Polyhydroxyalkanoate production from crude glycerol by newly isolated Pandoraea sp.
⁎Corresponding author. Fax: +55 19 3534 0009. jconti@rc.unesp.br (Jonas Contiero)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new bacterial strain was isolated from Atlantic rainforest in Brazil for polyhydroxyalkanoate (PHA) production utilizing crude glycerol from biodiesel industry (CG) and it was identified as Pandoraea sp. MA03. Shake flask experiments were performed at 10–50 g L−1 carbon source and showed the best values of poly(3-hydroxybutyrate) (P3HB) production from CG cultivations compared to pure glycerol, with a polymer accumulation ranging from 49.0% to 63.6% cell dry weight (CDW). The results obtained from this study showed a positive effect of contaminant NaCl on P3HB synthesis up to 30 g L−1 CG. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] production was obtained from CG plus propionic acid with up to 25.9 mol% 3HV. Since it is interesting the utilization of CG for obtaining added-value products along with biodiesel, this study reported a novel and promising PHA-producing bacterial strain as an additional effort to enhance the viability of a sustainable industry based on biofuels and biopolymers.
Keywords
Polyhydroxyalkanoates (PHAs)
Biodiesel
Glycerol
Pandoraea
Biopolymer
1 Introduction
Polyhydroxyalkanoates (PHAs) represent a class of microbial polyesters accumulated as intracellular granules of energy reserve materials. Most genera of Bacteria and members of Archaea show the ability to synthesize PHAs under nutrient imbalance such as excess carbon with limited nitrogen, phosphorus or oxygen (Anderson and Dawes, 1990). The PHAs are among the most well known bioplastics, being recognized as completely biosynthetic and biodegradable with zero toxic effects and recyclable into organic waste (Chanprateep, 2010). On the other hand, the petrochemical-based plastics not only take many decades to be decomposed in nature, but also produce toxins during the degradation process. Hence there is special interest for PHAs since the allure of biopolymers is also linked to the depletion of petrochemical reserves (Gross and Kalra, 2002; Da Silva et al., 2009).
PHAs are composed of 3-hydroxy fatty acid monomers varying from one carbon (C1) to over 14 carbons (C14) and although approximately 150 different constituents of PHAs have been identified, the poly(3-hydroxybutyrate) (P3HB) is the most common type and extensively studied (Steinbüchel and Füchtenbusch, 1998; Suriyamongkol et al., 2007). The P3HB homopolymer resembles the characteristics of petrochemical-based polypropylene (PP) such as molecular weight, brittleness, stiffness, melting point and glass transition temperature. Therefore, the P3HB can replace some non-biodegradable plastics though its application range has been limited due to its high crystallinity and low extension to break (Braunegg et al., 1998; Grothe et al., 1999). These P3HB unfavorable characteristics can be improved by co-monomers incorporation such as 3-hydroxyvalerate (3HV) or 3-hydroxyhexanoate (3HHx) to produce the copolymers poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HHx)] thereby resulting in suitable properties for thermoplastic processing such as lower crystallinity and greater flexibility as compared to P3HB (Qiu et al., 2006; Lau et al., 2010).
Despite the PHAs have been recognized as good candidates to replace conventional petrochemical plastics, their use in a wide range of applications is still limited by their expensive production price. Alternative solutions have been proposed from the development of production processes based on waste carbon sources or industrial by-products instead of noble ones, since 40–48% of the total production cost are designated to raw materials (Choi and Sang, 1997; Cavalheiro et al., 2009). Currently, the biodiesel production generates about 10% (w/w) glycerol as the main by-product. This means that a 100,000-ton-per-year plant produces about 10,000 tons of crude glycerol (CG) (Yang et al., 2012).
Since purified glycerol is a high-value chemical, the surplus of CG presents opportunities for new applications. Further, most of the smaller scale biodiesel producers require alternative solutions to the CG refining which is economically counterproductive (Ashby et al., 2011). Therefore, development of sustainable processes for utilizing this by-product is desirable in order to promote the biodiesel industrialization in a large scale (Yang et al., 2012). One such possibility is the CG utilization as a carbon source for the fermentative production of value-added products such as biopolymers generating a production set dissociated from petrochemical compounds. In this study, a new PHA-producing bacterial strain utilizing CG was isolated from Atlantic forest ecosystem and it was affiliated to Pandoraea genus, a recent taxon whose new species have been described (Coenye et al., 2000; Sahin et al., 2011). Since to our knowledge there are no reports about PHA accumulation from industrial by-products by this established taxon, here it is presented the first results on PHA production from CG by a representative of the Pandoraea genus.
