Translate this page into:
Pollen source preferences and pollination efficacy of honey bee, Apis mellifera (Apidae: Hymenoptera) on Brassica napus crop
⁎Corresponding author at: Unit of Bee Research and Honey Production, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. khalidtalpur@hotmail.com (Khalid Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Brassica napus is an insect-pollinated crop species and offers itself as the main nutrient source to many insects that consume floral nectar. This study was carried out to ascertain the pollen preferences of Apis mellifera among the available floral resources. We wanted to identify and quantify all types of pollen collected by honeybee during the flowering period. Moreover, the foraging rate and pollination efficacy of managed honey bee colonies (placed at 250 and 500 m distance away) on B. napus crop were determined. The results revealed that A. mellifera foraged efficiently on 18 plants species belonging to 11 families during the flowering period of the Brassica crop. The Asteraceae family was represented by six plants species as pollen sources followed by Solanaceae, Malvaceae, Fabaceae, and Rosaceae represented by two plant species from each family. One floral source included Brassicaceae, Convolvulaceae, and Poaceae families. Among 18 identified plant species, 6 were weeds, 4 herbs, 4 shrubs, and 2 species each were from crops and ornamental plants. In this study, weeds were reported as the major bee supporting bee flora followed by shrub and crops. The identified pollen grains had different morphology such as sub-spheroid, prolate shape, spheroid, ovate, glandular, triangular, round, and oval shape. The maximum foraging activities of bees on B. napus took place during day hours particularly at 12:00 PM followed by 14:00 PM, and then at 10:00 AM weekly. The study revealed that the total number of pods per plant, total number of seeds per 100 pods, and weight of seeds per 10 plants were significantly higher in bee-pollinated flowering plants compared to flowering plants with no bee-pollination.
Keywords
Honey bee
Foraging behavior
Pollen diversity
Canola
Hive distance
Yield
1 Introduction
Brassica napus is the second most essential oilseed crop and considered the main valuable nectar-producing plants in the world (Enkegaard et al., 2016; Song et al., 2020). It is primarily an oilseed crop, accounting for approximately13%-16% of vegetable oil globally (Wang et al., 2018). Further, it is allotetraploid species that was originated in the Mediterranean region about 7500 years ago by the natural hybridization of B. oleracea and B. rapa (Nagaharu, 1935; Lu et al., 2019). It provides edible oil that is important for human nutrition and high-quality animal feed, and it became a good choice for biodiesel production (Morse and Calderone, 2000; Marles and Gruber, 2004). Mostly commercial cultivar have brown to black and yellow seed color (Morse and Calderone, 2000). It is cultivated many areas in the world and contributes the domestic oil production among various oilseed crops (Minfal, 2014). Poor soil fertility, pests, diseases, water stress, and insufficient pollination are the causes of low canola productivity (Free, 1993).
As a cross-pollinated crop, its pollens are dispersed either through wind, gravity or animals (Thompson et al., 1999; Westcott and Nelson, 2001; Bommarco et al., 2012). Pollination by insects is very important to the persistence and reproduction of various plants including agricultural crops, medical herbal crops, horticultural and wild plants (Ollerton et al., 2011; Latif et al., 2019b; Shakeel et al., 2019). For the transfer of pollen grains, B. napus requires different groups of insects because canola is a self-incompatible crop that is why it needs different insects for the transportation of pollen from male to female flowers (Roy et al., 2014). Canola flowers secrete a large quantity of nectar and are highly attractive to insect pollinators especially to honeybees (Masierowska and Piętka, 2014). Further, bees improve the hybridization of the plants during nectar and pollen collecting processes and are suggested as an essential contributors to short distance pollination (Morse and Calderone, 2000). Insect pollination in canola, maximize seed production, larger grain, the formation of well-shaped grains, and more viable seed (Khan and Chaudhary, 1995).
