Potential functional and numerical response in a large sized raptor may be mediated by the abundance of an exotic lagomorph
⁎Corresponding author at: Grupo de Investigaciones en Biología de la Conservación, Laboratorio Ecotono, INIBIOMA – CONICET (Universidad Nacional del Comahue), San Carlos de Bariloche, Río Negro, Argentina. grinbic@comahue-conicet.gob.ar (Gonzalo O. Ignazi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Predators relying on a particular prey as their main food resource are especially susceptible to fluctuation in prey availability. When prey abundance decreases they show a functional response by adjusting their diet. After this, predators may suffer a numerical response. These responses have been poorly studied in presence of alien invasive preys. The black-chested buzzard-eagle (Geranoaetus melanoleucus) is a large raptor inhabiting open areas of South America. Here we present the first long-term study on this eagle population tendency, analyzing functional and numerical responses associated with changes in the abundance of the exotic European hare (Lepus europaeus). We measured breeding performance and studied eagles’ diet during the breeding seasons of 1991, 1992, 2006, 2011 and 2012. We also estimated the relative abundance of hares in those years. Eagles diets changed over the years with a decrease in hare consumption. The number of eagles decreased from 1992 to less than half in 2012. Forty one percent of the total eagles observed in 1991/92, and 27% in 2006 were immature, while in 2011 and 2012 no immature were observed. We found similar tendencies of decrease in the abundance of hares which suggest that the decrease in eagles population may be related with the decreasing tendency of hares. No other factor that may have affected immature eagles abundance was evident in the area during this period. Our data suggest that changes in the abundance of an exotic lagomorph may lead numerical and functional responses in a top predator by affecting its diet, age structure, and ultimately abundance.
Keywords
Numerical response
Functional response
Alien species
Top predator
Black-chested buzzard-eagle
European hare
1 Introduction
Predators that rely on a particular prey as their main food source may be affected in different ways according to shifts in the abundance of such prey (Jaksić, 1989; Marti et al., 1993; Dupuy et al., 2009). Generally, when the abundance of the most consumed prey decreases predators show a functional response adjusting their diet. According with the alternative prey hypothesis (Angelstam et al., 1984), predators may adapt their diet according to fluctuations in the abundance of their main preys. When this functional response fails to compensate the shortage of resources, a numerical response may take place altering different population parameters (Solomon, 1949; Redpath and Thirgood, 1999). For instance, predators that relied on native European rabbits (Oryctolagus cuniculus) in Spain suffered significant changes when an outbreak of the rabbit hemorrhagic disease (RHD) reduced rabbits population in the early 1980’s (Moreno et al., 2008; Lees and Bell, 2008; Moleón et al., 2009). Most predators changed their diets to cope with the lack of prey (Fernández, 1993; Moleón et al., 2009, 2012). The decrease of rabbits reduced the breeding performance of some species (Fernández, 1993), and produced the loss of breeding territories (Martínez and Zuberogoitia, 2001).
A large number of foreign species have been introduced in many ecosystems worldwide, causing impacts of different magnitudes (Vitousek et al., 1996; Lowe et al., 2000). Some of these alien species adapted fast to their new environments becoming primary prey for the native predators of the invaded community (Tylianakis et al., 2008). When a new prey decreases, this may impact native predators’ assemblages that currently rely on it strongly (Tablado et al., 2010).
In Patagonia, southern South America, the food source for the predator assemblage has been modified in the last century by the introduction the European hare (Lepus europaeus). It constitutes an abundant, relatively easy to hunt and rich in biomass prey for the medium-to-large sized carnivore assemblage extant in Patagonia. It was introduced in 1888 and by 1980 it was already present in most of the Argentinean territory (Grigera and Rapoport, 1983). Currently hares are consumed by several Patagonian raptors (Hiraldo et al., 1995; Monserrat et al., 2005; Lambertucci et al., 2009; Barbar et al., 2016a,b), as well as other terrestrial predators (Novaro et al., 2000). However, there are almost no studies evaluating the long-term effect of the hare population fluctuations on the predator populations from South America.
One of the predators that strongly feed on the European hare is the black-chested buzzard-eagle (Geranoaetus melanoleucus, hereafter “eagle”, Hiraldo et al., 1995). This is a large raptor that inhabits open areas from Venezuela to southern Argentina (Ferguson-Lees and Christie, 2001). In Patagonia it presents one of the highest densities of breeding territories for any eagle species (Hiraldo et al., 1995; Barbar et al., 2018). It is a generalist that consumes small mammals, birds, reptiles and arthropods. The European hare is the most important item consumed in northwestern Patagonia by both territory holding adults and non-reproductive immatures. Furthermore, immature eagles select patches where the density of hares is particularly high (Bustamante et al., 1997).
