Translate this page into:
Potential biomonitoring of the environmental contamination using snails as sentinel organism: A case study from the Manzala Lagoon, Egypt
⁎Corresponding author. sqaysi@KSU.EDU.SA (Saleh Qaysi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
For the first time, historical and recent records on fascioliasis in Egypt reveal the results of sympatric and allopatric fluke/snail interactions in the Manzala Lagoon area by exploring the susceptibility of the species Pseudosuccinea columella after exposure to the Fasciola hepatica isolates from Egypt. Many factors influence trace metal accumulation in Pseudosuccinea columella, including temperature and salinity. Meanwhile, other factors are important in decreasing the bioaccumulation of heavy metals as low pH, low temperature, and high organic content of the substrate. It is indicated, from this study, that the Fe and Cu contamination can be detected in Pseudosuccinea columella species. The abundance of these snails varied all over the seasons where the minimum value was recorded in summer while reaching the maximum in autumn. Moreover, the abundance increased with increasing rainfall and humidity during the winter season.
Keywords
Pseudosuccinea columella
Fasciola hepatica
Manzala Lagoon
Heavy metal pollution
Fascioliasis
Egypt
1 Introduction
Several factors such as demographic, socioeconomic, environmental, geographic, climatic, and meteorological factors affect the health of individuals and populations. In fact, all these factors crucially affect public health (Guerreiro et al., 2014; Ebi et al., 2017). Snails are excellent biomonitors of environmental contamination because of their easy sampling, areal distribution, high tolerance to stress, and ability to accumulate varied contaminants (Soltani, 2013). Furthermore, gastropods are essential taxonomic corporations capable of biomonitoring heavy metal pollutants. This case employed Pseudosuccinea columella as biomonitors of heavy metal pollution. Biomonitors which are organisms (groups of organisms) are essential precursors for qualitative and quantitative environmental pollutants (Fränzle, 2006).
In the Manzala Lagoon, increasing industrialization and agricultural activities contribute to the increase in the discharge of chemical pollution into the ecosystem. This leads to increased metal levels in natural waters that damage marine habitats (El-Nemr, 2012). Trace metals naturally exist in the Earth’s crust, where they are essential for biological systems considering that they participate in numerous enzymatic processes (Pellerin and Amiard 2009). However, cadmium, lead, and mercury typically bioaccumulate in tissues and cause intoxication, decreased fertility, and tissue damage (Nordberg, 2010; Stankovic and Jovic, 2012).
Observing the compatibility between different parasite and snail populations may thus help in better understanding and predicting the transmission of fascioliasis. The present resistant and sensitive Pseudosuccinea columella is due to the intermediate host snails of Fasciola hepatica in the Manzala Lagoon of Egypt (Fig. 1a). This study aims to evaluate the feasibility of applying certain disbursed snails to minimize the concentrations of contaminants in the ecosystem. The concept is that snails feeding on species directly exposed to dry and wet pollutant deposition and inhaling major water pollutants can be critical biomonitoring sentinels for determining the spatial distribution and bioavailability of pollutants and heavy metals. In this regard, Pseudosuccinea columella species amassed from the Manzala Lagoon can be used to determine the extreme toxicity and observe the bioconcentration of trace metals. These results could provide insights into the actual players in the host-parasite interactions in the Manzala Lagoon by exploring snail–parasite combinations where they are most significantly present. The spatial distribution of Lymnaeidae species in the Manzala Lagoon is beneficial to better understanding the fascioliasis distribution and delineating priority areas for control interventions. Therefore, this paper aims to provide such information on Pseudosuccinea columella as baseline data and verify the potential of these snails as excellent biomonitors.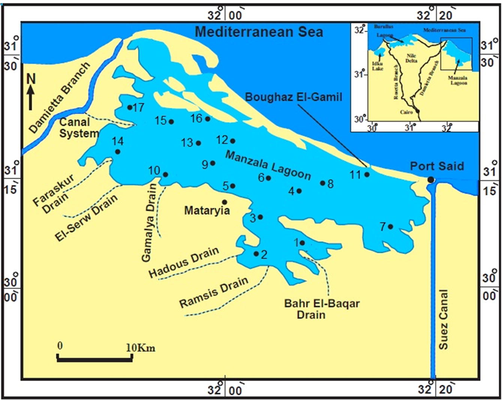
Location map.
2 Study area
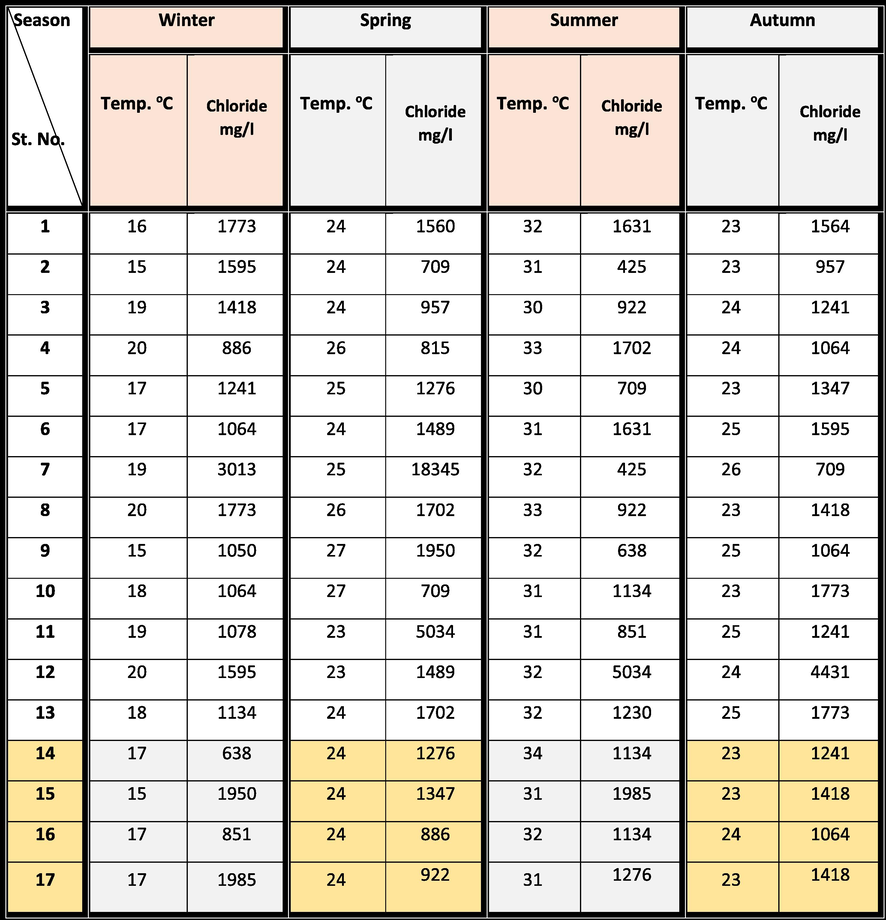
The Manzala Lagoon (Fig. 1a) covers almost 3 % of the area of Egypt and is positioned close to the Mediterranean coast of the Delta region. It serves as the primary assembly factor of numerous polluted water resources and is considered the most important natural resource for fish manufacturing in Egypt. It presents >7 % of the country’s total fish production (GAFRD 2006). Since the mid-19th century, the volume of water flowing seaward in the Damietta branch of the Nile, which forms the lagoon’s western border, has decreased markedly. This stems from the artificially accelerated channelization of this Nile branch and the diversion of the Nile water into an increasing number of complicated agricultural canal systems (Stanley, 1996). The maximum extents of the rectangular lagoon are 49 km (NW–SE) by 29 km (SW–NE). The annual water temperature ranges from 15 °C to 34 °C (Table 1) while the salinity is from 0.68 g l−1 in winter to > 3.0 g l−1 in summer (El-Hehyawi, 1977). The lagoon is separated from the Mediterranean Sea by a chain of broad, elongated, coalescing, and low-elevation sand ridges.