All insect pollinators do not show the same foraging behavior regarding their food collection from the availability of flora diversity (Bashir et al., 2018; Ahmad et al., 2021; Khan et al., 2021; Saleh et al., 2021a, 2021b). For instance, the difference occurs between the foraging pattern of bumblebees and honeybees. It has been found that bumblebees generally collect pollens with high protein contents during foraging activity in the spectra of plant species, whereas honeybees tend to exploit diverse plant resources to collect pollen quantity instead of pollen quality (Leonhardt and Blüthgen, 2012). Pollens from different floral resources have specific size, shape, and orientation (Shubharani et al., 2013). Pollen study has significant importance in recognition of bee flora (Shubharani et al., 2013; Al-Kahtani et al., 2020). Moreover, the diversity of pollen resources could affect the foraging behavior and longevity of the bees (Hanley et al., 2008; Latif et al., 2019a).
This study was conducted to determine the honeybee pollen preferences among the available floral diversity in B. napus. Also, to explain the foraging behavior and pollination efficacy of A. mellifera colonies (placed at 250 m and 500 m distances) in B. napus crop.
2 Materials and methods
The B. napus (canola) variety was cultivated in 2018. Row to row and plant to plant distance was maintained as 45 cm and 15 cm, respectively.
2.1 Identification of pollen sources collected by A. mellifera during the flowering period of B. napus
Five colonies of bees with equal strength were placed in the experimental area of 2 acres to perform this experiment. A field survey was conducted to identify the collected pollen sample as a reference from all flowering local plant species. For further investigation, the mature pollen grains of the identified plant species were collected and preserved in 70% ethanol (Shubharani et al., 2013).
To identify the pollen collected by bees, seven incoming forager bees with pollen load were collected from each hive at weekly interval. For pollen identification, the acetolysis protocol was used (Jones, 2014). Pollen suspension was made by washing the bee body in 70% ethanol. After that 2 mL glacial acetic acid was added in 5 mL pollen suspension for 10 min. Centrifuged it for 3 mins at 2400 rpm and the supernatant was removed. Then 10 mL of acetolysis mixture (glacial acetic acid and concentrated sulfuric acid 9:1) were added in it and heated the solution in the water bath at 70 °C for 12 mins. This solution was cool down for 5 mins and again centrifuged at 2400 rpm for 3 mins and the supernatant was discarded. After that, it was resuspended in distilled water, centrifuged and discarded the supernatant. After acetolysis, pollens were preserved for archival reference slides (Schmid, 1995). In this, a base stock of jelly was prepared by combining 10 g gelatin, 30 mL glycerin, and 35 mL distilled water. Then a drop of the prepared jelly and a sample of pollen and stain were added on a clean microscope slide and the slide were gently warmed, stirred to thoroughly homogenize the mixture. Then a coverslip was added, sealed with nail polish around the edges. Then pollens were identified using identification keys and an online image database (Kearns and Inouye, 1993).
2.2 Quantification of pollen collected by A. mellifera
From each colony, seven bees were collected weekly and pollen quantification was done by washing the bee body in a known quantity of ethanol. Ethanol was also used in the cleaning of the hemocytometer. With the help of a pipette, a small drop of pollen suspension was taken and placed in the center of the hemocytometer. The coverslip was placed properly over the hemocytometer. Pollen suspension was allowed to settle at the bottom chamber for 2 min before counting. The chamber is 0.1 mm high and divided into 25 medium squares of 0.04 mm2 each, which were further subdivided into 16 small squares of 0.0025 mm2 each. This means a volume of 0.1 mL in the chamber, 0.004 mL in each medium square and 0.00025 mL in each small one. For each pollen sample, the pollen grains of five medium squares at the center, left, and right corners at the top and bottom of the chamber were counted under binocular microscope 100 X magnifications (Human et al., 2013) which were repeated for making 107 individual observations. Pollen counting was done using a formula:
Pollens per mL = (Total number of pollens counted × Diluted factor/ Area of squares counted (mm2) × Chamber depth (mm))
2.3 Effect of beehive distance on colony-level pollination efficiency
For monitoring the pollination efficacy of A. mellifera, five colonies of equal bee strength were selected and that foraging activity on B. napus was observed during the whole blooming period. These colonies were placed at 250 m and 500 m away from the experimental area of 2 acres with the start of the blooming period till crop harvesting. The following parameters were investigated.