Here we evaluate how eagles respond to decreases in the population of the introduced hare, and if this response is similar to that of native predators when their native most important prey decrease in other systems. We hypothesized that the response of a predator before fluctuations of an established alien species will be similar to that shown by native predators and preys that had evolved together. We predict that a decrease in hare abundance will produce different effects on eagle’s population depending on the intensity of the decrease. First, we expect a decrease in the consumption of hares by the eagles when hares are less abundant. Then we expect a numeric response (Rohner, 1996), as follow: 1) as immature eagles are not attached to a territory, we expect a change in age structure of the local population, with less immature when hares are scarce, 2) a decrease in the breeding performance, with lower numbers of pairs attempting to breed and/or an increased nestling mortality, and 3) ultimately, a reduction of the adult abundances with its consequent loss of breeding territories.
2 Methods
2.1 Study area
We worked in the northwest of Patagonia, Argentina (ca. 39° 57′ S; 71° 05′ W). The landscape consists of extensive steppe plains with mountain slopes, abundant rocky outcrops used by this and other raptors (e.g., Lambertucci et al., 2008), and bottom valleys with rivers and wet meadows. Vegetation consists in a heterogeneous matrix dominated by grass (Poa sp., and species from the Stipae tribe like Pappostipa speciosa) and cushions shrubs (Mullinum spinosum), with scattered shrubs (Chacaya trinervis, Berberis darwinii and Schinus molle) and some disperse trees (Maytenus boaria and Austrosedrus chilensis). The weather is cold and dry, with a mean temperature of 6 °C and rainfall of around 500 mm (Leon et al., 1998; Oyrazabal et al., 2018).
2.2 Nest locations and breeding performance
We surveyed an area of approximately 11,309 Km2 during the austral breeding seasons (September-February) of 1991, 1992, 2011 and 2012. The years refer to the reproductive seasons that start in September. We used the data for the breeding seasons that started in September of 1991 and in September of 1992 pooled (from here 1991/92) to calculate territory density, NND, and the breeding parameters (as well as for the food habits, see below). We did this since that data from the historical samples come from Hiraldo et al. (1995) and there it has not been discriminated by the year. Surveys were made from vehicle in both main and secondary roads using binoculars and spotting scopes. We recorded the location of territories with a Global Positioning System (GPS) and used this information to calculate the density of territories and the Nearest Neighbor Distance (NND; Barbar et al., 2018). We compared density of territories and NND in a sub-area of 415 Km2 in which the sampling effort regardless to the search of active nests was equal. This sub-area corresponds to the one referred as “plains” in Hiraldo et al (1995). For the rest of analyses we included data from all the territories found. We defined a territory as occupied whenever we observed adults defending nests on a cliff, and active when we observed breeding behavior by the adults (nest defense and maintenance).
To evaluate eagles breeding performance, each territory was visited twice, once during the early stage of the breeding period and another one when chicks where 3–4 weeks old. We considered that a pair was successful when at least one chick reached 40 days of age. We calculated productivity as the mean of chick per pair, per year.
2.3 Food habits
To study breeding eagles food habits we collected pellets under the surveyed nests and nearby perches within territories during the breeding seasons of 1991, 1992, 2006 and 2011. As in the former section, data for the breeding seasons of 1991 and 1992 was taken from the pooled information coming from Hiraldo et al. (1995), so it is referred as 1991/92 from now on. We removed pellets present at each site before our sampling period starts to avoid counting preys consumed previously. Pellets were dried and separated apart in the lab. Bones contained in the pellets were identified using reference collections and a mammal skull key (Pearson, 1995). When no bones were found, hair remains were identified by using the keys of Chehébar and Martín (1989). We counted each prey in pellets as one individual unless we found evidence of more than one individual (e.g., more than one skull in the same pellet).
Pellet analysis to study raptors diet have some limitations such as the inability to estimate prey age and the tendency to over-represent mammalian and under-represent avian composition (Redpath et al., 2001; Marti et al., 2007). It also has special quantification problems when prey are large and consumed over several meals when compared with other techniques like direct observation or counting prey remains under nests (Marti et al., 2007). However this technique allows comparing the diet spatially and temporally, in large areas with a simple comparative method. Moreover, pellet analysis has been used to study raptors diet worldwide (e.g., Resano-Mayor et al., 2015) including this species (Hiraldo et al., 1995; Bustamante et al., 1997; Trejo et al., 2006).