2 Materials and methods
2.1 Screening for PHA production
The bacterial strains were isolated from soil samples of Atlantic rainforest in Ubatuba, São Paulo State, Brazil (23°34′58.53″ S, 45°16′12.91″ W). The primary bacterial isolation was carried out by serial dilution, in which a volume of 0.1 mL was surface plated on solid mineral salts medium (MSM) (Ramsay et al., 1990), pH 7.0, containing (g L−1): Na2HPO4, 3.5; KH2PO4, 1.5; (NH4)2SO4, 1.0; MgSO4·7H2O, 0.2; CaCl2·2H2O, 0.01; Fe(III)NH4-citrate, 0.06; and 1 mL trace elements solution containing (g L−1): H3BO3, 0.3; CoCl2·6H2O, 0.2; ZnSO4·7H2O, 0.1; MnCl2·4H2O, 0.03; NaMoO4·2H2O, 0.03; NiCl2·6H2O, 0.02; CuSO4·5H2O, 0,01. 20 g L−1 pure glycerol (PG) was used as the sole carbon source and 1 g L−1 (NH4)2SO4 was added to the medium as a nitrogen source. The plates were incubated at 30 °C for up to 120 h and single colonies were transferred and streaked on nutrient agar (NA: 5 g L−1 peptone, 3 g L−1 meat extract and 15 g L−1 agar) until pure cultures were obtained. Screening for PHA-producing bacteria was performed in a nitrogen limiting MSM agar (0.06 g L−1 (NH4)2SO4) containing 20 g L−1 PG which was incubated at 30 °C and 37 °C for 5 days and after stained with Sudan Black B (Schlegel et al., 1970). PHA production capabilities were confirmed by GC analysis from 250 mL Erlenmeyer flask experiments. The bacterial isolates were transferred to NA for 72 h at 30 °C. Cells from agar plates were used to inoculate 100 mL nutrient broth (NB: 5 g L−1 peptone, 3 g L−1 meat extract) which was incubated for 24 h at 150 rpm and 30 °C. A volume of 3 mL NB culture was transferred to 100 mL MSM containing 20 g L−1 PG and 1 g L−1 (NH4)2SO4. Shake flasks were incubated for 72 h at 30 °C and 150 rpm.
2.2 Phylogenetic analysis
The isolate MA03 was cultivated in NB for 24 h at 30 °C and 150 rpm for 16S rRNA gene analysis. Cells were harvested by 15 min centrifugation at 10,000g and their genomic DNA was extracted. The 16S rRNA gene was amplified by PCR using the Pure Taq Ready-To Go PCR Beads with the universal primers for prokaryotes 27F (5′-AGAGTTTGATCA/CTGGCTCAG-3′) and 1492R (5′-ACGGT/CTACCTTGTTACGACT-T-3′). The PCR products were purified using the GFX PCR DNA and Gel Band Purification kit (GE Healthcare, Little Chalfont, UK). The BigDye Terminator v3.1 Cycle Sequencing kit was used to prepare the sequencing according to procedures provided by the manufacturer. The 16S rRNA gene sequencing was carried out on automatic capillary sequencer ABI 3500 (Applied Biosystems, Whaltam, Massachusetts, USA). The consensus sequences were aligned with previously published sequences of bacterial strains using the BLAST function available on the National Center for Biotechnology Information (NCBI) database. Phylogenetic and molecular evolutionary analyses were conducted using the software MEGA 6.06 (Tamura et al., 2013).
2.3 Bacterial characterization
The biochemical properties of the isolated strain MA03 were tested using the API 20 NE (Biomerieux, France) to determine nitrate reduction; indole production; glucose fermentation; arginine dihydrolase, β-galactosidase and urease activities; and hydrolysis of gelatin and esculin. The carbon substrate assimilation tests were determined using the API 50 CH (Biomerieux, France). The biochemical characterization was performed in duplicates at 30 °C according to the manufacturer’s instructions. Morphological characteristics were identified by bright-field microscopy and cultivations on agar plates were performed to verify salt tolerance and temperatures of growth.
2.4 PHA synthesis
The bacterial strain MA03 was streaked from −80 °C stock cultures on NA for 24 h at 30 °C. The inocula were prepared from agar plates through the transferring of bacterial colonies to NB for 24 h at 30 °C and 150 rpm. The isolated strain was then cultivated in 250 mL Erlenmeyer flask containing 100 mL MSM with 1 g L−1 (NH4)2SO4 for up to 120 h at 30 °C and 150 rpm. The pH was adjusted to 7.0 using 2 M hydrochloric acid and 2 M sodium hydroxide. CG was synthesised from the alkaline-catalyzed transesterification of soybean oil and it was obtained from Petrobras biodiesel plant (Candeias, Brazil). CG or PG was added to the sterile medium at concentrations between 10 and 50 g L−1. Moreover, shake flask experiments were carried out utilizing 10 g L−1 sugar cane molasses (SCM), permeate cheese whey (PCW) and waste cooking oil (WCO) to compare the PHA production from these alternative carbon sources. To investigate the ability of isolate MA03 on accumulating 3HV or 3HHx monomers, this strain was cultivated in MSM with 10 g L−1 CG plus 1 g L−1 propionic acid (PA) or 1 g L−1 hexanoic acid (HA), respectively, both added to the culture broth after 24 h. Cells were harvested by 15 min centrifugation at 10,500 g and 4 °C, washed twice, frozen and lyophilized. Biomass was determined gravimetrically as cell dry weight (CDW) expressed in grams per liter of culture broth.
2.5 PHA extraction
The intracellular PHAs were extracted from 1 g lyophilized cells by 20 mL chloroform extraction for 48 h at 30 °C and 200 rpm. Cellular debris was removed by filter paper filtration and the chloroform was evaporated from the filtrate to obtain the crude polymer. Thereupon, the extracted polymer was dissolved in a small volume of chloroform and precipitated by dropwise addition of cold ethanol. The polymer was recovered, placed into a tared vial and dried overnight at 40 °C for GC/MS analysis.