2.4 Colony foraging rate
Two distances 250 m and 500 m of honeybee colonies away from B. napus crop were taken as treatments. In each treatment, five bee colonies were placed as replication. The number of bee foragers from each colony was estimated from hives distance 250 m and 500 m by counting the foragers returning with pollen loads to the hive for 10 min at 10:00 AM, 12:00 PM, and 14:00 by standing at the side of the bee hive (Baker and Jay, 1974).
2.5 Pollination effect of by A. mellifera in yield of B. napus crop
To find out the contribution of managed honey bees to the pollination of B. napus, five colonies of bees were placed in the corner of the field. The data were taken from pre-blooming to pod harvesting of the crop. Before the commencement of flowering, ten plants per replication were covered by muslin cloth to limit the access of insect pollinators. These plants remained covered throughout the blooming period. In the second treatment, plants remained uncovered for the whole flowering period. At the end of the crop cycle, the plants were harvested for assessment of the following parameters.
2.6 Number of pods
The total number of pods was counted from ten selected canola plants in each treatment at the time of harvest and their average was calculated.
2.7 Number of seeds per 100 pods
Hundred pods were randomly selected from each sample of each treatment at the time of harvest. Then after threshing, recorded the number of seed by counting and their average was calculated.
2.8 Weight of seed per 10 plants
From each treatment, ten plants were randomly selected. Then after threshing, recorded the seed weight of 10 plants from each treatment by using an electrical weighing balance (Mettler Toledo, Colombus, USA) to calculate their average.
2.9 Statistical data analysis
All results were presented in mean ± standard error (SE) and statistical data analyzed through statistical package SPSS (version 26). The effect of colony foraging rate at different beehives distance, data about the number of pods per 10 plants, number of seeds per 100 pods, and seed weight per10 plants were measured using analysis variance (ANOVA). The student’s t-test was used to calculate the significant difference between the two groups, one-way ANOVA followed by Tukey post-hoc test was used to determine the difference between more group. All means value were compared at the 95% (p < 0.05) confidence level.
3 Results
3.1 Identification of pollen sources collected by A. mellifera during the blooming period of B. napus
In the laboratory, different pollen sources were identified which were collected by A. mellifera during the flowering period of B. napus (Table 1). The result revealed that A. mellifera foraged efficiently on 18 plants species belonging to 11 families during the flowering period of Brassica crop. Information on pollen morphology and each floral source is also given in Table 1. Asteraceae family provided six plants species as pollen sources followed by Solanaceae, Malvaceae, Fabaceae, and Rosaceae which provided two plant species with each. One floral source was included by Brassicaceae, Convolvulaceae, and Poaceae families. Among 18 identified plant species, 6 species belonged to weeds, 4 species each from herbs and shrub were represented, and 2 each belonged to crops and ornamental plants, respectively (Table 2). In this study, weeds were reported as the major bee supporting flora followed by shrubs and crops. The bulk of diet was taken from weeds during the mass flowering periods (33.33%) and is therefore thought to play a key role at this time. In the absence of major flora, minor flora such as ornamental plants also provide food to the bees.
Sr. No
Scientific name and
Family
Morphology
Forage source
1.
Brassica napus
(Brassicaceae)
Sub-spheroid, Monoporate
Crop
2.
Taraxicum officinalis
(Asteraceae)
Bilateral symmetry
Weed
3.
Calendula officinalis
(Asteraceae)
Pantoporate, pores 32, Echinate, radial symmetry, reticulate exine
Ornamental plant
4.
Chrysanthemum indicum
(Asteraceae)
Exine reticulate, porate, spheroid, radial symmetry
Ornamental plant
5.
Calotropis procera
(Asteraceae)
Thicker outer layer but smaller in size than that of Brassicaceae
Weed
6.
Calendula arvensis
(Asteraceae)
Round shape porate, spheroid, spinolous,radial symmetry
Weed
7.