3 Materials and methods
3.1 Fieldwork
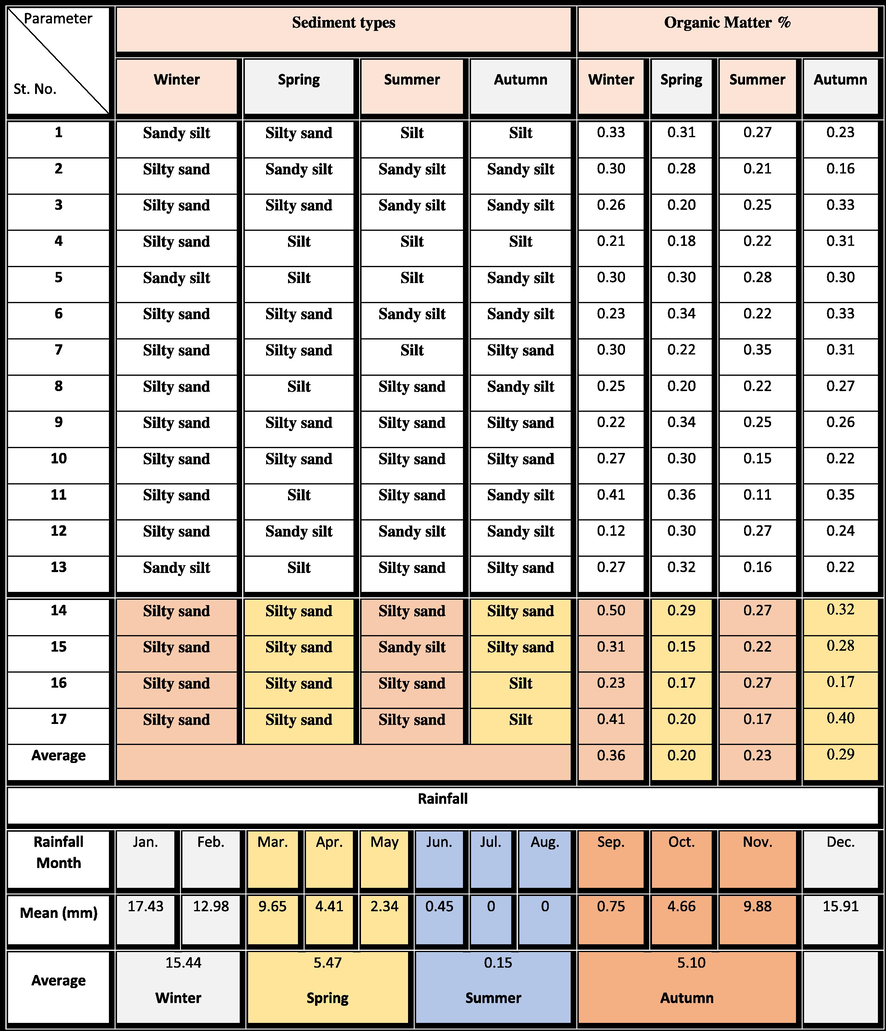
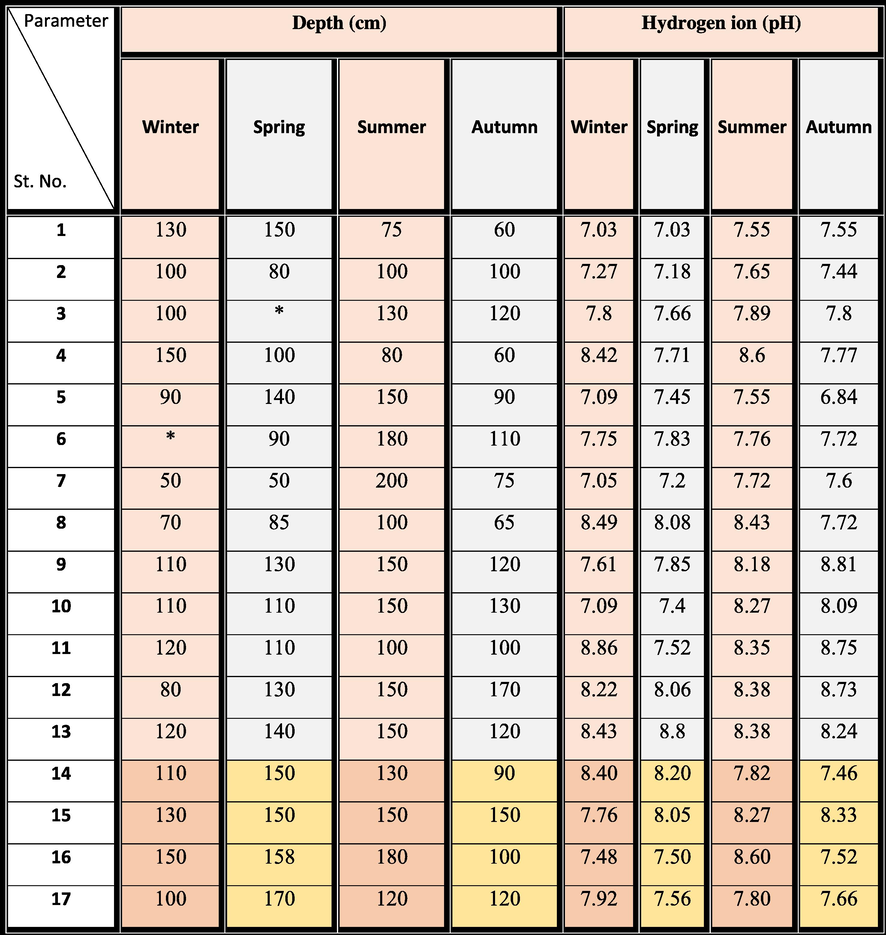
The current bottom sediment samples in the Manzala Lagoon were amassed seasonally from 17 selected stations along with profiles chosen including the entire area (Fig. 1a). The sampling began in the winter of 2018 to the spring, summer, and autumn of 2019. The bottom sediments were obtained from the lagoon using a grab sample (Ekman type) immersed to a depth ranging from 85 to 180 cm. The hydrogen ion concentrations (pH) of the selected samples were measured immediately (Table 3) using a movable pH sensor (HANNA model, HI 9125). While the mercury thermometer was used for measuring the bottom water temperature which should be lower than 5 °C. Table 2 displays the rainfall, organic matter, and sediment types in the Manzala Lagoon for the four seasons.
3.2 Determination of sediment organic carbon content
The organic carbon content was assessed using the acid/dichromate titration approach designated by Gaudette et al. (1974). The results of the analysis were obtained using the equation:
In the equation, T is the sample titration (ml ferrous solution), and S is the standardization blank titration (ml ferrous solution). The T/S factor cancels out the effect of the ferrous solution normality; the value 0.003 refers to the milliequivalent weight of carbon, 1.0 N is the normality of K2Cr2O7, and 10 is the volume of K2Cr2O7 in ml. W is the weight of the sediment sample in grams. The organic carbon is converted to organic matter by multiplying the organic carbon values by the factor of 1.8.