2.6 GC and GC/MS analyses
The PHA quantification and monomer composition were determined by gas chromatography (GC) of propyl esters. Samples of about 10 mg lyophilized cells were subjected to propanolysis reaction, using 2 mL of 1-propanol added 2 mL of 1,2-dichloroethane (Riis and Mai, 1988). A volume of 1 μL organic phase containing the propyl esters was analyzed after split injection (1:25) by a flame-ionization detector (FID). PHA content was determined using Agilent 7890A GC System (Agilent Technologies, USA) with a HP-5 capillary column (5% phenyl/methylpolysiloxane, 30 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA). The temperatures of injection and detector were 250 and 300 °C, respectively. The following temperature program was applied: 100 °C for 1 min, increasing 8 °C.min−1 up to 185 °C which was kept for 15 min. Helium was used as the carrier gas at a constant flow of 0.8 mL.min−1. Benzoic acid was used as internal standard. P(3HB-co-3HV) containing 12 mol% 3HV (Aldrich, USA), PHAMCL produced by Pseudomonas putida ATCC 29347 from fatty acids and P. putida IPT 046 from glucose were used as external standards. The monomer composition was confirmed by coupled GC/MS using a Shimadzu 14B/QP5050A with a quadrupole analyzer. A volume of 0.4 μL organic phase was splitless injected in a BPX-5 capillary column (30 m × 0.25 mm × 0.25 μm; SGE, Australia). Helium was used as the carrier gas (2 mL·min−1). The injection and detector temperatures were 280 e 320 °C, respectively. The column oven temperature was 100 °C, increasing 8 °C·min−1 up to 210 °C, which was kept for 15 min. Mass spectra of propyl esters were obtained by electron impact (EI) at 70 eV. The PHA content was expressed in grams per liter and the percentage (w/w) of the cell dry weight (% CDW).
2.7 Carbohydrates determination
Glycerol and PA quantifications were performed by HPLC apparatus (Prominence, Shimadzu, Japan) with a Rezex ROA column (8% cross-linked sulfonated styrene–divinylbenzene; 300 mm × 7.8 mm; Phenomenex, USA). Samples of 20 μL were injected and eluted with 0,0025 M H2SO4 at a flow rate of 0.6 mL·min−1. The column oven temperature was kept at 65 °C. The UV/Vis and RID-10A detectors (Shimadzu, Japan) were used to determine the PA and glycerol concentrations, respectively. HA was determined by GC (Agilent 7890A GC System) with a 0.2 μL supernatant portion injected on a FFAP column (30 m × 0.53 mm × 1.33 μm; Agilent Technologies, USA). The injection and FID temperatures were 250 °C e 300 °C, respectively. The carrier gas was helium at 3 mL·min−1 and the oven temperature program was applied with 130 °C for 5 min increasing at 10 °C·min−1 rate up to 185 °C. Glycerol, PA and HA (Merck, Germany) were used as external standards.
3 Results and discussion
3.1 Identification of isolate MA03
The strain MA03 was obtained among 76 isolates from soil samples of Atlantic rainforest, which 5 strains were confirmed as PHA-accumulating bacteria from glycerol as the sole carbon source. The partial 16S rRNA gene sequence (1391 bp) has been deposited in the GenBank database under the accession number KC111957. The sequence was aligned and compared with those available in this public database. A high similarity level was detected with 16S rRNA gene sequences of Pandoraea genus: Pandoraea pnomenusa (99%), Pandoraea sputorum (99%), Pandoraea pulmonicola (99%), Pandoraea apista (99%), Pandoraea norimbergensis (99%), Pandoraea vervacti (99%), Pandoraea faecigallinarum (99%), Pandoraea oxalativorans (99%) and Pandoraea thiooxidans (96%). The phylogenetic tree (Fig. 1) was constructed using MA03 sequence and other species belonging related taxa. The molecular evolutionary analyses also demonstrated high similarity with Pandoraea species and the isolate is therefore referred to as Pandoraea sp. MA03.