We present the data as frequency of appearance for each prey category (i.e., number of pellets in which each category was found), and as percentage of pairs in which the category is consumed. To compare the shift in the diet over the years, we constructed a web of interactions with the proportion of each prey consumed, in every territory and each year. However, as the number of territories varied between years, we used the nine territories that where occupied in all sampling periods to avoid a sample size bias.
2.4 Eagle and hare abundance
To estimate eagles abundances we performed point counts at 21 locations close to roads during the late nestling season before fledges left the nest. The 21 points were included in 24 points originally selected in 1992 by Bustamante et al. (1997), from which 12 were located less than 2 km away from nearest active nest, and 12 more than 2 km away from nearest nest at that time. All points were more than 2 km apart from each other (see Bustamante et al., 1997). At each point we conducted two 30 min counts for the breeding seasons of 1992, 2006, 2011 and 2012, one in the morning (between 08:00 and 11:00 hs) and the other in the afternoon (between 16:00 and 19:00 hs) on different days. Observations were made by one observer. We recorded the number of contacts with eagles and the age class of individuals. Adult and immature were differentiated since adults are white and grey while immature are mostly brown (Ferguson-Lees and Christie, 2001). Then we added all contacts at a given point during the morning count with those contacts made at the same point during the evening count. In addition in 2011 we visited the communal roost found by Hiraldo et al. (1995) and Bustamante et al. (1997) to check if they were still being used by immature eagles.
We performed a 30 min foot transect to count hares in order to estimate relative abundance at the 21 count points following the methodology used by Bustamante et al. (1997). Transects to count hares were made between 19:00 hs and 21:00 hs by one observer walking at a consistent speed through the predominant environment of the area.
2.5 Statistical analysis
We evaluated how the intensity of consumption of different preys varied over the years using an interaction web. The graphical web representations were made with “bipartite” package on R-statistical software (Dormann et al., 2008; R Development Core and Team, 2012). To analyze the changes in hare and eagles abundances over the years we used Generalized Linear Mixed Models (GLMM). As all response variables are counts we used a Poisson distribution of the errors. We performed individual GLMM’s to assess the relationship between hares, total eagle (adults + immature), adult and immature eagles’ abundances using the different years as explanatory variable. Site and hares abundance (this last just for the models of eagles abundances) were included as random effects. We also used GLMMs to assess the total eagle, adult and immature eagles’ abundances with the abundance of hares as explanatory variable; using as random effects the site and year. We used Poisson distribution and checked for overdispersion in our data. However, none of the ratios between the residuals of the variance and the residuals of the degrees of freedom differed from 1, thus evidencing that our data were not affected by overdispersion (Harrison, 2014). Analyses were done with “lme4” package in R-statistical software (Bates et al., 2015; R Development Core and Team, 2012).
3 Results
3.1 Food habits
In 1991/92 we collected 1097 pellets from 26 territories, in 2006 228 from 12 territories, and in 2011 96 from 9 territories. Hares represented 58.29% of the diet in 1991/92, 51.36% in 2006, and 41.26% in 2011 (Table 1). We were able to collect pellets for all years in the same 9 territories in 1991/92, 2006 and 2011. Therefore, we used those territories to make comparisons between years to avoid biases due to the differences in sampling size. Interaction links between territories and prey items varied over the years, with a reduction of 17% in the consumption of hares between 1991/92 and the formation of new links in 2006 and 2011 with the arrival or increase in numbers of European rabbits (Oryctolagus cuniculus) and the red deer (Cervus elaphus, Fig. 1).
