Translate this page into:
Potential antiosteoporotic effect of ginkgo biloba extract via regulation of SIRT1-NF-kB signaling pathway
⁎Corresponding author: Orthopedics Department, The First Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, Kunming, Yunnan 650021, China. liyankm812@sina.com (Yan Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Osteoporosis is bone disorder that happens when too much bone is damaged by the body, causing too little bone or both. As a consequence, bones are brittle and may crack from dropping or sneezing or small bumps in extreme situations. Ginkgo biloba is a Traditional Chinese Medicine (TCM) and commonly used for the treatment of various bone related disease. The current experimental study aimed to scrutinize the anti-osteoporosis effect of Ginkgo biloba and explore the possible mechanism of action.
Methods
The rats were divided into following groups; Control, Osteoporosis, osteoporosis received ginkgo biloba (25, 50 and 100 mg/kg) and rats were received the treatment after the surgery for 8 weeks. Bone parameters, osteocalcin (OC), alkaline phosphatase (ALP), calcium (Ca), phosphorus (P) and cytokines were estimated. The expression of sirtuin 1 (SIRT1) and nuclear factor kappa B (NF‑κB) were estimated.
Results
Dose dependently treatment of ginkgo biloba extract significantly altered the bone parameters such as increase the number of trabecular bone, trabecular bone thickness, connectivity density, ration of BV/TV and decrease the trabecular separation. Ginkgo biloba extract decreased the serum level of ALP, OC, Ca and P at dose dependent manner. ginkgo biloba extract decreased the cytokines at dose dependent manner. Ginkgo biloba extract also reduced the expression of SIRT1 and NF-kB.
Conclusion
We can conclude that ginkgo biloba extract showed the anti-osteoporosis effect via activation of SIRT1-NF-kB pathway.
Keywords
Osteoporosis
Ginkgo biloba extract
Bone loss
SIRT1
NF-kB
- TCM
traditional Chinese medicine
- OC
osteocalcin
- ALP
alkaline phosphatase
- Ca
calcium
- P
phosphorus
- SIRT1
sirtuin 1
- NFkB
nuclear factor kappa B
- WHO
World Health Organization
- ERT
estrogen replacement therapy
- HRT
hormones replacement therapy
- BMD
bone mineral density
- BMSCs
bone marrow stromal cells
Abbreviations
1 Introduction
Osteoporosis is a bone disorder disease and has the characteristics of bone strength reduction, osteopenia and damage of bone microstructural. In the last 2 decades, the osteoporosis related morbidity increase (Lewiecki, 2019). Its lead the enhanced susceptibility to bone frailty and fractures due to disturbance in the micro-architecture of bone tissue (Lewiecki, 2019; Sambrook and Cooper, 2006). According to the World Health Organization (WHO), the osteoporosis suffer patient in million throughout China, Japan, USA and Europe. The prevalence of osteoporosis enhances dramatically with life expectancy. The osteoporotic fractures risk increase and their related cost boost due to the population aging (Wang et al., 2018). The hip fractures related mortality is very common in elder patient. In USA, patient suffer from osteoporosis or osteopenia reach almost near to 61 million in 2020, while the patient suffer from osteoporosis in china already reach 69.44 million in 2006. The current experiment has been focus on the significant feature of osteoporosis, especially the reduction of bone mineral content and bone tissue absorption. Additionally estimated the enhance osteopsathyrosis, bone microstructural damage, bone load and suppression of biomechanical properties in osteoporosis patient (Feng et al., 2014; Wang et al., 2017). Moreover, the complete fractures and micro fractures can easily identified in patient suffered with osteoporosis.
During the expansion of osteoporosis, the lack of estrogen hormones after the menopause is related to the bone fracture (Feng et al., 2014; Wang et al., 2017). Osteoporosis is a metabolic bone disease categorized via deterioration of micro-architecture and low bone mass, leading to the high risk for fragility fracture (Feng et al., 2014; Wang et al., 2017). Studies suggest that combination of some exogenous (exercise and nutrition) and some endogenous (metabolic and genes hormones) are controlled the skeletal of bone. Osteoporosis exhibits the serious health problem that affects the younger postmenopausal women and elderly women (Howe et al., 2011). Furthermore, menopause considerably increases the risk of osteoporosis (Compston et al., 2019; Lewiecki, 2019). Previous studies suggest that the postmenopausal osteoporosis occurs due to an imbalance among the osteoclastic bone resorption, osteoclastic bone resorption and osteoporosis occurs as a resultant estrogen loss (Dontas et al., 2006; Mei et al., 2012).