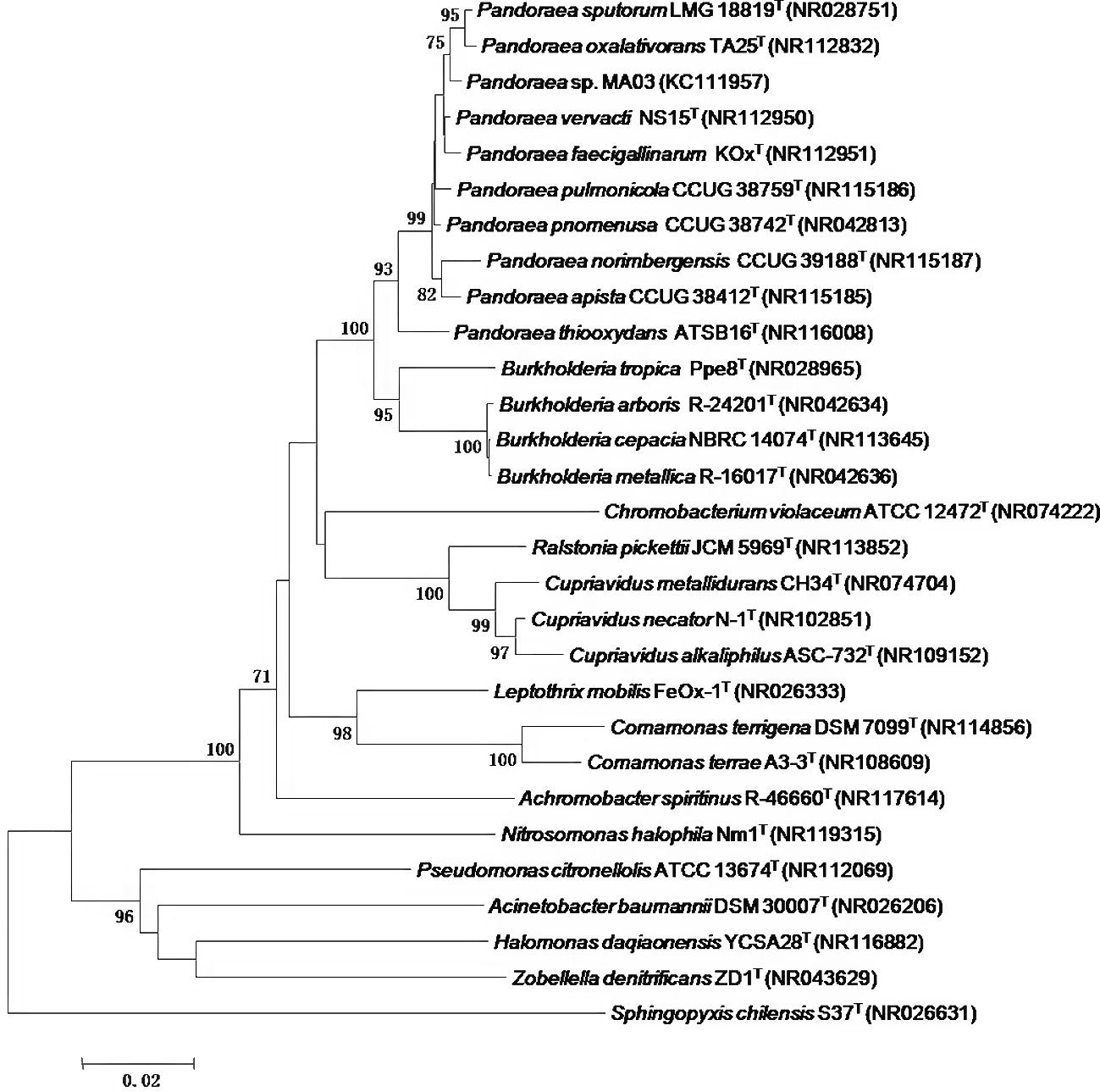
- Phylogenetic tree derived from neighbor-joining analysis showing phylogenetic position of isolate MA03 based on 16S rRNA gene sequences comparison. Numbers above nodes represent bootstrap confidence values above 70% from 1000 resamplings. Bar scales indicate 0.02% sequence dissimilarity.
3.2 Biochemical and physiological characterization
Pandoraea sp. MA03 is Gram-negative, rod-shaped, and nonspore-forming, colonies are off-white due to PHA production. Catalase, oxidase and β-glucosidase were detected.
Table 1 shows data from API 20NE and API 50CH, respectively, for carbohydrates assimilation. Bacterial growth was observed at NaCl concentrations from 1% to 5% (w/v). Nitrate reduction; indole production; gelatin hydrolysis; urease, β-galactosidase and arginine dihydrolase activities were not detected. Glucose was assimilated aerobically but not anaerobically. No growth was observed after 5 days at 10 and 42 °C. The biochemical and physiological characterization showed similarity with the species Pandoraea norimbergensis (Coenye et al., 2000) and Pandoraea oxalativorans (Sahin et al., 2011).
| Characteristic | Pandoraea sp. MA03 |
|---|---|
| Gram staining | − |
| Cell shape | rod |
| Cell size | 0.6–0.8 × 1.2–1.8 μm |
| Catalase | + |
| Oxidase | + |
| Nitrate reduction | − |
| Indole production (Tryptophane) | − |
| Glucose fermentation | − |
| Arginine dihydrolase | − |
| Urease | − |
| β-Glucosidase (esculin) | + |
| Gelatin | − |
| β-Galactosidase | − |
| Growth in: | |
| 3% and 5% NaCl | +,+ |
| Growth at 42 °C | − |
| d-glucose | + |
| d-mannose | + |
| d-mannitol | + |
| N-acetyl-glucosamine | + |
| d-maltose | + |
| Potassium gluconate | + |
| Capric acid | + |
| Adipic acid | − |
| Malic acid | + |
| Glycerol | + |
| d-arabinose, l-arabinose | + |
| d-galactose | + |
| d-fructose | + |
| d-cellobiose | + |
| d-melibiose | − |
| Glycogen | − |
3.3 PHA production from crude glycerol
Pandoraea sp. MA03 was cultivated in MSM at five different concentrations of PG or CG (1–5% w/v) in order to observe the P3HB production from both carbon sources and verify the versatility of the isolated strain on utilizing this biodiesel by-product for biopolymer production. Best values of intracellular polymer accumulation (% CDW) and final P3HB production (g L−1) were obtained from 2% CG, showing values of 63.6% CDW and 2.9 g L−1, respectively, after 72 h cultivation. From 1% to 3% by-product concentrations it was possible to verify higher polymer accumulation using CG instead of PG (Fig. 2A). P3HB production (g L−1) was higher in CG than PG media at all studied concentrations (Fig. 2B). In PG media, the CDW and residual biomass decreased slightly as the initial concentration was increased to 5%. On the other hand, CG cultivations showed CDW values varying from 4.3 to 4.7 g L−1 with increasing CG concentrations at a range of 1–3%. Higher CG concentrations showed a decrease in the CDW to 3.9 g L−1 up to 5% following the P3HB accumulation profile, while residual biomass tended to stabilize at an average of approximately 2 g L−1 between 1% and 5% CG. Further, cultivations performed in PG media showed an increase in P3HB accumulation from 1% to 2% PG (from 37.1% to 44.3% CDW), with stabilized values from 2% to 5% PG ranging 44.3% to 48.4% CDW. All these results suggest that CG is a better carbon source than PG for bacterial growth and an increase in CG concentrations from 1% up to 3% promoted a consequent increase in P3HB accumulation of 29.8% CDW.