| Species | 1991/92 | 2006 | 2011 | |||
|---|---|---|---|---|---|---|
| Frequency of appearance | Percentage of pairs | Frequency of appearance | Percentage of pairs | Frequency of appearance | Percentage of pairs | |
| Mammals | ||||||
| Lepus europaeus | 58.29 | 100 | 51.36 | 100 | 41.26 | 100 |
| Oryctolagus cuniculus | 0 | 0 | 13.21 | 38 | 23.3 | 22 |
| Rodents | 19.16 | 85 | 25.85 | 72 | 30.3 | 22 |
| Other mammals | 3.36 | 40 | 11.46 | 30 | 30 | 33 |
| Birds | 16.43 | 100 | 31.2 | 100 | 35.16 | 100 |
| Reptiles | 1.84 | 40 | 1.38 | 25 | 1.56 | 22 |
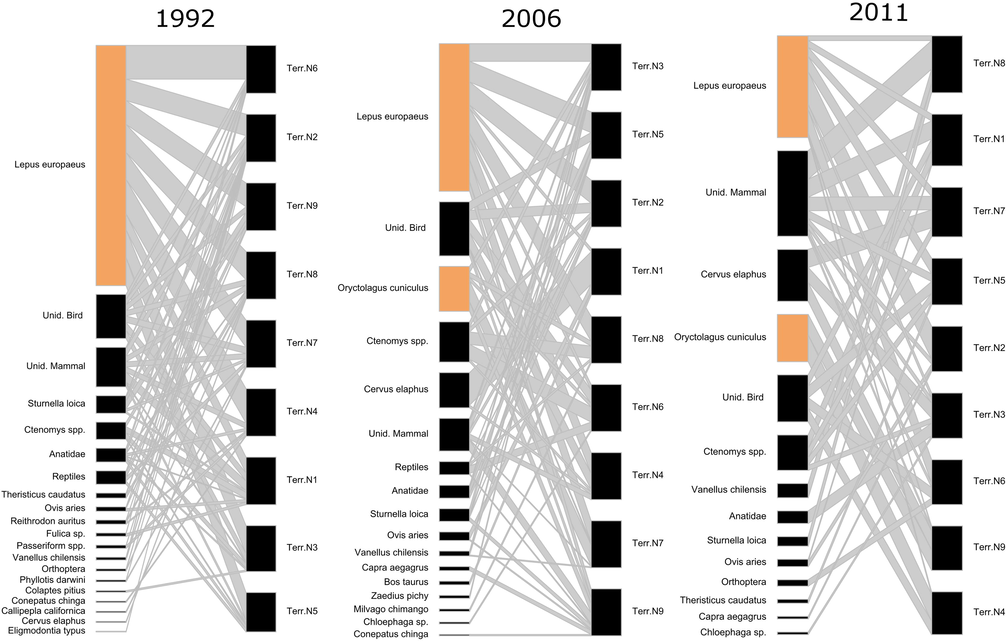
- Trophic interactions of nine territories of black-chested buzzard-eagles during the breeding seasons of 1991/92 (1992), 2006 and 2011. The sizes of boxes represent the percentage of the diet in each year, for every territory. Thickness of interaction lines between each territory and the different prey items represents the strength of each predator prey interaction. The two exotic lagomorphs are in brown boxes.
3.2 Breeding attempts and breeding performance
Density of territories and the NND remained relatively constant over the different periods. The percentage of pairs that attempted to breed was also similar between different periods. The percentage of pairs that successfully raised at least one fledgling was lowest in 2011, while the highest value was recorded for 2012. The main productivity per active pair was also lowest in 2011 (Table 2). In 1991/92 and 2012 every active pairs successfully raised at least one chick. Meanwhile, in 2011 just the 54.5% of active pairs were successful.
| Period | Density of territories | Mean NND (m) | Percentage of pairs that attempted to breed | Percentage of successful pairs | Active pairs main productivity (chicks/pair/year) |
|---|---|---|---|---|---|
| 1991/92 | 1 pair/13.2 km2 | 2081 (SD = 916, range 900–3900, N = 17) | 64.3 (N = 43) | 62.8 (N = 43) | 1.1 |
| 2011 | 1 pair/15.5 km2 | 2806 (SD = 1888, range 593–7143, N = 10) | 64.7 (N = 17) | 35.3 (N = 17) | 0.5 |
| 2012 | 1 pair/15.5 km2 | 2806 (SD = 1888, range 593–7143, N = 10) | 60.7 (N = 14) | 78.6 (N = 14) | 1.3 |
3.3 Relationship between eagle and hare abundance
The abundance of hares decreased over the years, as well as the total abundance of eagles (Table 3). There was a significant decrease of hares as well as immature eagles over the years (Table 3). There was a positive relationship between the abundance of adult eagles and the abundance of hares. This pattern of decreasing abundances over the years is evident when comparing the abundance of eagles and with the abundance of hares (Fig. 1). Overall, the total abundance of eagles decreased over time but this is mainly driven by the decrease in immature eagles (Table 3; Fig. 2). We found no activity in 2011 in the communal roosts found by Hiraldo et al. (1995) and Bustamante et al. (1997) in 1991.