Capsicum annuum
(Asteraceae)
Porate, spinolous, oval shape. radial symmetry
Weed
8.
Cichorium intybus
(Asteraceae)
Round shape, bilateral symmetry
Weed
9.
Petunia spp.
(Solanaceae)
Prolate, sub- spheroid, thin bilateral symmetry
Shrub
10.
Solanum melongena
(Solanaceae)
Sub-spheroid,exinepsilate and thin,bilateral symmetry
Vegetable
11.
Alcea rosea
(Malvaceae)
Pantoporae, spheroid, radial symmetry
Herb
12.
Callistemons rigidus
(Myrtaceae)
Triangular shape, radial symmetry
Tree
13.
Convolvulus arvensis
(Convolvulaceae)
Spinlouse pollens, bilateral symmetry, thick layer on either side of the structures.
Weed
14.
Trifolium alexandrianum
(Fabaceae)
Porate, echinate pollen, bilateral symmetry spinlous round shape
Crop
15.
Acacia nilotica
(Fabaceae)
Oval in shape and multi symmetrical
Tree
16.
Eriobotrya japonica
(Rosaceae)
Ovate, pointed, glandular shaped pollens
Fruit
17.
Rosa indica
(Rosacae)
Ovate, pointed, glandular shaped pollens, bilateral symmetry, prolate
Shrubs
18.
Sorghum halepense
(Poaceae)
Prolate, spheroid, radial symmetry
Weeds
Plant species
No. of species
Percentages
Crops
2
11.11
Shrubs
4
22.22
Herbs
4
22.22
Ornamental plants
2
11.11
Weeds
6
33.33
Total
18
100
The pollen species differed in morphology, structure, symmetry, exine structure, and sculpture. Pollen grains of B. napus and Solanum melongena were sub-spheroid. Echinate type of pollen grains belonged to the Trifolium alexandrianum and Calendula officinalis. Petunia spp. and Sorghum halepense had prolate shape pollen grains. Spheroid types of pollen grains were found in Chrysanthemum indicum, Calendula arvensis, and Alcea rosea. Asteraceae family had echinate, spheroid, and spinolous type of pollen grains. Eriobotrya japonica and Rosa indica had the ovate and glandular type of pollen grains. Callistemons rigidus, Cichorium Intybus, and Acacia nilotica had the triangular, round, and oval shape of the pollen grain, respectively.
3.2 Quantification of pollen collected by A. mellifera workers
The pollens collected from different floral sources by A. mellifera during the flowering period of B. napus were separated from their bodies and quantified (Table 2).
The results indicated that pollen grains observed from forager bees represented Brassicaceae, Asteraceae, Malvaceae, Solanaceae, Convolvulaceae, Fabaceae, Rosaceae, and Poaceae families. Among these observed families, maximum numbers of B. napus pollens were from Brassicaceae. The maximum pollens collected by bees differed significantly between the week intervals (F (5116) = 11.35, P = 0.001). During the B. napus blooming period, the maximum pollen grains collected by honeybees were 37333.33 mg in the first week, whereas less pollen grains were 27911.11 mg during the sixth week (Fig. 1).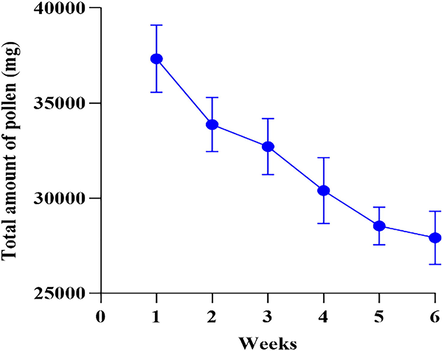
The total amount of pollen (mg/week) collected by Apis mellifera during the flowering period of Brassica napus.