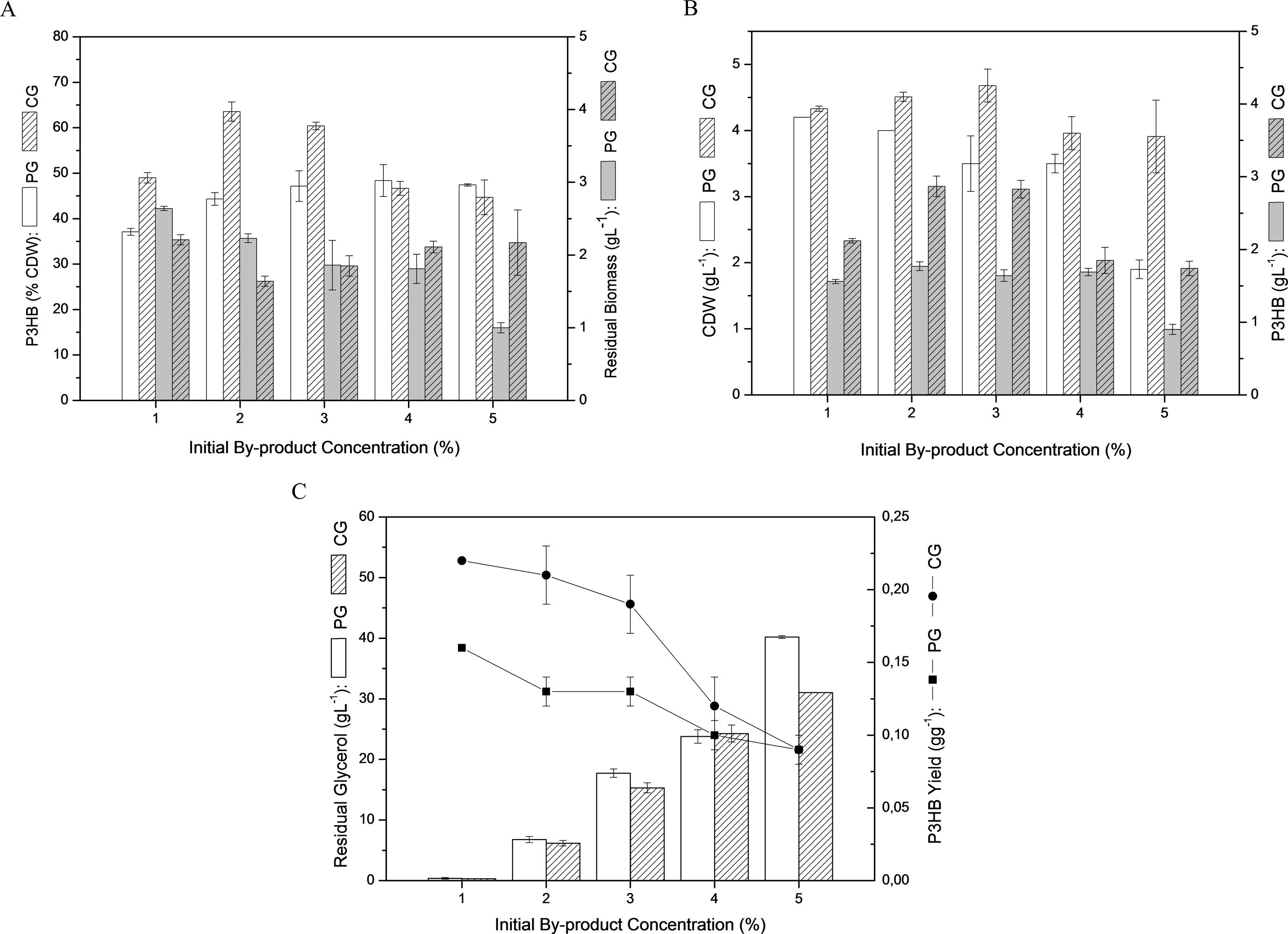
- Profile of P3HB production by Pandoraea sp. MA03 at different by-product concentrations (PG and CG) after 72 h. (A) P3HB accumulation and residual biomass. (B) CDW and P3HB production. (C) Residual glycerol and P3HB yield.
Mothes et al. (2007) tested P3HB production using CG by Cupriavidus necator and Paracoccus denitrificans. Their experiments released a negative effect on the growth and polymer yield due to the presence of sodium ions in the CG from different biodiesel plants. The same was observed by Cavalheiro et al. (2009) with a decrease in the growth rate observed at CG concentrations above 40 g L−1 which may be caused by sodium accumulation in culture media. However, the authors did not report a significant effect on growth rate at PG concentrations up to 70 g L−1. In this present study, the increase in PG concentrations showed a negative effect over the residual biomass and CDW values within the studied range of by-product concentrations. On the other hand, CG did not affect the bacterial growth and an increase in this by-product concentration up to 30 g L−1 demonstrated a positive effect on polymer accumulation. NaCl stress was previously investigated for PHAs production and under specific culture conditions it was reported an enhancing on polymer accumulation by C. necator DSMZ 545 with highest P3HB production at 9 g L−1 NaCl, which was 30% higher than the control (Passanha et al., 2014). Natarajan et al. (1995) described a P3HB content 6-fold increased under NaCl stress by Rhizobium DDSS 69. The β-ketothiolase activity was 3.5-fold higher under stress condition compared to control and showed the potential of NaCl stress effect on key enzymes involved in PHA metabolism of Rhizobium.