| Modeled variable | Fixed effects | Estimate (±S.E.) | p | Random variables |
|---|---|---|---|---|
| Hare abundance | Year | −0.07 (±0.19) | <0.01 | Site |
| Total eagle abundance | Year | −0.02 (±0.01) | n.s. | Site, hare abundance |
| Adult eagle abundance | −0.01 (±0.01) | n.s. | ||
| Immature eagle abundance | −0.15 (±0.03) | <0.01 | ||
| Total eagle abundance | Hare abundance | 0.13 (±0.05) | 0.01 | Site, year |
| Adult eagle abundance | 0.11 (±0.06) | 0.01 | ||
| Immature eagle abundance | 0.12 (±0.12) | n.s. | ||
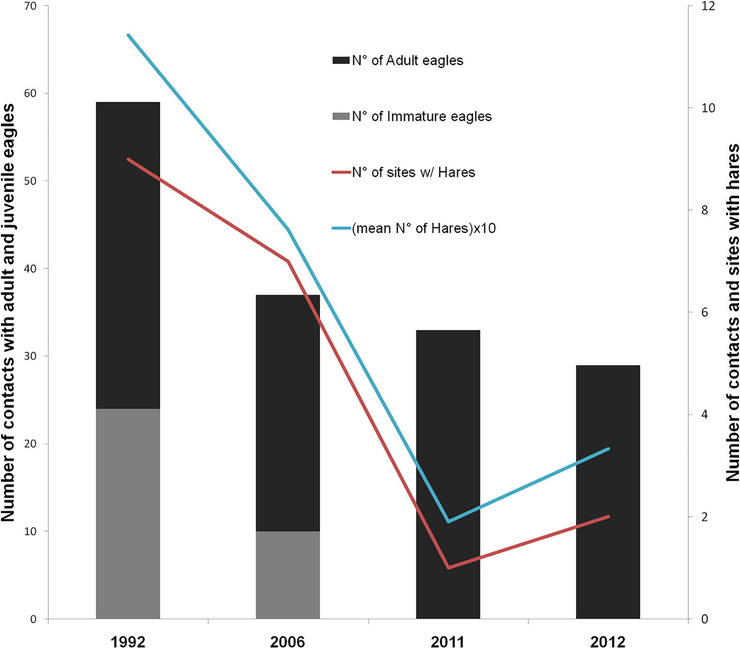
- Abundance of adult and immature black-chested buzzard-eagles, abundance of European hares and number of sites with hares in the years 1992, 2006, 2011 and 2012.
4 Discussion
We found an apparent reduction in hare consumption by eagles over time suggesting that a functional response may have taken place. This is reflected in a decrease in frequency of occurrence of hares in the diet, but is more evident in the reduction of strength of interaction links in the diets of each territory (Fig. 2). However, in 2006 and 2011, it seems that there was a replacement of hares by rabbits. There was also a sustained consumption of carrion (large mammals that cannot be hunted by the eagle) through time, which was more intense in 2011 possibly as a response to a decrease of their main prey availability. The difference in sample size between the years (especially between 1991/92 and 2011) was a consequence of the lack of pellets in the territories in the later years. The season of 2011 was the one with the lowest abundance of hares we recorded, so it is possible that eagles may have had to move larger distances to forage and therefore pellets were no longer concentrated nearby nests as in 1991/92, when hares were more abundant. The Spanish Imperial eagle (Aquila adalberti) which is considered a specialist in catching rabbits showed a similar response we found with the black-chested Buzzard-eagles, when the RHD affected rabbits in the 80′s (Sánchez et al., 2009).
Paired with the apparent decrease in hare consumption over the years, we found a decrease in the abundance of immature suggesting a numerical response, affecting population age structure. The abundance of immature birds decreased over the years while adults remained constant. Furthermore, in 2011 we visited the places that were used as communal roosts by immature individuals in 1991/92 (Hiraldo et al., 1995), but we found no eagles nor any evidence suggesting any kind of use (i.e. pellets or feathers). This could be because adults took advantage of other exotic alternative preys as rabbit and they scavenge on large mammals. These responses differ from those shown by other raptors which drastically decrease the numbers of adults when their main prey became scare. For instance, around half of the breeding territories of the eagle owl (Bubo bubo) in the Iberian Peninsula where lost in two consecutive seasons when the RHD strongly diminished the abundance of rabbits (Martínez and Zuberogoitia, 2001). However, a generalist species as the black kite (Milvus milvus) had shown similar responses as eagles, since breeding parameters decreased, but not adult density (Viñuela and Veiga, 1992).