During the osteoporosis, estrogen deficiency is the significant marker of osteoclastic bone loss and studies suggest that the osteoclastic bone loss have been linked with the osteoporosis. Osteoporosis can be avoid via reduced the smoking, caffeine intake, excessive alcohol and maintain the body weight. Estrogen deficiency is a major basis for postmenopausal osteoporosis, which contributes to enhance the bone turnover and consequently decreasing the bone structure destruction and bone mineral content (Feng et al., 2014; Leonard et al., 2003). Estrogen replacement therapy (ERT) and hormones replacement therapy (HRT) have been established as a therapy for postmenopausal osteoporosis. Through ERT have exhibited the therapeutic effects on osteoporosis management, their potential effects are cooperated via serious side effects such as breast cancer and uterine cancers and having high cost (Feng et al., 2014; Leonard et al., 2003; Zhang et al., 2015).
Sirtuins (SIRT1) protein is the 1st class of Sirtuin family, a class III histone acetylation enzymes dependent on NAD+ dependent deacetylases, could exert protective effects in various diseases due to its critical role in the metabolism (Feng et al., 2014; Zhang et al., 2015). SIRT1 is able to contribute in deacetylation between non-histone and histone lysine residue, and circulate the nuclear factor ((NF)‑κB, p53 and other transcription factors (Feng et al., 2014; Leonard et al., 2003; Zhang et al., 2015). The SIRT1 activation affects the various pathological and physiological process, such as differentiation, cell proliferation, osteoporosis, anti-oxidative response and inflammation (Liang et al., 2009; Yang et al., 2006). Additionally, these genes standardize differentiation, metabolism, tumor morbidity and cell proliferation. Current investigation has confirmed that SIRT1 also serve a significant role in the morbidity and inhibition of osteoporosis. Various plant extracts have been exhibit a potential role in orthopedic and cardiovascular diseases.
Ginkgo biloba is commonly found in the East Asia especially in China and its extract having long history to treat against various diseases and use in the medicine recipes (Belwal et al., 2019; Dziwenka and Coppock, 2016). Ginkgo biloba rich source of various phyto-constituents such as glycosides (quercetin, isorhamnetic and kaempferol) and terpenoids (bilobalide and ginkgolides) exhibited the potential antioxidant effect. Ginkgo biloba demonstrated the antioxidant effect of the organ particularly superoxide dismutase and glutathione peroxidase (Liu et al., 2014). Previous research investigation suggests the numerous pharmacological effects such as anti-inflammatory (Dziwenka and Coppock, 2016), antiapoptotic (Evans, 2013), antidiabetic (Droy-Lefaix and Packer, 1999) and antioxidant effect (Zhang et al., 2010). Ginkgo biloba extract exhibited the potent antioxidant effect via potent free radical scavenger effect (Dziwenka and Coppock, 2016; Mahdy et al., 2011). Ginkgo biloba also decrease the platelet aggregation induced via hydrogen proxide and tert-butyl hydroperoxide via its antioxidant effect (Birks and Evans, 2009; Droy-Lefaix and Packer, 1999; Mahadevan and Park, 2008). Moreover, Ginkgo biloba extract showed the antagonistic effect against the plateletactivating factor. Its also showed the potential effects against the myocardial ischemia/reperfusion damage (Diamond et al., 2000; Droy-Lefaix and Packer, 1999; Zhang et al., 2013). Moreover, it has been shown that Ginkgo biloba was able to rescue the cardiac phenotype in streptozotocin-induced diabetic rats (Birks and Evans, 2009; Droy-Lefaix and Packer, 1999; Evans, 2013). Due to its antioxidant and anti-inflammatory effect, in this experimental study, we scrutinized the osteoporosis effect of Ginkgo biloba extract against osteoporosis and explore the possible mechanism of action.
2 Material and method
2.1 Collection of plant material
The leaves of the Ginkgo biloba was collected from the Department Herbal Garden and plant sample were authenticated from the Botanist (Prof. Kyum Si) and one reference sample submitted in the department for further reference.