Fig. 2C shows P3HB yield at different concentrations of CG and PG, which demonstrates the best results for CG ranging from 0.22 to 0.19 gg−1 at 10–30 g L−1 CG, respectively. Considering the maximum theoretical yield of 0.47 gg−1 for P3HB production from glycerol via acetyl-CoA pathway (Moralejo-Gárate et al., 2011), the results obtained in these experiments represent up to 46.8% of the maximum yield. However, the theoretical yield assumes that the carbon source is totally used to produce this polymer. Yamane (1993) and Gomez et al. (1996) have proposed the calculation of P3HB yield regarding the usage of the carbon source to build up cells in addition to polymer accumulation, which is known as P3HB overall yield. The obtained P3HB% values showed that Pandoraea sp. MA03 accumulated P3HB with an efficiency of 93% of the maximum overall yield at 10 g L−1 CG.
Cultivations in MSM added sugar cane molasses (SCM), permeate cheese whey (PCW) and waste cooking oil (WCO) were performed and confirmed the preference of Pandoraea sp. MA03 to accumulate P3HB using CG (Table 2). Costa et al. (2009) obtained polymer accumulation values ranging from 43% to 50.4% CDW using WCO by Pseudomonas aeruginosa strains. However, these authors reported lower PHA% values when glycerol was utilized as the sole carbon source and the studied strains reached intracellular accumulation of 4.6–22.2% CDW. In order to test the ability of Pandoraea sp. MA03 to produce co-monomers such as 3HV and 3HHx, it was conducted cultivations with PA and HA added to the media after 24 h. Pandoraea sp. MA03 was not able to accumulate 3HHx monomer from HA but the P(3HB-co-3HV) production was observed from CG plus PA. However, the addition of PA in the MSM after 24 h was inhibitory for growth and total polymer accumulation resulted in 1.1 g L−1 CDW with 18.5% polymer after 72 h (Fig. 3B). Maximum 3HV content was achieved after 120 h with 25.9 mol% of 3HV. These results compared to kinetic studies in MSM added 10 g L−1 CG as the sole carbon source have confirmed the toxic effect of PA over growth and PHA production metabolism of Pandoraea sp. MA03. CG cultures showed a higher polymer production and CDW at the same CG concentration after 72 h reaching values of 49% CDW and 4.3 g L−1, respectively (Fig. 3A). On the other hand, various bacterial strains achieved poor 3HV yields from PA with approximated ratios of 0.10 gg−1, which are lower in comparison to the maximum theoretical yield of 1.35 gg−1 (Gomez et al., 1996). Pandoraea sp. MA03 experiments for 3HV production from PA resulted in a 3HV yield of 0.19 gg−1. This value could be considered higher than a variety of ratios reported for wild strains though it was distant from those obtained by mutant strains of Burkholderia sp., which showed 3HV yields up to 0.81 gg−1 in similar culture conditions using sucrose and PA as 3HV precursor (Silva et al., 2000). Nevertheless, the experiments from the present study demonstrated a significant potential of Pandoraea sp. MA03 to produce P(3HB-co-3HV) co-polymer.
| Carbon source | CDW (g L−1) | PHA (% CDW) | PHA (g L−1) | Polymer yielda(gg−1) | Residual biomassb(g L−1) |
|---|---|---|---|---|---|
| SCM | 2.10 | 12.35 | 0.26 | 0.05 | 1.84 |
| PCW | 2.92 | 12.01 | 0.35 | 0.05 | 2.57 |
| WCO | 2.58 | 10.37 | 0.27 | 0.04 | 2.31 |
| PG | 4.20 | 37.11 | 1.56 | 0.16 | 2.64 |
| CG | 4.33 | 49.00 | 2.12 | 0.22 | 2.21 |
| CG + PA | 1.13 | 18.55 (19.24 mol %c) | 0.21 | 0.05 (0.19d) | 0.92 |
| CG + HA | 3.22 | 45.27 | 1.46 | 0.15 | 1.76 |
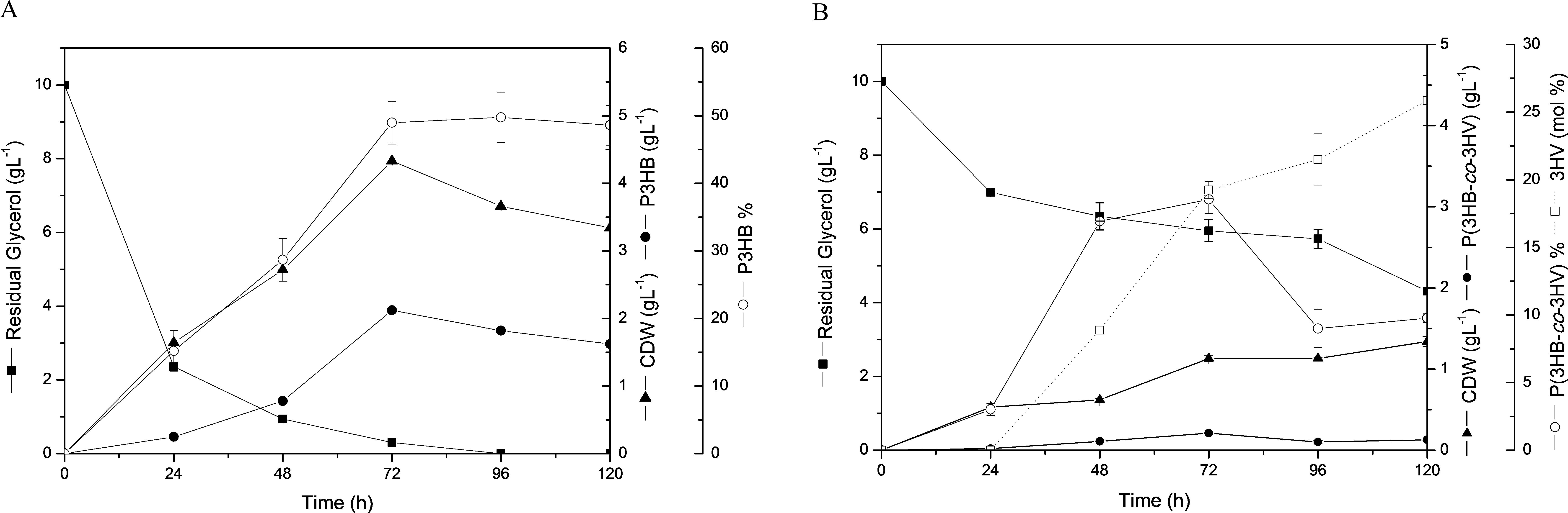
- Residual glycerol, CDW and polymer production profiles at 10 g L−1 CG (A) or 10 g L−1 CG plus 1 g L−1 PA (B) during 120 h.
