunol.. 2011;31(1):81-89.
- [CrossRef] [Google Scholar]
- Molecular cloning, characterization and gene expression of an antioxidant enzyme catalase (MrCat) from Macrobrachium rosenbergii. Fish Shellfish Immunol.. 2012;32(5):670-682.
- [CrossRef] [Google Scholar]
- Prophenoloxidase activating enzyme-III from giant freshwater prawn Macrobrachium rosenbergii: Characterization, expression and specific enzyme activity. Mol. Biol. Rep.. 2012;39(2):1377-1386.
- [CrossRef] [Google Scholar]
- Macrobrachium rosenbergii cathepsin L: Molecular characterization and gene expression in response to viral and bacterial infections. Microbiol. Res.. 2013;168(9):569-579.
- [CrossRef] [Google Scholar]
- Antidiabetic activity of Ficus amplissima Smith. bark extract in streptozotocin induced diabetic rats. J. Ethnopharmacol.. 2013;147(2):302-310.
- [CrossRef] [Google Scholar]
- Determination and characterization of gallotannin by high performance liquid chromatography. Anal. Chem.. 1977;49(2):238-242.
- [CrossRef] [Google Scholar]
- Immunological role of C4 CC chemokine-1 from snakehead murrel Channa striatus. Mol. Immunol.. 2014;57(2):292-301.
- [CrossRef] [Google Scholar]
- Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10(3):178-182.
- [Google Scholar]
- Molecular importance of prawn large heat shock proteins 60, 70 and 90. Fish Shellfish Immunol.. 2016;48:228-238.
- [CrossRef] [Google Scholar]
- In-silico analysis and mRNA modulation of detoxification enzymes GST delta and kappa against various biotic and abiotic oxidative stressors. Fish Shellfish Immunol.. 2016;54:353-363.
- [CrossRef] [Google Scholar]
- Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem.. 1987;162(1):156-159.
- [CrossRef] [Google Scholar]
- Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (falfa) rats. Diabetes. 2002;51(10):2903-2910.
- [CrossRef] [Google Scholar]
- Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol.. 2000;7(10):793-803.
- [CrossRef] [Google Scholar]
- HPLC / ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal.. 2008;47:59-67.
- [CrossRef] [Google Scholar]
- Glucose transporters and insulin action — implications for insulin resistance and diabetes mellitus. N. Engl. J. Med.. 1999;341(4):248-257.
- [CrossRef] [Google Scholar]
- A Review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant. Evid. Based Complement. Altern. Med. 2014:2-32.
- [CrossRef] [Google Scholar]
- Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47(3):345-351.
- [Google Scholar]
- In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine. 2014;21(4):415-422.
- [Google Scholar]
- Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev. Comp. Immunol.. 2021;114:103863.
- [CrossRef] [Google Scholar]
- Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol. Biol. Rep.. 2021;48:5857-5872.
- [CrossRef] [Google Scholar]
- Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci.. 2021;283:119864.
- [CrossRef] [Google Scholar]
- Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice : molecular characterization in liver and muscle. Pharmacology. 2006;316(1):17-24.
- [CrossRef] [Google Scholar]
- Insulinomimetic activity of two new gallotannins from the fruits of Capparis moonii. Bioorg. Med. Chem.. 2010;18(11):3940-3945.
- [CrossRef] [Google Scholar]
- Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement. Altern. Med.. 2013;13(1):222.
- [CrossRef] [Google Scholar]
- Antihyperglycemic and antihyperlipidemic activity of Jatropha gossypifolia methanolic extract in streptozotocin-nicotinamide induced diabetic rats. Asian J. Pharm. Clin. Res.. 2017;10(11):326-330.
- [Google Scholar]
- Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced type 2 diabetic rats. Asian Pac. J. Trop. Med.. 2011;4(11):904-909.
- [CrossRef] [Google Scholar]
- A murrel cysteine protease, cathepsin L: Bioinformatics characterization, gene expression and proteolytic activity. Biologia (Poland). 2014;69(3):395-406.
- [CrossRef] [Google Scholar]
- A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol. Immunol.. 2015;68(2):421-433.
- [CrossRef] [Google Scholar]
- Tannins: an antinutrient with positive effect to manage diabetes. Res. J. Recent Sci.. 2012;1(12):70-73.