3.3 Colony-level pollination efficacy of A. mellifera on B. napus
3.3.1 Colony foraging rate
The foraging rate of A. mellifera on B. napus was investigated at 10:00 AM, 12:00 PM, and 14:00 on weekly intervals at 250 m and 500 m distance from crops (Fig. 2 A, B). During the five-week intervals at 10:00 AM, maximum foraging rate (617 bees) was observed during the 1st weekly interval which was highly significant from other weekly intervals at 250 m distance (F (4,16) = 32.46, P = 0.001). In contrast the less foraging rate was found at the 5th weekly interval that was 491 bees. In the case of five-week intervals at 12:00 PM, maximum foraging rate (816 bees) was observed during the 1st weekly interval which was highly significant from other weekly intervals, while less foraging rate was 601 bees during the 5th week interval at distance of 250 m. During the five-week intervals at 14:00, maximum foraging rate (718 bees) was observed during the 1st weekly interval and less foraging rate (561 bees) was observed at the 3rd weekly interval (Fig. 2 A).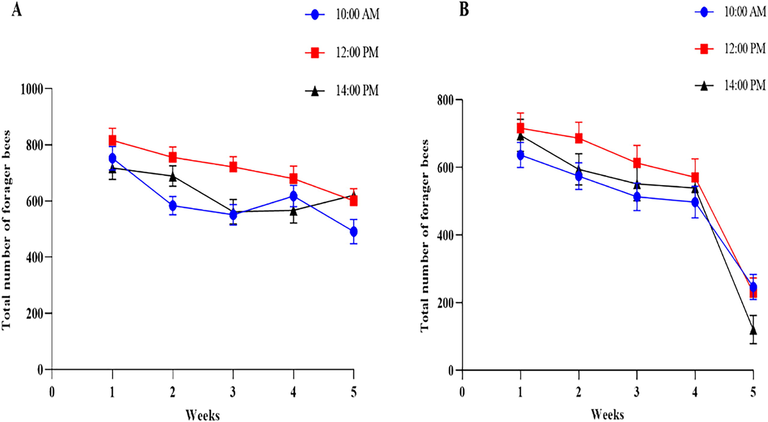
Foraging rate of Apis mellifera on Brassica napus during the flowering period at weekly interval. (A), the total number of bees visit (Mean ± Standard error) on Brassica napus at 10:00 AM, 12:00 PM, and14:00 PM from 250-meter distance. (B) The total number of bees visit (Mean ± Standard error) on B. napus at 10:00 AM, 12:00 PM, and 14:00 PM from 500-meter distance.
The effect of 500 m hive distance on the foraging rate of honeybees during the flowering period of B. napus at different week intervals was described (Fig. 2 B). During the five-week intervals at 10:00 AM, the maximum foraging rate (636 bees) was observed during the 1st weekly interval which was highly significant from other weekly intervals and less rate was (246 bees) at the 5th weekly interval. The maximum foraging rate (716 bees) was observed during the 1st weekly interval while less foraging rate (229 bees) during the 5th week at 12:00 PM. Similarly, the maximum foraging rate (695 bees) was observed during the 1st weekly interval whereas less foraging rate (120 bees) during the 5th week at 14:00.
3.4 The effect of pollination efficacy on yield parameters of B. Napus at 250- and 500-meter distances
3.4.1 Total number of pods per plant
The number of pods per plant is a major yield determining component that contributed towards yield. In the case of 250 m distance, the total number of pods per plant differed significantly from bee-pollinated B. napus plants in comparison to without insect-pollinated plants (F (1,4) = 176.81, P = 0.002). The mean number of pods per plant was 1270 ± 3.12 in the bee-pollinated plant whereas 1096 ± 5.26 pods in without insect pollination (Table 3).