3.4 Polymer characterization
Lyophilized cells from MSM cultures containing CG plus PA were subjected to chloroform reaction and extracted P(3HB-co-3HV) was analyzed by GC/MS aiming to confirm and characterize the PHAs monomers synthesised by this new producing strain Pandoraea sp. MA03. The peak profiles for 3HB and 3HV can be observed in the chromatogram presented in the Fig. 4A. The peaks at 2.95 min and 4.46 min were identified as 3HB and 3HV, respectively, based on mass spectra analysis. Fig. 4B and C show the electron ionization mass spectra for propyl-esters of 3-hydroxyalkanoates corresponding 3HB and 3HV peaks. The fragment m/e 131 is characteristic of propyl-esters of 3-hydroxyalkanoates formed by α-cleavage of the hydroxyl functional group (Lee and Choi, 1995). The monomers 3HB and 3HV could be identified by the analysis of fragments of m/e [M-59] (Silva-Queiroz et al., 2009). Therefore, the fragment m/e 87 is related to 3HB and fragment m/e 101 is characteristic of 3HV.
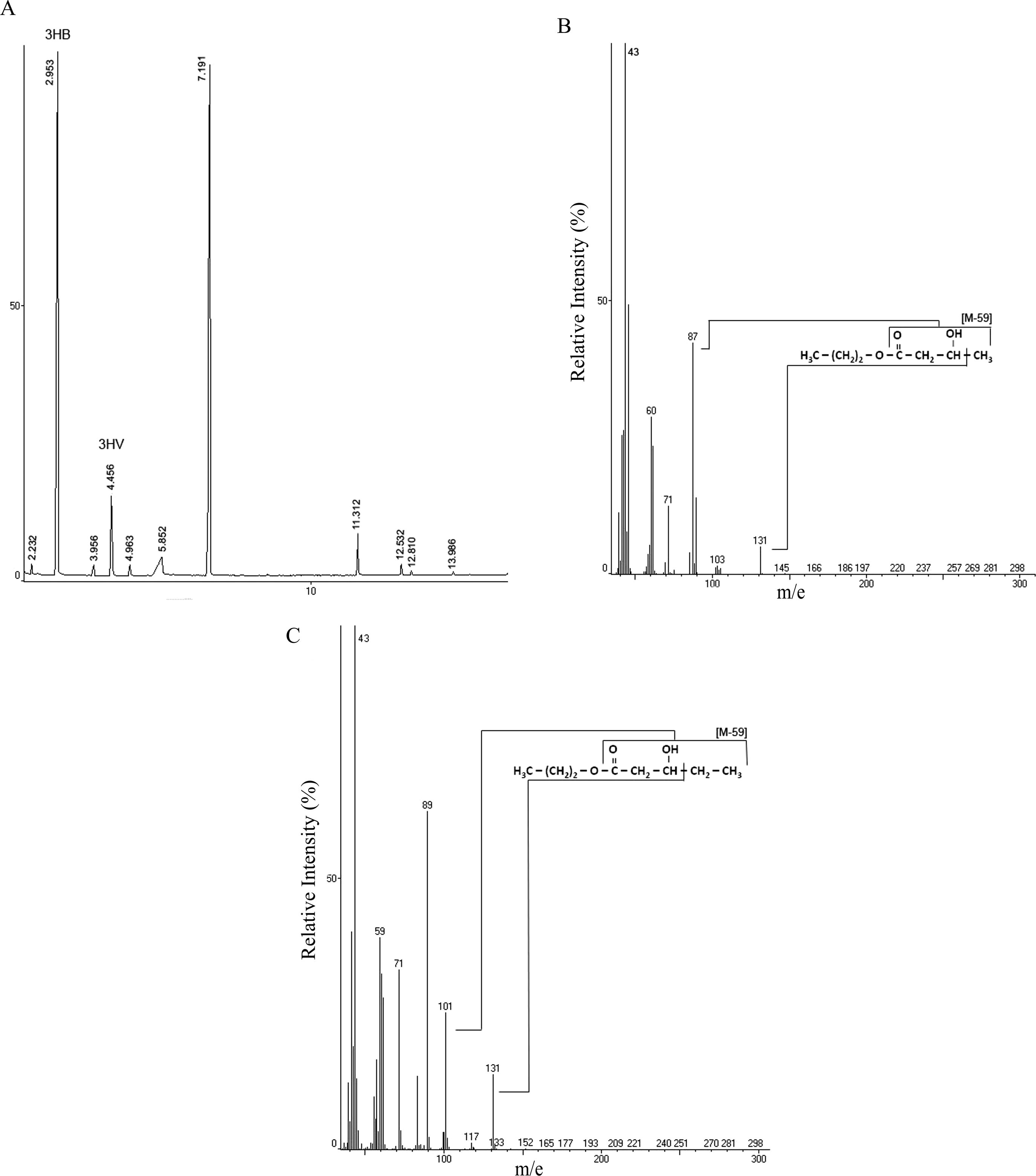
- (A) Gas chromatographic analysis of extracted P(3HB-co-3HV) produced by Pandoraea sp. MA03. Peaks at retention time 2.95 and 4.46 min were identified as 3HB and 3HV, respectively. Mass spectra of electron ionized propyl-esters of 3-hydroxyalkanoates: 3HB (B) and 3HV (C).