3.3 Snail study
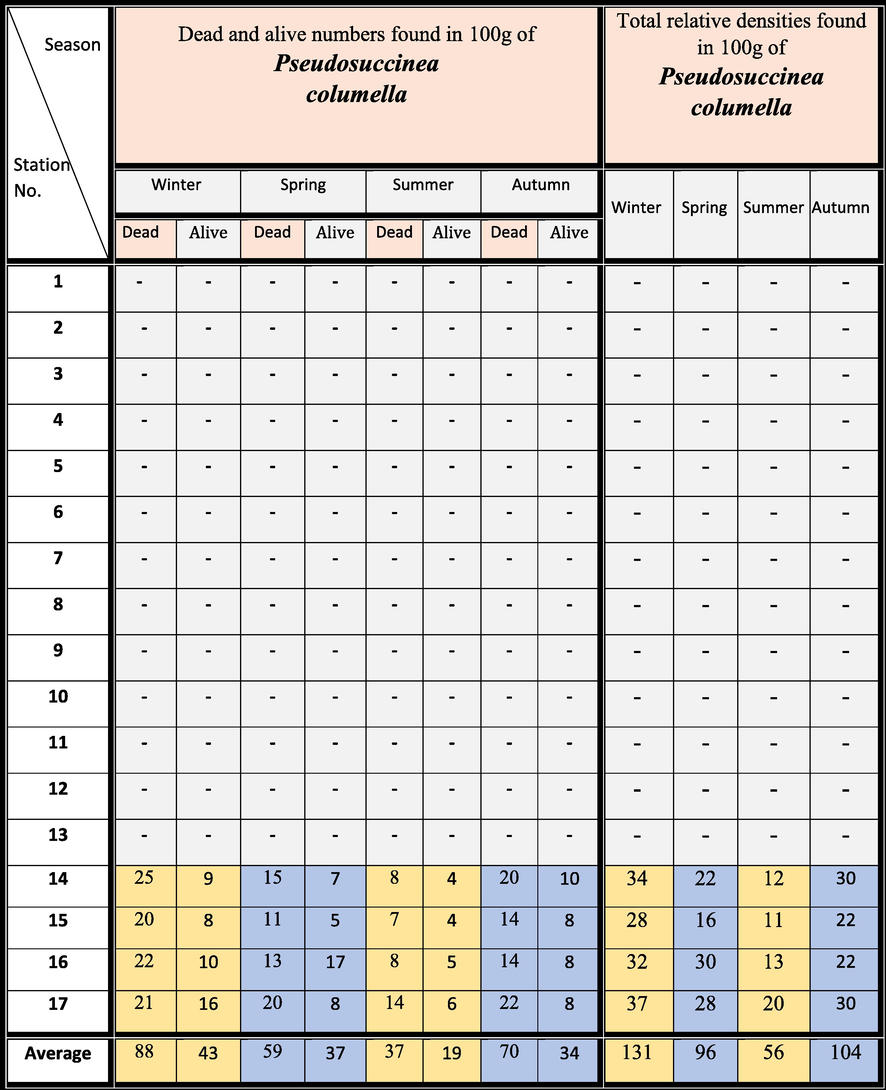
The snails were diagnosed using morphological markers (i.e., shells with short spires, periostracum ornamented with spiral sculptures, and ureter with two distant flexures; Pointier 2008). The snail species of Pseudosuccinea columella were mostly gathered only at four stations (14, 15, 16, and 17) in front of the most important drains of Faraskur El-Serw and Gamalya, which have poured into the Manzala Lagoon for the duration of the four seasons (winter of 2018 and spring, summer, and autumn of 2019) (Fig. 1a). The snail samples have been gathered through handpicking. The freshwater gastropods of Pseudosuccinea columella are recorded as mid-way hosts for Fasciola hepatica. The acquired soft sediments were then analyzed to count the living and dead faunas. Then, approximately 100 g of individual sample was dehydrated in an oven at 80–100 °C for 12 h and finally, soaked in a 10 %–15 % hydrogen peroxide solution for 24 h. The analyzed samples were sieved using a 2- to 125-mm screen mash and then dehydrated again. Mollusks identification was carried out through binocular microscopic (Fig. 1b) and counting them for each station (Table 4).