2.2 Experimental animal
For the current experimental study, Wistar rats (175–200 g, sex-male, 3–4 old month) were procured from the Institutional animal house and kept in the single polyethylene cage. The rats were kept in the standard laboratory condition (temperature 22 ± 2 °C, humidity 60 ± 10% and 12 h dark/light cycle). The rats were received the standard diet (Rodent Chow, Beijing) and water ad libitum. The whole experimental study was approved from the institutional animal ethical committee (13708458556).
2.3 Preclinical study
Previously published method was used for the induction of an osteotomy in the right femur of rats with minor modification (Dontas et al., 2006; Galanis et al., 2019). All the group rats were anesthetized with pentobarbital (30 mg/kg) and surgical procedure performed such as tibia tail fixation and post osteotomy.
2.4 Experimental animal
The rats were divided into following groups: Group I: normal control, Group II: osteoporosis control, Group III: osteoporosis + ginkgo biloba (25 mg/kg), Group IV: osteoporosis + ginkgo biloba (50 mg/kg), Group V: osteoporosis + ginkgo biloba (100 mg/kg), respectively. All group rats received the treatment after the 7 days of surgery for 8 weeks. Normal and osteoporosis group rats received the normal saline till end of the experimental study (Wang et al., 2017).
2.5 Bone mineral density (BMD) estimation
All groups of rats were screened by using a dual energy x-ray absroptiometery system via using the previously published method. Briefly, BMD value was estimated for the proximal 3rd of the right tibia of a scan pitch (1.5 mm) at a speed of 60 mm/s. The femur bone of the rats was divided into 3 equal portions for X-ray analysis, which included the femoral shaft and distal femoral epiphysis (Feng et al., 2014; Wang et al., 2017).
2.6 Bone mechanical test
Previous reported method was used for the estimation the bone mechanical test. All the bone samples were assessed for the femur strength through 3 point bending test. Briefly, the femur was successfully removed from the all group rats and stored in the −80 °C and finally estimated the length via using the venire caliper. Digital scale was used for the estimation the hydrated weight of bones. Finally, the specimen’s samples were kept on the 2 supports (12 mm) and bent until the bone fractured through lowering the crosshead positioned at the mid shaft. The ultimate stiffness and peak load of the bone were obtained from the load placement curve (Feng et al., 2014; Wang et al., 2017).
2.7 Biochemical parameter
At the end of the experimental study, the blood samples of all group rats collected via puncturing the retro orbital plexue and collected the blood into the Eppendrof tubes. The blood samples were kept into the room temperature for 4 h, followed by the centrifugation at 3000g for 20 min at 48 °C. After that the aliquots of the supernatant were successfully removed and stored at 28 °C before assay. Osteocalcin and alkaline phosphatase levels were estimated via using the manufacture’s instruction (BIKW, Beijing, China).
2.8 In vitro differentiation of osteoblasts
For the invitro osteoblastogenesis, the cells were cultures by medium supplemented with 10 nM dexamethasone, L-ascorbic acid (50 mg/I) and Nab-glycerophosphatase (10 mM) and finally the staining of ALP on the culture (day 14) and ALP activity was estimated via following the standard method (Zur Nieden et al., 2003).
2.9 Real-time PCR
Total RNA was successfully isolated from the Bone marrow stromal cells (BMSCs). Commercially available kits were used for the isolation of cDNA (Prime Scriptw RT, Dalian, China). The real time was performed using the real time PCR. Data were standardized via using the house keeping gene GAPDH. Primers were prepared according to the sequence in previous reported method. The primers as follow osteopontin forward 50-CTACAGTCGATGTCCCCAAC-30 and reverse 50-GACTCCTTAGACTCACCGCT-30; collagen 1 forward 50-TCTCCACTCTTCTAGTTCCT-30 and reverse 50-TTGGGTCATTTCCACATGC-30; SIRT1 forward 50-ACAACCTCCTGTTGGCTGATG-30 and reverse 50-GCTTGCGTGTGATGCTCTGT-30 and GAPDH forward 50-GGCACAGTCAAGGCTGAGAATG-30 and reverse 50-ATGTGGTGAAGACGCCAGTA-30.