4 Conclusions
Pandoraea is a recently established bacterial genus and new species have been described. Therefore, it is relevant to report the metabolic features and possible production of added-value metabolites of industrial interest or medical applications besides the characterization of new described species from this genus. In this study, Pandoraea sp. MA03 showed a good potential to produce P3HB and P(3HB-co-3HV) from CG as the sole carbon source and CG plus PA, respectively. The utilization of CG for the production of PHAs is an interesting alternative to support the biodiesel production from fats and oils. Further, the implementation of biofuels plants along the biodegradable plastics could be a green outlet for petrochemical plastics and fuels. Pandoraea sp. MA03 is a new PHA-producing strain adapted to convert CG to these microbial polyesters with high polymer accumulation rates which leads to future prospects concerning the optimization of cultivation parameters and the increase of cellular productivity.
Acknowledgments
The authors thank São Paulo Research Foundation (FAPESP) for financial support.
References
- Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev.. 1990;54:450-472.
- [Google Scholar]
- Efficient utilization of crude glycerol as fermentation substrate in the synthesis of poly(3-hydroxybutyrate) biopolymers. Am. Oil Chem. Soc.. 2011;88:949-959.
- [Google Scholar]
- Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol.. 1998;65:127-161.
- [Google Scholar]
- Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem.. 2009;44:509-515.
- [Google Scholar]
- Current trends biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng.. 2010;110:621-632.
- [Google Scholar]
- Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng.. 1997;17:335-342.
- [Google Scholar]
- Description of Pandoraea gen. nov. with Pandoraea apista sp. nov., Pandoraea pulmonicola sp. nov., Pandoraea pnomenusa sp. nov., Pandoraea sputorum sp. nov. and Pandoraea norimbergensis comb. nov. Int. J. Syst. Evol. Microbiol.. 2000;50:887-899.
- [Google Scholar]
- Cassava wastewater as a substrate for the simultaneous production of rhamnolipids and polyhydroxyalkanoates by Pseudomonas aeruginosa. J. Ind. Microbiol. Biotechnol.. 2009;36:1063-1072.
- [Google Scholar]
- Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol. Adv.. 2009;27:30-39.
- [Google Scholar]
- Evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Appl. Microbiol. Biotechnol.. 1996;45:785-791.
- [Google Scholar]
- Fermentation optimization for the production of poly(β-hydroxybutyric acid) microbial thermoplastic. Enzyme Microbiol. Technol.. 1999;25:132-141.
- [Google Scholar]
- Biosynthesis and mobilization of a novel polyhydroxyalkanoate containing 3-hydroxy-4-methylvalerate monomer produced by Burkholderia sp. USM (JCM15050) Bioresour. Technol.. 2010;101:7916-7923.
- [Google Scholar]
- Gas chromatography-mass spectrometry analysis and its application to a screening procedure for novel bacterial polyhydroxyalkanoic acids containing long chain saturated and unsaturated monomers. J. Ferment. Bioeng.. 1995;80:408-414.
- [Google Scholar]
- Microbial community engineering for biopolymer production from glycerol. Appl. Microbiol. Biotechnol.. 2011;92:631-639.
- [Google Scholar]
- Characteristics of NaCl stress associated proteins of Rhizobium under varying cultural conditions. J. Basic Microbiol.. 1995;35:413-420.
- [Google Scholar]
- The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour. Technol.. 2014;163:287-294.
- [Google Scholar]
- Metabolic engineering of Aeromonas hydrophila for the enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Appl. Microbiol. Biotechnol.. 2006;69:537-542.
- [Google Scholar]
- Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl. Envirom. Microbiol.. 1990;56:2093-2098.
- [Google Scholar]
- Gas chromatography determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J. Chromatogr.. 1988;445:285-289.
- [Google Scholar]
- Pandoraea oxalativorans sp. nov., Pandoraea faecigallinarum sp. nov. and Pandoraea vervacti sp. nov., isolated from oxalate-enriched culture. Int. J. Syst. Evol. Microbiol.. 2011;61:2247-2253.
- [Google Scholar]
- The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Arch. Mikrobiol.. 1970;71:283-294.
- [Google Scholar]
- Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. J. Biotechnol.. 2000;76:165-174.
- [Google Scholar]
- PHAMCL biosynthesis systems in Pseudomonas aeruginosa and Pseudomonas putida strains show differences on monomer specificities. J. Biotechnol.. 2009;43:111-118.
- [Google Scholar]
- Bacterial and other biological systems for polyester production. Trends Biotechnol.. 1998;16:419-427.
- [Google Scholar]
- Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants – a review. Biotechnol. Adv.. 2007;25:148-175.
- [Google Scholar]
- MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol.. 2013;30:2725-2729.
- [Google Scholar]
- Yield of poly-D(-)-3-hydroxybutyrate from various carbon sources: a theoretical study. Biotechnol. Bioeng.. 1993;41:165-170.
- [Google Scholar]
- Value-added uses for crude glycerol – a byproduct of biodiesel production. Biotechnol. Biofuels. 2012;5:13.
- [Google Scholar]