Snail shell samples were examined by the binocular microscope.
3.3.1 Classification
Biota.
Kingdom: Animalia.
Phylum: Mollusca; Linnaeus 1758.
Class: Gastropod; Cuvier 1795.
Subclass: Heterobranchia; Gray 1840.
Infraclass: Euthyneura; Spengel 1881.
Superorder: Hygrophila; Férussac 1822.
Superfamily: Lymnaeoidea; Rafinesque 1815.
Family: Lymnaeidae; Rafinesque 1815.
Subfamily: Lymnaeinae; Rafinesque 1815.
Genus: Pseudosuccinea; Baker 1908.
Species: Pseudosuccinea columella; Say 1817.
3.3.2 Identification, distribution, and habitat
The shells were horny brown, thin, translucent, and finely striated. Their apices were pointed and had 3.5–4 weakly convex whorls with shallow sutures. The last whorls predominated with ovate apertures. Columellate margins were reflected in their upper sections, and lower margins were sharp and straight. The shell widths ranged from 8 to 13 mm, and the heights ranged from 15 to 20 mm. The shells were found in Lower Egypt (the Nile and its essential canals) in the Damietta and Rosetta branches of the Nile, particularly in the El Qaluobyia, El Menoufia, and Damietta regions. The snail species occur in shallow, stagnant water (with an abundance of lily pads) and on rocky beaches, which can protect them from wave action.
3.4 The heavy metals analysis of water samples
Water sampling (200 ml) was processed by 5 ml of a diacid mixture (HNO3:HCLO4, 9:4 ratio) on a hot plate and sorted through the Whatman No. 42 papers. Double distilled water was then added to increase the volume to 50 ml. The heavy metals in the water samples were analyzed following the method given by the Association of Official Analytical Chemistry (Association of Official Analytical Chemistry (AOAC), 1995) using an atomic absorption spectrophotometer (SolarM600531 v1.27).
3.5 Analysis of heavy metals in sediment samples
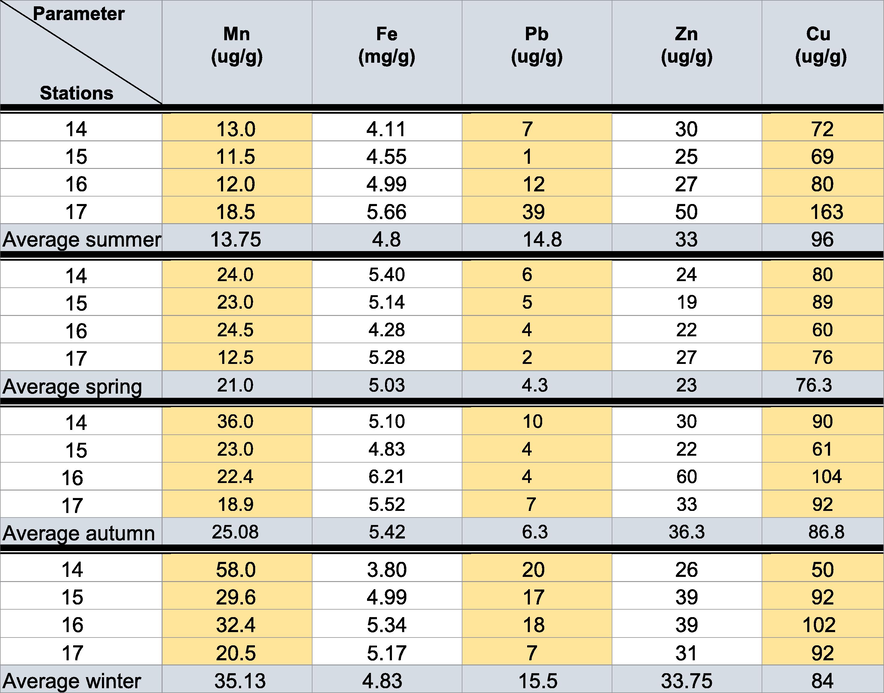
Portions of 1 g from the dehydrated sample were weighed and moved into a hot block digester. Further, they were treated with HNO3 solution (Analar grade, BDH 69 %) in two steps; low temperature (40 °C) for 1 h and high temperature (140 °C) for 3 h. Then, diluted with purified deionized water. Finally, the values of Mn, Fe, Cu, Pb, and Zn have been counted through the AASs (Pb through the furnace AAS Model 67OG and Cu and Zn by the flame AAS Model 67OG). Relevant data were illustrated in parts per million (Table 5).
3.6 Analysis of heavy metals in snail tissues
Live Pseudosuccinea columella snails have been acquired to identify the bioconcentrations of trace metals in their soft tissues in every station (Tables 6-9). The soft tissues of Pseudosuccinea columella snails have been carefully unglued from their shells and rinsed with distilled water and methanol to minimize contamination in mantle fluids and remove any sediment particles. About 0.01 g of the soft tissues of each snail sample was dehydrated at 50 °C, weighed, and then dissolved through 1 ml of concentrated HNO3 at 70 °C for 2 h. In the end, these samples were diluted in 5 ml of ultrapure deionized water to evaluate their heavy metal contents (Federici et al., 2007).