2.10 Statistical analysis
All the statistical analysis was performed by Graphpad Prism (Graphpad Prism 7, U.S.A). All the data are presented as mean ± SD standard deviation. One way analysis of variance and Dunnett’s test was performed. Statistical significance was set at *P < 0.05 is consider as the significant, **P < 0.01 is consider as the more significant and ***P < 0.001 is consider as the extreme significant.
3 Result
3.1 Effect of ginkgo biloba extract on body weight
Fig. 1 demonstrated the effect of ginkgo biloba extract on the experimental rats. Normal control group rats showed the increased body weight as compared to treated group rats. Osteoporosis control group rats showed the reduced body weight as compared to other treated group and normal control group rats. Ginkgo biloba extract (25 and 50 mg/kg) group showed the increased body weight as compared to the osteoporosis control. Ginkgo biloba extract (100 mg/kg) increased the body weight almost near to the normal control.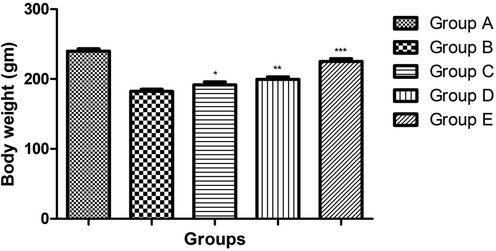
showed the effect of body weight on the different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
3.2 Effect of ginkgo biloba extract on BMD
Fig. 2 showed the effect of ginkgo biloba extract on the bone mineral density of osteoporosis rats. Osteoporosis control group rats demonstrated the reduced bone mineral density due to expansion of disease and dose dependently treatment of Ginkgo biloba significantly (P < 0.001) increased the bone mineral density.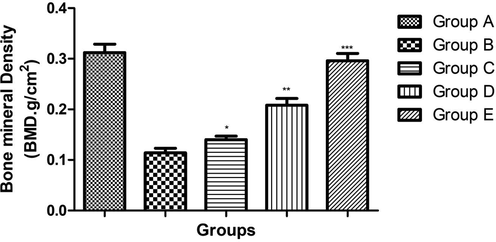
showed the effect on the Bone mineral density of different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
3.3 Effect of ginkgo biloba extract on femoral porosity
On the comparison with the normal group rats, pixel intensity such as distal, mid shaft and proximal epiphysis was considerably increased in the osteoporosis group (Figs. 2, 3). Ginkgo biloba extract (25 and 50 mg/kg) group rats showed the little different in the pixel intensity. Ginkgo biloba extract (100 mg/kg) group rat showed the significantly (P < 0.001) reduction in the pixel intensity as compared to osteoporosis (Figs. 3, 4).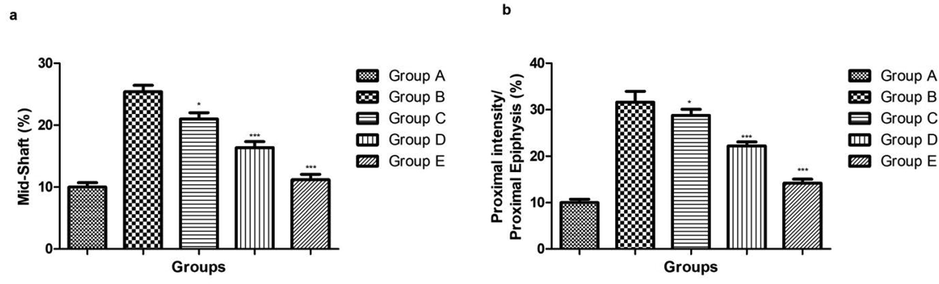
showed the effect on the Femoral porosity based on the pixel intensity on the different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
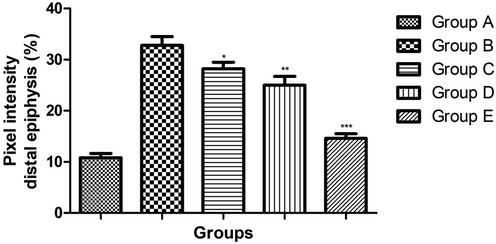
showed the effect on the Pixel intensity of different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
3.4 Effect of ginkgo biloba extract on ALP and OC
During the osteoporosis, the level of ALP and OC was considerably increased due to decrease the bone density. Osteoporosis control group rats showed the increased level of ALP and OC as compared to the normal control group rats. Dose dependently treatment of ginkgo biloba extract significantly (P < 0.001) reduced the level of ALP and OC in the serum as compared to osteoporosis control group rats (Fig. 5).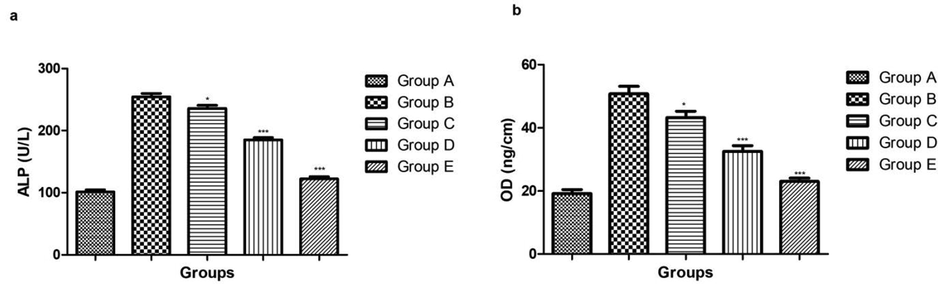