Treatment
The effect of pollination efficacy on yield parameters at 250 m distance
The effect of pollination efficacy on yield parameters at 500 m distance
Pods per plant
Seeds per 100 pods
Seeds weight per 10 plants (g)
Pods per plant
Seeds per 100 pods
Seeds weight per 10 plants (g)
Mean ± S. Error
Mean ± S. Error
Mean ± S. Error
Mean ± S. Error
Mean ± S. Error
Mean ± S. Error
Honey bee pollination
1270 ± 3.12
2632 ± 13.77
67.26 ± 0.21
1026 ± 4.52
2365 ± 9.35
59.94 ± 1.23
Without pollination
1096 ± 5.26
2135 ± 5.22
47.97 ± 0.74
893 ± 10.54
1792.60 ± 11.20
49.66 ± 4.94
Similarly, at a distance of 500 m, the total number of pods per plant were significantly higher in the case of bee-pollinated B. napus plants in comparison to without insect-pollinated plants (F (1,4) = 50.50, P = 0.002). The mean number of pods per plant was 1026 ± 4.52 in the bee-pollinated plant while 893 ± 10.54 pod in without insect pollination.
3.4.2 Total number of seeds per 100 pods
The number of seeds per 100 pods were significantly higher in bee-pollinated plants compared to without bee pollination (F (1, 4) = 14276.53, P = 0.001). The number of seeds per 100 pods was 2632 ± 13.77 in bee-pollinated flower whereas 2135 ± 5.22 in the net without pollination (Table 3).
At 500 m distance, the number of seeds per 100 pods were significantly different in bee-pollinated plants as compared to without bee pollination (F (1, 4) = 245.25, P0 = 0.001). The number of seeds per 100 pods (2365 ± 9.35) in bee-pollinated flower while net caged plants without bee pollination (11.20 ± 17.92).
3.4.3 Weight of seeds per 10 plants
Seed weight per 10 plants was significantly higher in managed pollination of A. mellifera at distance of 250 m as compared to plants covered with net (F (1, 4) = 976.72, p = 0.001). Means seed weight per 10 plants obtained from A. mellifera pollinated plants were (67.26 ± 0.21 g) whereas without pollination was (47.97 ± 0.74 g).
Seed weight per 10 plants was significantly higher in managed pollination of A. mellifera at a distance of 500 m as compared to plants covered with net (F (1, 4) = 8.22, P = 04). Means seed weight per 10 plants obtained from A. mellifera pollinated plants were (59.94 ± 1.23 g) while without pollination was (49.66 ± 4.94 g) (Table 3).
4 Discussion
In the present study, it was aimed to identify all available pollen sources during the flowering period of the Brassica crop. Result revealed that bees collected pollens from the availability of various floral resources. Among them, B. napus was the main crop, however, honeybees also collected pollen from different ornamental plants, weeds, shrubs, and herbs. It was observed that the foraging activity of honeybee was slow at the start of the blooming period. Honeybee foraging activities increased during the flowering period of crops. Pollen and nectar quantities were high in the blooming period. This basic information could be provided to the beekeepers to inform them about the main crop and other pollen sources.
It was evident from pollen quantification, A. mellifera foragers collected maximum pollens from B. napus flowers. Natural plantation such as weeds can fulfil pollen requirements of honeybees. In this study, six weeds including T. officinalis, C. procera, C. annuum, C. intybus, C. arvensis, and S. halepense were reported as the bee supporting flora. Wild blooming plants and other natural vegetation can serve as an alternate food source for A. mellifera. The bulk of diet was taken from weeds during the mass flowering periods (33.33%) and is therefore thought to play a key role during flowering periods. Forager bees visited on different floral resources due to seasonal availability of resources, the presence of brood, and the amount of stored food in the hive (Camazine, 1993; Bilisik et al., 2008). The pollen preference may depend upon the interaction of macronutrients rather than the signal nutrients (Ghosh et al., 2020). The pollen identification and quantification were also useful to determine the geographical and botanical origin of the honey.
The results also highlighted that the foraging rate of A. mellifera on B. napus was significantly more at 12:00 PM followed by 14:00 PM and 10:00 AM at weekly intervals, respectively. Ghosh et al. (2020) found similar results that foraging activity of honeybee was highest on Brassica crops during 12:00 PM of the days. The amount of pollen collection was higher in the afternoon as compared to the morning. Similarly, Pernal and Currie (2001) reported that the foraging rate of A. mellifera was higher in the afternoon than in the morning. Yucel and Duman (2005) documented that forager bees had more foraging activity and pollen collection in onion crop from 11:00 AM to 12:00 PM of the days. In another study reported similar results that the foraging rate of the honeybee was higher afternoon than in the morning (Shakeel et al., 2019; Saleh et al., 2021a, 2021b). Khan et al. (2021) found that highest number of bees with pollen entered into the hive at 12:00 PM of the day.