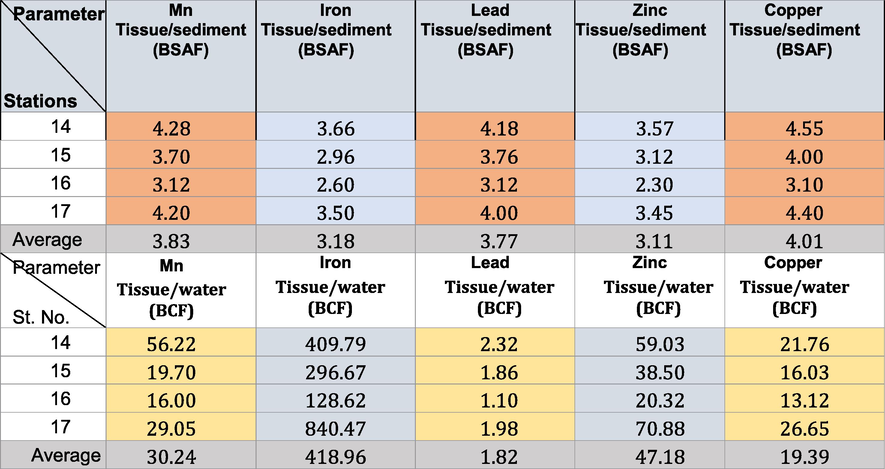
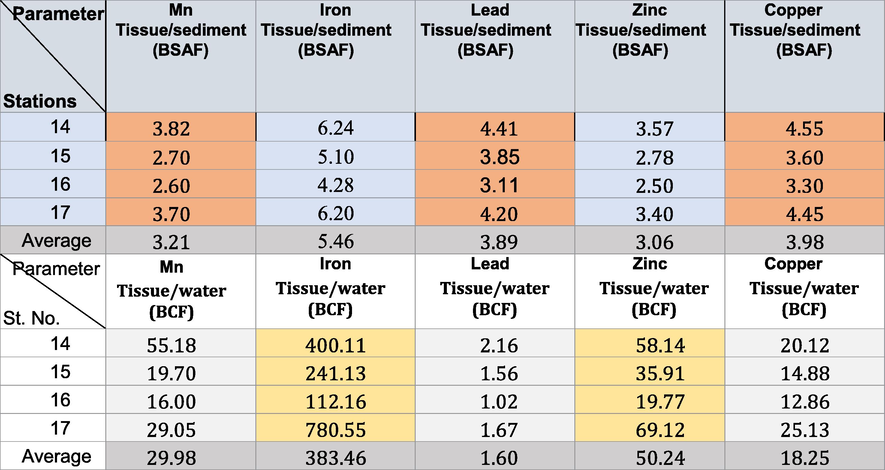
The concentrations of chemical substances in aquatic organisms may be calculated using two different factors: the bioconcentration factor (BCF) and the bioaccumulation factor (BAF). BCF refers to concentration levels of chemical substances in organisms only due to uptake from the surrounding water, whereas BAF additionally includes uptake from food. In this regard, the bioaccumulation sediment factor was calculated as an organism’s average metal-to-average sediment concentration ratio at a given time (Zhao et al., 2012) to evaluate the performance of metal bioaccumulation in organisms. The BCF of Gobas and Morrison (2000) (Tables 6-9) was calculated as follows:
The biota sediment accumulation factor (BSAF) was estimated for designated metals in the snails through the following relationship to estimate the proportion of metals in the organism to the associated sediments (Szefer et al., 1999): where Cx and Cs are the concentrations of the designated metals in the snail tissues and the accompanying sediments, respectively. The tissues of snails were categorized as macroconcentrators (BSAF > 2), microconcentrators (1 < BSAF < 2), or deconcentrators (BSAF < 1), following Dallinger (1993). The deposition of mollusk shells has been illustrated in Fig. 1b.
4 Results
4.1 Life and dead snails
Table 4 displays a comparison between the density of living mollusks and dead fauna (Table 4). This reveals the quantity of living Pseudosuccinea columella snails found only in the four samples from the west and northwest regions (Samples 14–17). The quantity of living Pseudosuccinea columella snails from the surface bottom deposits indicates the ecological explanations and inspection of relations amongst species and physicochemical environmental variables. In Egypt, Pseudosuccinea columella became one of the most widely distributed freshwater snail species. It is also an efficient midway host of Fasciola hepatica (Coelho and Lima, 2003). This could increase the risk of the emergence of fascioliasis in another area and its occurrence in endemic areas due to the presence of greater numbers of susceptible hosts.
4.2 Trace metals in water samples
The Cu, Pb, Fe, Mn, and Zn (ppm) concentrations in the various samples of water in all seasons vary between 4.10–8.45, 5.21–7.67, 4.30–7.54, 3.97–5.98, and 0.28–0.34, respectively. The maximum trace metal levels were recorded in front of the El-Serw drain, where the values of trace metals in both freshwater and sediment samples enhanced significantly through the high effluent discharge level into this drain from the industrial region of Damietta.
4.3 Trace metals in bottom sediments
The percentages of all heavy metals were higher in the bottom sediment than in the water. This revealed that the metals precipitated in the sediment instead of dissolving in water (Table 5), where sediments are important sinks for numerous pollutants such as pesticides and heavy metals. Also, sediments play an extensive function in remobilizing contaminants in aquatic systems under favorable situations and in water–sediment interactions (Hyun et al., 2007).
Iron: In summer, the minimum average concentration value of iron at the four stations was 4.80 mg/g. It increased slowly in winter (4.83 mg/g) and reached 5.03 mg/g in spring and 5.42 mg/g in autumn. The maximum annual average concentration values of iron (6.21 mg/g) were recorded at Station 16 during autumn. Zinc: The maximum average concentration value of zinc was recorded (36.3 μg/g) in autumn. It decreased gradually in winter and summer (33.75 and 33 μg/g, respectively) and reached its minimum average value in spring (23 μg/g). The maximum annual average concentration values of zinc (60 μg/g) were recorded at Station 16 in autumn. Copper: The highest concentration value for copper was monitored in summer (96 μg/g). In contrast, the lowest value was recorded in spring (76.3 μg/g). It decreased gradually in autumn and winter (86.8 and 84 μg/g, respectively). The maximum annual average values were recorded at Station 17 (163 μg/g) in summer. The most concentrated trace metal recorded were Fe and Zn, whereas the least was Cu. These findings agree with the previous observations of Abd El-Azim et al. (2018) in Lake Timsah.