showed the effect on the Serum ALP and OC parameters of different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
3.5 Effect of ginkgo biloba extract on bone loss
Table 1 showed the effect of ginkgo biloba extract on the bone loss. Osteoporosis induced group rats showed the decrease number of trabecular bone, trabecular bone thickness, connectivity density, ration of BV/TV and increase the trabecular separation and dose dependently treatment of ginkgo biloba extract significantly increase the number of trabecular bone, trabecular bone thickness, connectivity density, ration of BV/TV and decrease the trabecular separation. One way ANOVA test was used for the statistical analysis. N = 6 rats in each group. *P < 0.05, **p < 0.01 and **p < 0.001 versus OVX group.
S.No
Parameters
Groups
Group A
Group B
Group C
Group D
Group E
1
Trabecular bone number (Tb.N) (1/mm)
3.03 ± 0.67
2 ± 0.83
2.15 ± 0.56*
2.38 ± 0.45**
2.89 ± 0.16***
2
Trabecular bone thickness (Tb.Th) (mm)
0.075 ± 0.031
0.031 ± 0.023
0.045 ± 0.019**
0.052 ± 0.018***
0.069 ± 0.016***
3
ratio of BV to tissue volume (BV/TV)
0.083 ± 0.022
0.022 ± 0.012
0.032 ± 0.011**
0.045 ± 0.009***
0.078 ± 0.002***
4
Trabecular separation (Tb.Sp) (mm)
0.44 ± 0.09
0.74 ± 0.12
0.70 ± 0.11*
0.59 ± 0.10**
0.47 ± 0.08***
5
Connectivity density(Conn. D)
16 ± 0.73
2 ± 0.11
4.5 ± 0.34***
9.2 ± 0.76***
15.3 ± 0.73***
3.6 Effect of ginkgo biloba extract on calcium and phosphorus
During the osterosporis, increased the level of calcium and phosphorus. A similar effect was observed in the osteroporsis control group rats. the increased level of calcium and phosphorus was observed and ginkgo biloba extract significantly (P < 0.001) reduced the level of calcium and phosphorus (Table 2). One way ANOVA test was used for the statistical analysis. N = 6 rats in each group. *P < 0.05, **p < 0.01 and **p < 0.001 versus OVX group.
S.No
Parameters
Groups
Group A
Group B
Group C
Group D
Group E
1
Ca (mg/dL)
10.34 ± 0.28
13.94 ± 0.38
13 ± 0.43*
12.11 ± 0.28**
11.87 ± 0.26***
2
P (mg/dL)
3.65 ± 0.15
6.56 ± 0.28
6.03 ± 0.22*
5.11 ± 0.25**
4.12 ± 0.16***
3.7 Effect of ginkgo biloba extract on pro-inflammatory cytokines
Osterosporis rats showed the increased level of cytokine such as TNF-α, IL-6 and I-1β. Osteoporosis control group rats received the ginkgo biloba extract significantly (P < 0.001) reduced the level of cytokine such as TNF-α, IL-6 and I-1β (Table 3). One way ANOVA test was used for the statistical analysis. N = 6 rats in each group. *P < 0.05, **p < 0.01 and **p < 0.001 versus OVX group.