Moreover, the present results suggested that the total number of pods per plant, total number of seeds per 100 pods, and weight of seeds per 10 plants were significantly higher in bee-pollinated flower plants than without the bee-pollinated plants. Tara and Sharma (2010) compared the qualitative and quantitative effects of pollination on controlled (covered) and open-pollinated plants of B. campestris. Their results indicated that the fruit set was higher in open-pollinated (88.10%) as compared to controlled (80.00%) plots. The mean number of seeds per pod in open-pollinated plants was 11.2 whereas in controlled pollinated plants was 10.2. Similarly, the mean weight of 100 seed was higher in open-pollinated plants (0.42 g) as compared to controlled pollinated plants (0.17 g). Goswami and Khan (2014) found similar results that open pollination increased the number of pods (142.83) and percent pod set (83.42) as compared to the number of pods (96.64) and percent set (62.80) in caged mustered. Shubharani et al. (2013) found that average yields of canola were 189.3 ± 1.7 pods per plant in bee-pollinated plots whereas 142.2 ± 2.4 pods per plant in the covered plots. Present results are also in accordance with the findings of Stanley et al. (2013) who revealed significant differences between open-pollinated and covered canola for three yield parameters (i.e., number of seeds per pod, the weight of 1000 seeds and total yield of seeds). Similarly, Thakur (2005) reported that the highest number of pods per plant (495) in A mellifera pollinated plants, (438) in A cerana (417) in open-pollinated plants whereas covered plants without pollinators produced (290) pods per plant. We could suggest that the managed bee colonies play critical role to ensure continued provision of pollination services to Brassica crop.
5 Conclusions
The results indicated that A. mellifera foraged efficiently on a total of 18 plants species belonging to 11 families during the blooming period of B. napus. Asteraceae family provided six plants species as pollen sources, whereas Solanaceae, Malvaceae, Fabaceae, and Rosaceae provided two plant species with each. Brassicaceae, Convolvulaceae, and Poaceae families provide one floral pollen source with each. Moreover, within18 identified plant species, 6 species belonged to weeds, four species belonged to herbs and shrub each, and 2 belonged to crops and ornamental plants each, respectively. The maximum foraging rate of A. mellifera was observed at 12:00 PM followed by 14:00 and 10:00 AM on B. napus at weekly intervals. Furthermore, the total number of pods per plant, total number of seeds per 100 pods and weight of seeds per 10 plants have differed significantly in bee-pollinated plants as compared to without bee-pollinated plants. However, further study is needed to investigate the difference of pollen sources preference of A. mellifera. The authors acknowledge Muhammad Amjad Bashir, for continuous assistance in research trials.
Acknowledgments
The authors extend their appreciation to the Scientific Research Deanship at King Khalid University and the Ministry of Education in KSA for funding this research work through the project number IFP-KKU-2020/5.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of native pollinator communities on the physiological and chemical parameters of loquat tree (Eriobotrya japonica) under open field condition. Saudi Journal of Biological Sciences 2021
- [Google Scholar]
- Effect of harvest season on the nutritional value of bee pollen protein. PLoS ONE. 2020;15(12):e0241393
- [Google Scholar]
- Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum) Saudi Journal of Biological Sciences. 2018;25(7):1291-1297.
- [Google Scholar]
- Seasonal variation of collected pollen loads of honeybees (Apis mellifera L. anatoliaca) Grana. 2008;47(1):70-77.
- [Google Scholar]
- Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia. 2012;169(4):1025-1032.
- [Google Scholar]
- The regulation of pollen foraging by honey bees: how foragers assess the colony's need for pollen. Behav. Ecol. Sociobiol.. 1993;32(4):265-272.