Manganese: The highest average concentration value of manganese (35.1 μg/g) was reported in winter while the lowest was in summer (13.75 μg/g). It decreased slowly in autumn and spring (25.08 and 21.0 μg/g, respectively) till reached the minimum in summer (13.75 μg/g). The extreme annual average concentration value of manganese was recorded at Station 14 (58.0 μg/g), and the lowest value was 18.5 μg/g at Station 17 in summer. Lead: The highest average concentration values of lead were recorded in winter and summer and reached 15.5 and 14.8 μg/g, respectively. These gradually decreased in autumn and spring (6.3 and 4.3 μg/g, respectively). The lowest average value reached 4.3 μg/g in spring. The maximum annual average concentration value of lead was recorded at Station 17 (39 μg/g). Lead is a toxic metal originating from natural processes and anthropocentric activities (coal power plants, mining, waste gas fuel, leather whipping, paint, and battery factories). It is detrimental to the environment and human health.
4.4 Bsaf
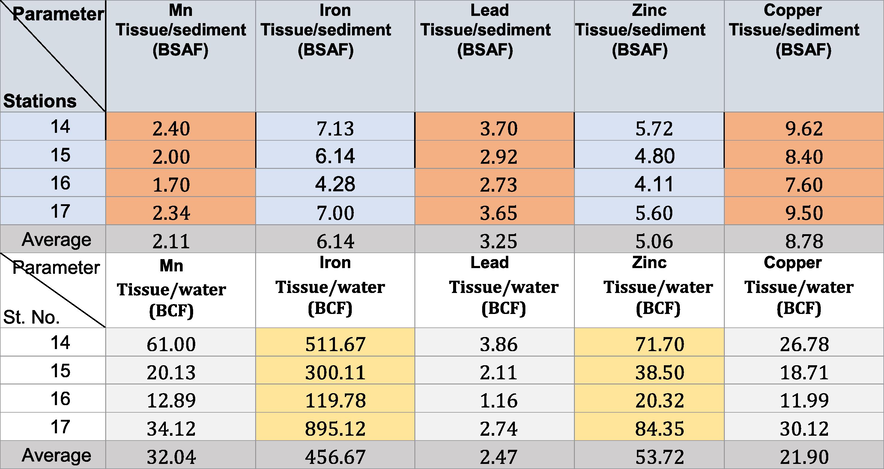
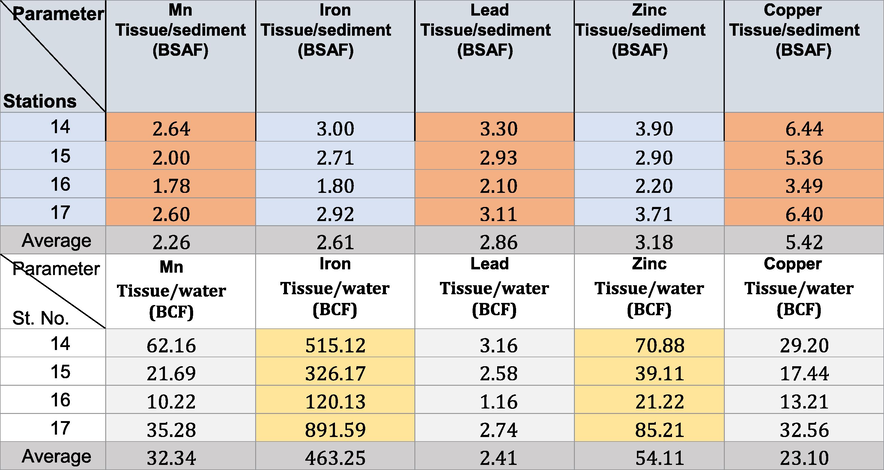
The BSAF in winter, spring, summer, and autumn in the area under investigation revealed that all the soft tissues of the Pseudosuccinea columella snails were macroconcentrators (BSAF > 2) (Tables 6–9) except for Mn (where they were deconcentrators in summer and autumn; BSAF < 1) and Fe (where they were deconcentrators in autumn; BSAF < 1). These show that selective tissues are thus good biomonitors of pollution rates. The minimum BSAF values for Cu in the soft tissues varied from 3.1 to 4.55 in winter. These increased to 3.30–4.55 in spring. The maximum values for Cu were recorded in summer (7.60–9.62). The minimal BSAF values for Zn in the soft tissues varied from 2.20 to 3.90 in autumn. These increased to maximum values during summer (4.11–5.72). The maximum BSAF values for Pb in the soft tissues ranged from 3.11 to 4.41 in spring, whereas the minimum values (2.10–3.30) were recorded in autumn.
The minimum BSAF values for Fe in the soft tissues ranged from 1.80 to 3.00 in autumn. The maximum values ranged from 4.28 to 7.13 in summer. The minimum BSAF values for Mn in the soft tissues ranged from 1.70 to 2.40 in summer. The maximum values ranged from 3.12 to 4.28 in winter. Laszczyca et al. (2004) illustrated that enzymatic activities are responses to environmental stress. Thus, they can be used to estimate pollution at population and ecosystem levels. Meanwhile, El-Khayat et al. (2015) used physiological and biochemical parameters as indicators of histopathological changes. These parameters have also been widely used as biomarkers in the health evaluation of animals.
4.5 Bcf
The BCF confirmed that the highest concentrations (ppm) of Cu ranged from 13.21 to 32.56 in autumn. Those of Fe ranged from 119.78 to 895.12 in summer, and those of Mn ranged from 10.22 to 62.16 in autumn. The highest concentrations of Zn ranged from 21.22 to 85.21 in autumn, and those of Pb ranged from 1.16 to 3.86 in summer (Tables 6-9). The BCF values obtained for Cu and Pb were>1.00, indicating that both were highly bioaccumulated and biomagnified in the tissues of Pseudosuccinea columella snails (Cu has higher BCF than that of Pb). Copper is essential in some enzymes for organisms (Yap et al. 2016) and serves as a micronutrient for cellular metabolism (Ismarti et al. 2017). The cuproprotein hemocyanin (Cheung and Wong, 1992) functions similarly to Fe in the metabolism of molecular oxygen in snails because the oxygen-carrying pigment in mollusks’ blood is not hemoglobin. This study confirmed the high variations in BCF limits depending on the metal type (Tables 6-9). This is due to the tolerance effect of snails to the persistent toxic chemicals and can be considered effective biomonitors (Rehman et al., 2016). The concentration factors (BCF) exceeding 1 indicated that the freshwater snail Pseudosuccinea columella may be used to clean up the trace metal contamination caused by human activity.