S.No
Parameters
Groups
Group A
Group B
Group C
Group D
Group E
1
TNF-α (pg/ml)
70.43 ± 3.45
176.4 ± 5.84
152.34 ± 4.95*
128.3 ± 5.03**
84.93 ± 4.35***
2
IL-1β (pg/ml)
32.56 ± 3.65
70.34 ± 5.03
61.04 ± 4.98*
50.12 ± 3.92**
34.56 ± 3.94***
IL-6 (pg/ml)
112.3 ± 6.43
485.3 ± 10.34
400.3 ± 9.34*
287.3 ± 7.54**
134.53 ± 4.82***
3.8 Effect of ginkgo biloba extract on SIRT1 and NF-kB expression
The SIRT1 and NF-kB expression increased during the osteoporosis. In the current study, osteoporosis control group showed the increased level of SIRT1 and NF-kB and dose dependently treatment of ginkgo biloba significantly decreased the expression of SIRT1 and NF-kB (Fig. 6).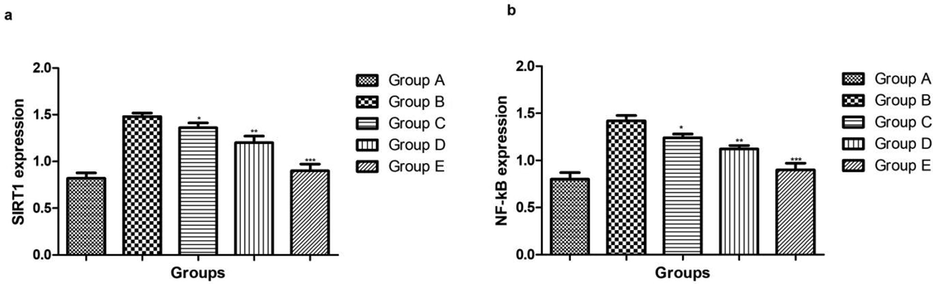
showed the effect on the SIRT1 and NF-kB level of different group of rats. Bars are means ± SEM. The data were compared with the Osteoporosis control (Group B) with the ginkgo biloba treated group rats (Group C, D and E), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001. Where Group A: Normal control, Group B: Osteoporosis control, Group C: Osteoporosis control treated with ginkgo biloba (25 mg/kg), Group D: Osteoporosis control treated with ginkgo biloba (50 mg/kg) and Group E: Osteoporosis control treated with ginkgo biloba (100 mg/kg).
4 Discussion
Systemic skeletal disease such as osteoporosis is categorized via decreased bone mass and deterioration of microarchitectural structure of bone tissue with consequent enhance in bone fragility and propensity to fractures (Feng et al., 2014; Wang et al., 2017). According to the WHO, millions of people suffer from the osteoporosis throughout the China, USA and Europe. The high frequency, severe complications, increase financial burden and vividly decreased the living quality show the severity of osteoporosis in humans (Feng et al., 2014; Wang et al., 2017; Zhang et al., 2016). HRT is the current treatment to cure the osteoporosis, but this therapy having the limitation due to induce the side effects of long treatment history (Bhalla and Bono, 2018). Moreover, to scrutinize the novel protective and therapeutic drug for the treatment of osteoporosis is urgently needed. Previous studies suggest that the Chinese herbal medicine herbal extracts have been expansively scrutinized for their pharmacological effects associated to the bone protective effects.
Reduced the body weight was commonly observed during the osteoporosis due to loss of the bone structure deformation (Feng et al., 2014). During the osteoporosis, reduced the body weight and uterine weight, a similar result was observed in the osteoporosis group rats. A similar result was observed in the osteoporosis control group rats and dose dependently treatment of ginkgo biloba significantly increased the body weight. Ginkgo biloba (100 mg/kg) increased the body weight almost near to the normal control.