- [CrossRef] [Google Scholar]
- Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J. Biol. Chem.. 2005;280(6):4422-4428.
- [CrossRef] [Google Scholar]
- NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biol. Intl.. 2021;45:2331-2346.
- [CrossRef] [Google Scholar]
- Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47(2):224-229.
- [Google Scholar]
- Phenolic content and antioxidant activity of olive extracts. Food Chem.. 2001;73(1):73-84.
- [CrossRef] [Google Scholar]
- Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J. Basic Med. Sci.. 2014;17(4):263-270.
- [Google Scholar]
- Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine. 2013;20(3–4):237-245.
- [Google Scholar]
- Comparative epidermal morphology of West African species of Jatropha L. (Euphorbiaceae) Bot. J. Linnean Soc.. 1993;111(2):139-154.
- [CrossRef] [Google Scholar]
- Functional roles and gene regulation of tumor necrosis factor receptor 1 in freshwater striped murrel. Mol. Immunol.. 2015;66(2):240-252.
- [CrossRef] [Google Scholar]
- Proc. Natl. Acad. Sci. USA. 1982;79(22):6792-6796.
- [CrossRef]
- The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1985;25(1):223-230.
- [CrossRef] [Google Scholar]
- Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol. Biol. Rep.. 2020;47:6727-6740.
- [CrossRef] [Google Scholar]
- TL15 of Arthrospira plantensis sulphite reductase scavenges free radicals demonstrated in zebrafish (Danio rario) as drug delivery model. Intl. J. Biol. Mac. Mol.. 2020;166:641-653.
- [CrossRef] [Google Scholar]
- Jatrophenone, a novel macrocyclic bioactive diterpene from Jatropha gossypifolia. Chem. Pharm. Bull.. 2003;51(7):870-871.
- [Google Scholar]
- Antihyperglycemic activity of TarralinTM, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550-557.
- [CrossRef] [Google Scholar]
- Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry. 2013;85:7-29.
- [CrossRef] [Google Scholar]
- Antidiabetic activity of different extracts of Dalbergia sissoo DC stem bark on streptozotocin- nicotinamide induced type 2 diabetes mellitus rats. Int. J. Pharm. Pharm. Sci.. 2013;5(2):382-387.
- [Google Scholar]
- Molecular approach to identify antidiabetic potential of Azadirachta indica. J. Ayurveda Integr. Med.. 2015;6(3):165-174.
- [CrossRef] [Google Scholar]
- Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci. Technol. Int.. 2006;12(5):385-395.
- [CrossRef] [Google Scholar]
- Pharmacologic downregulation of protein arginine methyltransferase1 expression by adenosine dialdehyde increases cell senescence in breast cancer. Eur. J. Pharmacol.. 2021;891:173697.
- [CrossRef] [Google Scholar]
- Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int. J. Diab. Mellitus. 2010;2(2):101-109.
- [CrossRef] [Google Scholar]
- Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr. Sci.. 2002;83(1):30-38.
- [CrossRef] [Google Scholar]
- Antihyperglycemic effect of Codariocalyx motorius modulated carbohydrate metabolic enzyme activities in streptozotocin-induced diabetic rats. J. Funct. Foods.. 2014;11(C):517-527.
- [CrossRef] [Google Scholar]
- GR15 peptide of S-adenosylmethionine synthase (SAMe) from Arthrospira platensis demonstrated antioxidant mechanism against H2O2 induced oxidative stress in in-vitro MDCK cells and in-vivo zebrafish larvae model. J. Biotechnol.. 2021;342:79-91.
- [CrossRef] [Google Scholar]
- Cholecalciferol and metformin protect against lipopolysaccharide-induced endothelial dysfunction and senescence by modulating sirtuin-1 and protein arginine methyltransferase-1. Eur. J. Pharmacol.. 2021;912:174531.
- [CrossRef] [Google Scholar]
- Antidiabetic effects of Swertia macrosperma extracts in diabetic rats. J. Ethnopharmacol.. 2013;150(2):536-544.
- [CrossRef] [Google Scholar]
- Anti-hyperglycemic effect of single administered Gardeniae Fructus in streptozotocin-induced diabetic mice by improving insulin resistance and enhancing glucose uptake in skeletal muscle. Chinese Med.. 2013;4(4):157-165.
- [CrossRef] [Google Scholar]