5 Discussion
5.1 Ecological factors affecting the abundance of P. Columella
5.1.1 pH parameter
The canonical correspondence analysis performed using the ecological data recorded in the north and northwest sites of the Manzala Lagoon confirmed the relation of the abundance of Pseudosuccinea columella with pH variations. We observed a strong negative association between snail abundance and pH, implying the snails’ certain tolerance for slightly acid waters. The average pH values were rounded to 8.15 (7.48–8.60). Globally, snails can tolerate environments with pH values ranging from 7 to 8. In this study, the number of snails decreased in summer (Samples 14, 16, and 17), which is characterized by relatively low pH (Tables 3 and 4). This agrees with the findings of Gutiérrez (2004) and Prepelitchi et al. (2011).
5.1.2 Rainfall parameter
The abundances of snails were low in summer, increased in autumn, peaked in winter, and decreased in spring (Table 4); i.e., the abundance increased with increasing rainfall. Another factor explaining the population boom of Pseudosuccinea columella in winter would be the almost complete disappearance of Biomphalaria spp. (Prepelitchi et al., 2011). The soft tissues of Pseudosuccinea columella could be used as biomonitors because of their favorable aquatic behavior, tolerance to wide temperature ranges, pH, salinity, and a relatively high intrinsic rate of natural increase (DeKock et al., 1989).
5.1.3 Organic matter parameter
We determined the organic matter content, including Pseudosuccinea columella snails, in the samples in the four seasons. The sample amassed in winter ranged from 0.23 % and 0.50 %, with an average of 0.36 %. In autumn, the organic matter content varied from 0.17 % to 0.40 %, with an average of 0.29 %. In summer, it ranged from 0.17 % to 0.27 %, with an average of 0.23 %. In spring, it decreased from 0.15 % to 0.29 %, with an average of 0.20 % (Table 2). The area was characterized by low organic matter contents in spring and relatively high content in winter. This explained the relative densities of snail species found in particularly excessively organic contents in Samples 14, 15, and 17 in winter (0.50 %, 0.31 %, and 0.41 %, respectively). Similarly, Samples 14 and 17 in autumn were characterized by relatively high numbers of snails because of their relatively high organic matter contents (0.32 % and 0.40 %, respectively). These findings agree with previous observations recorded by Sami et al. (2020) in their study on the bivalves of Lake Timsah (Egypt).
5.2 Factors controlling the distribution of trace elements
5.2.1 Metabolic parameters
The presence of excessive levels of Fe, Cu, and Pb in comparison with the low levels of Zn and Mn in the soft tissues of Pseudosuccinea columella is attributed to the roles of these metals as constituents of metabolically important biomolecules according to Langston et al. (1998). Notably, the soft tissues of the Pseudosuccinea columella snails could store Cu and Zn because their concentrations exceeded the environmental concentration and were attributable to bioaccumulation (Gundacker, 2000).
Marine gastropods typically collect and store Cu and use it to synthesize hemocyanin, a blood pigment. Thus, the Cu concentrations in the soft tissues of Pseudosuccinea columella may be attributed to Cu in hemocyanin (Dallinger and Wieser, 1984). So, the Cu concentration was high in the soft tissues since these were nurtured with blood. The soft tissues of the Pseudosuccinea columella snails can thus be used as biomonitoring agents for Cu and Fe contamination in water samples (Cheung and Wong, 1992). Furthermore, Pseudosuccinea columella is a beneficial bioindicator of trace metal pollution in aquatic ecosystems.
5.2.2 Physicochemical parameter
Factors critical in reducing the bioaccumulation of heavy metals are low pH, low temperature, and excessive organic content of the substrate. The temperature and pH effects can be explained by increased stress at decreased temperature and pH, resulting in reduced food intake (Elder and Collins, 1991). The pH parameter is vital in the immobilization of Pb by microbes in Pb-contaminated environments, affecting the microbial quantity and enzyme activity. It is suggested that biomineralization is swift and that the crystal structures of its products are proper when the pH was 8–9. Similarly, temperature is an important factor influencing the growth rate and metabolic activity level of microorganisms. The activity of enzymes, including urease and phosphatase, varies with temperature, resulting in changes in the biomineralization ability.
6 Conclusions
Human activities are principal factors responsible for freshwater mollusks’ invasion of aquatic environments, and this phenomenon has increased in the last decades. Thus, the presence of Pseudosuccinea columella in Egypt in the wild is not surprising. The average concentration of Zn was higher in autumn in sediment (36.3 μg/g) than those in the water and tissues. Meanwhile, the average concentration of Pb was higher in winter in sediment (15.5 μg/g) than those in the water and tissues. The average concentration levels of Pb were higher in winter than those in the other seasons. The average concentration level of Fe was higher in autumn (5.42 μg/g) than those in the water and tissues. Nevertheless, the Cu average concentration was higher in summer in sediment (96 μg/g) than those in the water and tissues. This observation showed that the average concentration levels of Cu and Fe in the tissues were higher in winter than those in the other seasons.
This study suggests that Pseudosuccinea columella can be a potential bioindicator for metal pollution and toxicity testing. In all seasons, the trace metal concentrations in the samples in all stations were greater than those in the snails’ tissues. This snail can be observed in various habitats, such as small streams, canals, and lakes, but seems to prefer standing running freshwaters. In Egypt, Pseudosuccinea columella may be increasing in distribution. Given that it is an efficient intermediate host of Fasciola hepatica, this situation could increase the risk of the emergence of fascioliasis in new areas and the prevalence of the disease in endemic ones because of the presence of greater numbers of susceptible hosts.
The BSAF identified that Pseudosuccinea columella were macroconcentrators for Fe and Pb, whereas its soft tissues were macroconcentrators for Cu and Zn. These findings indicated that the differences in metal distribution could be attributed to the differences in tissue physiology and metal handling, storage, and detoxification strategies. The BCF results confirmed the ability of Pseudosuccinea columella snails to accumulate heavy metals. The snails had a higher BCF category for Cu and Pb in water but a low category for sediment. This revealed that Pseudosuccinea columella snails are good accumulators of Cu and Pb in seawater. The abundance of living Pseudosuccinea columella snails in the front of the El-Serw drain in winter due to the content of organic matter and increasing rainfall, the high concentrations of chloride and alkalinity owing to sewage, high pH value, and the concentrations of nitrites.