Immature and adult eagles differ in their patterns of habitat selection. Immature may have more freedom of movement and can adjust their spatial distribution according to density of their main prey (Bustamante et al., 1997), while adults behave as central place foragers and are attached to their territory. Our results suggest that this pattern maybe evident also at a time scale, with immature present only in periods when hares were abundant. On the other hand adults remained in the area tolerating changes in hare abundance. It seems that adults are more tolerant to a shortage of prey in order to maintain their territory. Non territorial immature could select patches where preys are abundant and abandon these areas. This pattern was found in raptors from the Northern Hemisphere as the great horned owls (Bubo virginianus) in which immature non-territorial members of the population coexist with territorial breeding adults, while the abundance of snowshoe hares (Lepus americanus) was high. When hares abundance decreased, adults remained in their territory while immature abundance decreased (Rohner, 1996).
The decrease in immature abundance could be due to an increase of mortality or because they are moving to areas with more prey. If an increased mortality is the case, the area may be working as an ecological sink in which case recruitment is limited and the population is maintained due to the arrival of individuals from other areas. However, raptors use to move long distances during the natal dispersal period (Penteriani and Delgado, 2009). Eagles reach maturity at 4–5 years of age, therefore they have a long natal dispersal period in which they could move and establish somewhere else. Dispersal of immature individuals may also be a consequence of increased intra-specific competition with adults associated to the shortage of prey (Bustamante and Hiraldo, 1988; Negro et al., 1997). Furthermore, unusual concentrations of dispersal immature individuals have been recorded in areas were nesting activity is low in the center of Argentina, suggesting that individuals born in other areas may travel long distances searching for prey (López et al., 2017). The patterns of dispersal movements of immature remain unknown. In any case it seems that the abundance of their main prey produces changes in the presence and use of the area.
Although there is a slight decrease in territory density and NND, active territories remain abundant in the area with some pairs breeding less than 1000 m from their nearest active neighbors. Therefore, this does not reflect a numerical response at the level of breeding pairs. Such density remains between the highest recorded for this kind of species worldwide (Hiraldo et al., 1995; Barbar et al., 2018). However, we recorded a decrease in breeding performance when hare abundance was the lowest (during the year 2011). According to our hypothesis, this could be indicating that the disturbance caused by the shortage of hares affected breeding success and the abundance of immature individuals, but it was not strong enough to affect neither the abundance of adults nor the density of breeding territories. Alternatively, the disturbance caused by the eruption of the Puyehue-Cordón Cauye volcanic complex during 2011 (Gaitán et al., 2011) may have indirectly affected eagles. Ashes deposition affects plant productivity by accumulation and abrasion by the finest particles. Then herbivores may suffer from starvation, among other impacts (Wilson et al., 2011). This may eventually affect predators and produce a decrease in breeding performance as recorded in 2011. This is reinforced by the fact that in 2012, after some recovery of the eruption impact, breeding performance was higher. However, there is a need of more information to evaluate the effect volcanic ashes deposition may have on eagles.
There are, however, other factors different from hares abundance that may impact eagles population. For instance an unusual aggregation of black-chested buzzard-eagles (mainly immatures), possibly associated with fluctuation in prey abundance in central Argentina (López et al., 2017), may have favored recent events of large mortalities due to electrocution in that area, which show that those eagles are highly susceptible to this disturbance (Sarasola and Zanon Marínez, 2017). Electrocution in our study area could also have an impact on eagles, and more data in this regard is needed. However, even though we did not perform any systematic surveys under electric pools, neither new electric lines nor unusual congregation of eagles have been recorded in the study area. During our study period we only found one juvenile eagle dead under an electric line. This was one of two fledges that had recently left the nest constructed in a concrete made electric pool Therefore we have no evidence suggesting that electrocution as recorded by Sarasola and Zanon Marínez (2017) could be the explanation for our numerical response in juvenile eagles, although this subject merit special attention and specific studies.
Persecution by humans is another factor that may have a negative impact on eagles and could also be an alternative explanation to our findings. However, the fact that adult abundance and NND did not vary over the years suggests that if there is persecution towards this species it is mainly affecting immature individuals. This has been reported for similar species in other regions of Argentina (i.e. Sarasola and Maceda, 2006; Barbar et al., 2016a,b). We cannot discard this alternative hypothesis, however owners of two of the most extensive properties in the study area, were several of the count points and active nests were surveyed, claim that there is no persecution to this species in their land.