It is well proofed that bone matrix rich of organic and inorganic matters. Inorganic matter is referring to the bone minerals and contains the amorphous colloidal calcium phosphorus and crystalline hydroxypatite (Galanis et al., 2019). Organic matter contains the collagenous fibers such as type I primarily fibers and II collagenous fibers and also contain the amorphous matrixes. Previous studies suggest that the collagenous fibers made up proteins that build the bone structure and provide the bone mechanical strength (Feng et al., 2014). Consequently, the formation of bone is correspondent to bone mineralization and refers to the progression via which calcium phosphate and its mineral are accumulated into the organic matter of bone at regular time intervals. Moreover, estrogen boost the secretion of ALP, transforming growth factor and type I osteoblast collagen secretion, so as to encourage the bone formation (Compston et al., 2019; Klibanski et al., 2001). A similar result was observed in the osteoporosis control group rats, where the level of OC and ALP increase and dose dependently treatment of ginkgo biloba reduced and the level reach almost near to the normal values. Ginkgo biloba boosted the bone growth via the mediation of ALP level in the experimental rats. Due to reduce the level of OC and ALP suggest the anti-osteoporosis effect of extract. During the osteoporosis, alteration of OC and ALP expression altered during the serum and similar effect was observed in our experimental finding. ALP is considered as the significant marker of mineralization and osteoid formation (Wang et al., 2018; Zhao et al., 2018). ALP is the significant marker of bone formation, and changes with the balance between the osteoclast and osteoblast activity. Another parameter such as OC found in significant amount in bone and also takes part in the bone formation. Both the parameters (OC and ALP) considered as the protein maker for bone turnover, showing the relationship among the bone formation, bone mineralization and bone re-sorption (Zhao et al., 2018). Osteoporosis control group rats showed the increased level of ALP and OC and dose dependent treatment of Ginkgo biloba significantly reduced the level ALP and OC. Ginkgo biloba extract exhibited the possibility to prevent the bone loss via reduced bone turnover. This could be explicated because evidence has exhibited that ginkgo biloba was able to reducing the osteoclast activity. Result suggests that the ginkgo biloba reduced the bone loss and decreased the osteoclast differentiation. Our result indicates that ginkgo biloba is capable of enhancing the osteoblast differentiation and reducing the osteoclastogenesis.
It is well proofed that SIRTI is closed related with the bone mass and bone metabolism (Zhao et al., 2018). Previous study suggest that histone is able to reduce the level of ALP, OC in osteoblasts and also control the bone formation, also decrease the differentiation and proliferation of osteoblasts (Feng et al., 2014). Chinese herbal medicine use for the preventive medicine on the osteoporosis and these herbal medicines also boost the differentiation and osteoporosis (Bensky et al., 2004; Zhu et al., 2016). Previous studies suggest that the targeting the SIRT1 is the best approaches to treat the osteoblasts. The reduction of the SIRT1 expression, enhancing the BMD in the old age of rodent. In the current experimental study, ginkgo biloba extract reduced the expression of SIRT1 and suggest enhance the differentiation and proliferation of osteoblasts as well as decrease the osteoclasts quantity and formation of marrow adipose cells (Feng et al., 2014; Wang et al., 2017).
Previous studies suggest that the NF‑κB is closely related with the bone metabolism (Nakashima et al., 2012; Wada et al., 2006). During the osteoporosis, increase the level of NF‑κB and induce the weakness of bone proliferation and cellular differentiation and mineralization (Iotsova et al., 1997; Wada et al., 2006). Decreased the activity of NF‑κB in osteoblasts can strengthen mineralization, bone cellular differentiation and emphasize the bone formation (Kim et al., 2012). It is well proofed that the activation of NF‑κB depends on the NF‑κB/p65 phosphorylation. For the anti-osteoporosis effect of tested drug, researcher targeted the NF‑κB for scrutinize the preventive effect (Kim et al., 2012; Yu et al., 2017). Osteoporosis control group rats showed the increased level of NF‑κB and dose dependently treatment of ginkgo biloba significantly reduced the level of NF‑κB and suggest the anti-osteoporosis effect.
5 Conclusion
Taken together, our experimental study confirmed that ginkgo biloba extract could inhibit the osteoporosis is concentration dependent manner. Ginkgo biloba extract significantly altered the serum parameter, pro-inflammatory cytokines and bone parameters at dose dependent manner. Ginkgo biloba extract prevented the bone loss in OVX rats via systematic regulation of osteoblastogensis and osteoclastogenesis. Ginkgo biloba extract confirmed the protective effect against the osteoporosis was medicated via SIRT1-NF-kB signaling pathway. These finding confirmed that Chinese Traditional Medicine might be potential resources for new drugs development for osteoporosis.
Conflict of Interest
All the authors declares none Conflict of Interest.
References
- Bhalla, A., Bono, C.M., 2018. Osteoporosis, in: Orthopaedic Knowledge Update: Spine 5. 2(3), 5-11.
- Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev.. 2009;21(1):CD003120.
- [Google Scholar]
- Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil.. 2000;81(5):668-678.
- [Google Scholar]
- Protective effect of plant extract from Onobrychis ebenoides on ovariectomy-induced bone loss in rats. Maturitas. 2006;53(2):234-248.
- [Google Scholar]
- Antioxidant properties of ginkgo biloba extract. Antiox. Food Suppl. Human Health 1999:343-357.
- [Google Scholar]
- Ginkgo biloba. In: Nutraceuticals: Efficacy, Safety and Toxicity. 2016. p. :681-691.
- [Google Scholar]
- Ginkgo biloba extract for age-related macular degeneration. Cochrane Database Syst. Rev.. 2013;31(1):CD001775.
- [Google Scholar]
- Protective effects of resveratrol on Postmenopausal osteoporosis: Regulation of SIRT1-NF-κB signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai). 2014;46(12):1024-1033.
- [Google Scholar]
- Protective effect of Glycyrrhiza glabra roots extract on bone mineral density of ovariectomized rats. Biomed.. 2019;9(2):8.
- [Google Scholar]
- Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev.. 2011;6(7):CD000333.
- [Google Scholar]
- Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J. Cell. Biochem.. 2012;113(1):247-259.
- [Google Scholar]
- Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc.. 2001;285(6):785-795.
- [Google Scholar]
- Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun.. 2003;309(4):1017-1026.
- [Google Scholar]
- Lewiecki, E.M., 2019. Osteoporosis, in: Metabolic Bone Diseases: A Case-Based Approach. https://doi.org/10.1007/978-3-030-03694-2
- Protective effects of Gingko biloba extract 761 on myocardial infarction via improving the viability of implanted mesenchymal stem cells in the rat heart. Mol. Med. Rep.. 2014;9(4):1112-1120.
- [Google Scholar]
- Multifaceted therapeutic benefits of Ginkgo biloba L.: Chemistry, efficacy, safety, and uses. J. Food Sci.. 2008;73(1):R14-R19.
- [Google Scholar]
- The effect of Ginkgo biloba extract on 3-nitropropionic acid-induced neurotoxicity in rats. Neurochem. Int.. 2011;59(9):770-778.
- [Google Scholar]
- Protective effect of pycnogenol® on ovariectomy-induced bone loss in rats. Phyther. Res.. 2012;26(1):153-155.
- [Google Scholar]
- New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab.. 2012;23(11):582-590.
- [Google Scholar]
- RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med.. 2006;12(1):17-25.
- [Google Scholar]
- The systemic bone protective effects of Gushukang granules in ovariectomized mice by inhibiting osteoclastogenesis and stimulating osteoblastogenesis. J. Pharmacol. Sci.. 2018;136(3):155-164.
- [Google Scholar]
- Protective effects of resveratrol on osteoporosis via activation of the SIRT1-NF-κB signaling pathway in rats. Exp. Ther. Med.. 2017;14(5):5032-5038.
- [Google Scholar]
- Glutaminase acts in osteoblasts to regulate bone formation. J. Orthop. Res.. 2017;31(1):1-2.
- [Google Scholar]
- Purification of flavonoid from Gingko biloba extract by zinc complexation method and its effect on antioxidant activity. Sep. Purif. Technol.. 2010;71(3):273-278.
- [Google Scholar]
- Ginkgo biloba extract for patients with early diabetic nephropathy: A systematic review. Evidence-based Complement. Altern. Med.. 2013;2:689142
- [Google Scholar]
- Resveratrol attenuates acute inflammatory injury in experimental subarachnoid hemorrhage in rats via inhibition of TLR4 pathway. Int. J. Mol. Sci.. 2016;17(8):1331.
- [Google Scholar]
- Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol. Nutr. Food Res.. 2015;59(8):1443-1457.
- [Google Scholar]
- Oleanolic acid exerts bone protective effects in ovariectomized mice by inhibiting osteoclastogenesis. J. Pharmacol. Sci.. 2018;137(1):76-85.
- [Google Scholar]
- Chinese herbal medicine for menopausal symptoms. Cochrane Database Syst. Rev.. 2016;15(3):CD009023.
- [Google Scholar]
- In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71(1):18-27.
- [Google Scholar]