Acknowledgment
This research was supported by Researchers Supporting Project number (RSP2022R432), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Association of Official Analytical Chemistry (AOAC) (1995) Official method of analysis. Pesticide and industrial chemical residues, 16th edn. A.O.A.C. Int, Arlington.
- Trace metal contents of the Pacific oyster (Crassostrea Gigas) purchased from markets in Hong Kong. Environ. Manag.. 1992;16:753-761.
- [Google Scholar]
- Dallinger, R. (1993) In: Dallinger R, Rainbow PS (eds) Strategies of metal detoxification in terrestrial invertebrates. In ecotoxicology of metals in invertebrates. Lewis publisher, Boca Raton.
- Patterns of accumulation, distribution and liberation of Zn, Cu, Cd and Pb in different organs of the land snail Helix pomatia L. Comparative Biochemistry and Physiology. 1984;79:117-124.
- [Google Scholar]
- Geographical distribution and habitat preferences of the invader freshwater snail species Lymnaea columella (Mollusca: Gastropoda) in South Africa. Onderstepoort J. Vet. Res.. 1989;56:271-275.
- [Google Scholar]
- Ebi, K.L., Hess, J.J., Watkiss, P., 2017. Health Risks and Costs of Climate Variability and Change, in: Mock, C.N., Nugent, R., Kobusingye, O., Smith, K.R. (Eds.), Injury Prevention and Environmental Health. The International Bank for Reconstruction and Development/The World Bank, Washington (DC).
- Freshwater molluscs as indicators of bioavailability and toxicity of metals in surface-water systems. Rev. Environ. Cont. Toxicol. 1991:37-79.
- [Google Scholar]
- Some aspects of chemistry of lake Manzala water. Bull. Inst. Oceanogr. Fish ARE. 1977;7:2-30.
- [Google Scholar]
- “Snails and fish as pollution biomarkers in Lake Manzala and laboratory A: Lake Manzala snails”: Fish. Aquacul. J.. 2015;6(4):1000153.
- [Google Scholar]
- Environmental pollution and its relation to climate change. Nov. Sci. Pub. Inc. 2012:692.
- [Google Scholar]
- Toxicity of titanium dioxide to rainbow trout (Oncorhynchus mykiss): Gillinjury, oxidative stress, and other physiological effects. Aquat. Toxicol.. 2007;84:415-430.
- [Google Scholar]
- Complex bioindication and environmental stress assessment. Ecol. Ind.. 2006;6:114-136.
- [CrossRef] [Google Scholar]
- GAFRD (2006) General Authority for Fishery Resources Development. Year-Book of fishery statistics in Egypt (1990-2005), Cairo.
- An inexpensive titration method for the determination of organic carbon in recent sediments. J. Sedimentary Petrol.. 1974;44:249-253.
- [Google Scholar]
- Bio-concentration and biomagnifications in the aquatic environment. In: Boethling R.S., Mackay D., eds. Handbook of Property Estimation Methods for Chemicals. Lewis, Boca Raton: Environmental and health sciences; 2000. p. :189-231.
- [Google Scholar]
- Air quality status and trends in Europe. Atmos. Environ.. 2014;98:376-384.
- [CrossRef] [Google Scholar]
- Comparison of heavy metal bioaccumulation in freshwater molluscs of urban river habitats in Vienna. Environ. Pollut.. 2000;110(1):61-71.
- [Google Scholar]
- Intéractions Hôtes/Parasites dans le modèle Fasciola/Lymnaeidae: aspects dynamiques et génétiques. France: Université de Perpignan; 2004.
- New advances in IPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1(4):367-368.
- [Google Scholar]
- Ismarti, I., Ramses, R., Fitrah, A., Suheryanto, S. (2017) DEPIK 6 23.
- Metal handling strategies in molluscs. In: Langston W., Bebianno M.J., eds. Metal Metabolism in Aquatic Environments. London: Chapman and Hall; 1998. p. :219-283.
- [Google Scholar]
- Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland) Environ Int.. 2004;30(7):901-910.
- [Google Scholar]
- Biomarkers of exposure, effects and susceptibility in humans and their application in studies of interactions among metals in China. Toxicol. Lett.. 2010;192(1):45-49.
- [Google Scholar]
- Comparison of bioaccumulation of metals and induction of metallothioneins in two marine bivalves (Mytilus edulis and Mya arenaria) Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol.. 2009;150(2):186-195.
- [Google Scholar]
- Population structure and dynamics of (Say, 1817) (Gastropoda: Lymnaeidae) in wetlands of northeastern Argentina. Zool. Stud. (Taipei, Taiwan). 2011;50(2):164-176.
- [Google Scholar]
- Estimation of heavy metal of Mollusca Shell, water and soil collected from Darmalak dam, tehsil Lachi District Kohat. World J. Zool.. 2016;11(1):01-05.
- [Google Scholar]
- Impact of the size of commercial bivalves on bioaccumulation and depuration of heavy metals. Egypt. J. Aquat. Biol. Fisheries. 2020;24(7):553-573.
- [Google Scholar]
- Soltani, N., 2013. Diversity of the terrestrial gastropods in the Northeast Algeria: spatial and temporal distribution. European Journal of Experimental Biology, 3(4): 209-215. www.pelagiaresearchlibrary.com ISSN: 2248 –9215 CODEN (USA): EJEBAU. European Journal of Experimental Biology 3, 209–215.
- Health risks of heavy metals in the Mediterranean mussels as seafood. Environ. Chem.. 2012;10(2):119-130.
- [Google Scholar]
- Nile delta: extreme case of sediment entrapment on a delta plain and consequent coastal land loss. Mar. Geol.. 1996;129(3-4):189-195.
- [Google Scholar]
- Distribution and relationships of selected trace metals in molluscs and associated sediments from the Gulf of Aden, Yemen. Environ. Pollut.. 1999;106(3):299-314.
- [Google Scholar]
- Heavy metal concentrations in surface sediments and manila clams (Ruditapes philippinarum) from the Dalian coast, China after the Dalian Port oil spill. Biol. Trace Elem. Res.. 2012;149(2):241-247.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102341.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