The European hare altered the community in South America since its arrival by being an abundant food resource for many predators and a competitor for native herbivores (Grigera and Rapoport, 1983; Novaro et al., 2000; Barbar et al., 2018; Barbar and Lambertucci, 2018, 2019). In our study system hares had temporal and spatial fluctuations including important local decreases. Eagles seem to have responded by adjusting the diet and changing the proportion of age classes, with adults remaining in the area and immature decreasing in abundance. Further studies on the effect of exotic European hares on the demography of their consumers should be carried out since our results suggest that their fluctuations may produce numerical responses in a top predator population.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgments
We thank managers of “Rinconada” and “Estancia Collón Cura” for permissions to work in their lands. We also thank José Antonio Sánchez-Zapata, Pablo Alarcón, Pablo Plaza, Maricel Graña Grilli, Jorgelina Guido, Fernano Ballejo, Karina Speziale and Natalia Rebolo for their comments on this manuscript. Field work was carried on under the approval of Ministerio de Desarrollo Territorial, Provincia de Neuquén (Resolución 569/11). We thank PIP 0758-2014 (CONICET).
References
- Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecología. 1984;8:285-298.
- [Google Scholar]
- Direct persecution od Crowned Eagles (Buteogallus coronatus) in Argentina: a new call for their conservation. J. Raptor Res.. 2016;50:115-120.
- [Google Scholar]
- Medium-sized exotic prey create novel food webs: the case of predators and scavengers consuming lagomorphs. PeerJ. 2016;4:e2273
- [Google Scholar]
- Exotic lagomorph may influence eagle abundances and breeding spatial aggregations: a field study and meta-analysis on the nearest neighbor distance. PeerJ. 2018;6:e4746
- [CrossRef] [Google Scholar]
- The roles of introduced Leporids in the ecosystem: a review of their effects as native and exotic species. Mammal Rev. 2018 in press
- [Google Scholar]
- Introduced lagomorph produce stronger potential apparent competition in invaded communities than any other species in a similar but native food web. Biol. Invas.. 2019;21:3735-3740.
- [CrossRef] [Google Scholar]
- Package ‘lme4’.. Vienna: R Foundation for Statistical Computing; 2015.
- Factors influencing family rupture and parent-offspring conflict in the Black Kite Milvus migrans. Ibis. 1988;132:58-67.
- [Google Scholar]
- Differential habitat selection by immature and adult Grey Eagle-buzzards Geranoaetus melanoleucus. Ibis. 1997;139:322-330.
- [Google Scholar]
- Guía para el reconocimiento microscópico de pelos de los mamíferos de la Patagonia. Doñana Acta Vertebrata. 1989;16:247-291.
- [Google Scholar]
- Dormann, C.F., Gruber, B., Fründ, J., 2008. The bipartite package. Version 0.73.R Project for Statistical Computing, Vienna, Austria. Available from http://download.nextag.com/cran/web/packages/bipartite/bipartite.pdf.
- Numerical and dietary responses of a predator community in a temperate zone of Europe. Ecography. 2009;32:277-290.
- [Google Scholar]
- Raptors of the World. Houghton Mifflin Harcourt; 2001.
- Effect of the Viral Hemorrhagic Pneumonia of the wild rabbit on the diet end breeding success of the golden eagle Aquila chrysaetos. Rev. Ecol. Terre Vie. 1993;48:323-329.
- [Google Scholar]
- Cartografía del área afectada porcenizas volcánicas en las provincias de Río Negro y Neuquén. Bariloche (Argentina): Instituto Nacional de Tecnología Agropecuaria; 2011. p. :8. Informe PROEVO
- Status and distribution of the European hare in South America. J. Mammal.. 1983;64:163-166.
- [Google Scholar]
- Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ. 2014;2:e616
- [Google Scholar]
- Breeding biology of a Grey Eagle-buzzard (Geranoaetus melanoleucus) population in Patagonia. Willson Bulletin. 1995;107:675-685.
- [Google Scholar]
- Opportunism vs selectivity among carnivorous predators that eat mammalian prey: a statistical test of hypotheses. Oikos 1989:427-430.
- [Google Scholar]
- Use of communal roosts by Andean Condors in northwest Patagonia, Argentina. J. Field Ornithol.. 2008;79:138-146.
- [Google Scholar]
- Spatial and temporal patterns in the diet of the Andean condor: ecological replacement of native fauna by exotic species. Anim. Conserv.. 2009;12:338-345.
- [Google Scholar]
- A conservation paradox for the 21st century: the European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Rev.. 2008;38:304-320.
- [Google Scholar]
- Grandes unidades de vegetación de la Patagonia extra andina. Ecol. Austral. 1998;8:125-144.
- [Google Scholar]
- Unusual concentration of Black-chested Buzzard-eagles in central Argentina. J. Raptor Res.. 2017;51:489-491.
- [Google Scholar]
- 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Aukland: Invasive Species Specialist Group; 2000.
- Trophic structure of raptor communities: a three-continent comparison and synthesis. Current Ornithology 1993:47-137. Springer US
- [Google Scholar]
- Food habits. In: Bildstein K.L., Bird D.M., eds. Raptor Research and Management Techniques. Hancock House; 2007.
- [Google Scholar]
- The response of the Eagle Owl (Bubo bubo) to an outbreak of the rabbit hemorrhagic disease. J. Ornithol.. 2001;142:204-211.
- [Google Scholar]
- Respuesta dietaria de tres rapaces frente a una presa introducida en Patagonia. Rev. Chil. Hist. Nat.. 2005;78:425-439.
- [Google Scholar]
- Long-term decline of the European wild rabbit (Oryctolagus cuniculus) in south-western Spain. Wildl. Res.. 2008;34:652-658.
- [Google Scholar]
- Large-scale spatio-temporal shifts in the diet of a predator mediated by an emerging infectious disease of its main prey. J. Biogeogr.. 2009;36:1502-1515.
- [Google Scholar]
- Predator-prey relationships in a Mediterranean vertebrate system: Bonelli’s eagles, rabbits and partridges. Oecología. 2012;168:679-689.
- [Google Scholar]
- Causes of natal dispersal in the lesser kestrel: inbreeding avoidance or resource competition? J. Anim. Ecol.. 1997;66:640-648.
- [Google Scholar]
- Ecological extinction of native prey of a carnivore assemblage in Argentine Patagonia. Biol. Conserv.. 2000;92:25-33.
- [Google Scholar]
- Annotated keys for identifying small mammals living in or near Nahuel Huapi National Park or Lanín National Park, southern Argentina. Mastozoología Neotropical. 1995;2:99-148.
- [Google Scholar]
- R Development Core Team. 2012. R: A language and environment for statistical computing. Available from http://cran.case.edu/web/packages/dplR/vignettes/timeseries-dplR.pdf (accessed October 21, 2014).
- Numerical and functional responses in generalist predators: hen harriers and peregrines on Scottish grouse moors. J. Anim. Ecol.. 1999;68:879-892.
- [Google Scholar]
- Assesing raptor diet: compating pellets, prey remains and observational data at Hen harrier nests. Condor. 2001;103:303-309.
- [Google Scholar]
- Diet-demography relationships in a long-lived predator: from territories to populations. Oikos. 2015;125:262-270.
- [Google Scholar]
- The numerical response of great horned owls to the snowshoe hare cycle: consequences of non-territorial “floaters” on demography. J. Anim. Ecol.. 1996;65:359-370.
- [Google Scholar]
- Temporal and spatial differences in the feeding ecology of the Spanish Imperial Eagle Aquila adatberti during the non-breeding season: effects of the Rabbit population crash. Acta Ornithol.. 2009;44:53-58.
- [Google Scholar]
- Past and current evidence of persecution of the endangered Crowned Eagle Harpyhaeliaetus coronatus in Argentina. Oryx. 2006;40:347-350.
- [Google Scholar]
- Electrocución de aves en líneas eléctricas: la muerte silenciosa de las grandes rapaces. Informe Ambiental Anual FARN. 2017;2017:219-230.
- [Google Scholar]
- The paradox of the long-term positive effects of a North American Crayfish on a European Community of predators. Conserv. Biol.. 2010;24:1230-1238.
- [Google Scholar]
- Dieta del Águila Mora (Geranoaetus melanoleucus) en una transecta oeste-este en el ecotono norpatagónico. Hornero. 2006;21:31-36.
- [Google Scholar]
- Global change and species interactions in terrestrial ecosystems. Ecol. Lett.. 2008;11:1351-1363.
- [Google Scholar]
- Importance of rabbits in the diet and reproductive success of Black Kites in southern Spain. Ornis Scand.. 1992;23:132-138.
- [Google Scholar]
- Ash storms: impacts of wind-remobilished volcanic ash on rural communities and agriculture following the 1991 Hudson eruption, southern Patagonia, Chile. Bull. Volcanol.. 2011;73:223-239.
- [Google Scholar]