- [Google Scholar]
- Determinants of nectar production in oilseed rape. J. Apic. Res.. 2016;55(1):89-99.
- [Google Scholar]
- Insect Pollination of Crops. Academic Press; 1993.
- Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ.. 2020;44(1):4.
- [Google Scholar]
- Impact of honey bee pollination on pod set of mustard (Brassica juncea L.: Cruciferae) at Pantnagar. Bioscan. 2014;9(1):75-78.
- [Google Scholar]
- Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Funct. Ecol.. 2008;22(4):592-598.
- [Google Scholar]
- Pollen analyses for pollination research, acetolysis. J. Pollinat. Ecol.. 2014;13(21):203-217.
- [Google Scholar]
- Techniques for Pollination Biologists. University Press of Colorado; 1993.
- Foraging behavior of western honey bee (Apis mellifera) in different time intervals on Brassica campestrisc L. Fresenius Environ. Bull.. 2021;30:2606-2607.
- [Google Scholar]
- Diversity of pollinators and their role in the pollination biology of chickpea, Cicer arietinum L. (Fabaceae) J. Asia-Pac. Entomol.. 2019;22(2):597-601.
- [Google Scholar]
- Pollination biology of Albizia lebbeck (L.) Benth. (Fabaceae: Mimosoideae) with reference to insect floral visitors. Saudi J. Biol. Sci.. 2019;26(7):1548-1552.
- [Google Scholar]
- The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie. 2012;43(4):449-464.
- [Google Scholar]
- Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun.. 2019;10(1):1-12.
- [Google Scholar]
- Histochemical characterisation of unextractable seed coat pigments and quantification of extractable lignin in the Brassicaceae. J. Sci. Food Agric.. 2004;84(3):251-262.
- [Google Scholar]
- Variability in nectar and pollen production in flowers of double–low lines of white mustard (Sinapis alba L.) and their attractiveness to honey bees. Acta Sci. Pol. Hortorum Cultus. 2014;13(5):197-209.
- [Google Scholar]
- Minfal (2014). Agriculture statistics of Pakistan. Ministry of Food, Agric, and Livestock (MINFAL), Food and Agriculture Division (Economic Wing), Islamabad, Pakistan. pp. 36–37.
- The value of honey bees as pollinators of US crops in 2000. Bee Culture. 2000;128(3):1-15.
- [Google Scholar]
- Onion flowers anthesis and insect pollinators preferences on onion (Allium cepa L.) Crop. Fresenius Environ. Bull.. 2021;30:2580-2585.
- [Google Scholar]
- Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. J. Japanese Botany. 1935;7(7):389-452.
- [Google Scholar]
- The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.) Behav. Ecol. Sociobiol.. 2001;51(1):53-68.
- [Google Scholar]
- Saleh, M., Bashir, M.A., Khan, K.A., Mahmood, R., Sarwar, G., Rafiq, K., et al. (2021). onion flowers anthesis and insect pollinators preferences on onion (Allium Cepa L.) CROP.
- Schmid, R., 1995. Erdtman's Handbook of Palynology. JSTOR.
- Insect pollinators diversity and abundance in Eruca sativa Mill. (Arugula) and Brassica rapa L. (Field mustard) crops. Saudi J. Biol. Sci.. 2019;26(7):1704-1709.
- [Google Scholar]
- Pollen morphology of selected bee forage plants. Global J. Bio-Sci. Biotechnol.. 2013;2(01):82-90.
- [Google Scholar]
- Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants. 2020;6(1):34-45.
- [Google Scholar]
- Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: ecological and economic incentives for pollinator conservation. J. Insect Conserv.. 2013;17(6):1181-1189.
- [Google Scholar]
- Role of honeybees and other insects in enhancing the yield of Brassica campestris var. sarson. Halteres. 2010;2:35-37.
- [Google Scholar]
- Impact Of Insecticides And Mode Of Pollination On Yield Components Of Brassica Camprestris With Assessment Of Insecticidal Toxicity Influencing Behaviour Of Apis Mellifera L. Pantnagar: Govind Ballabh Pant University of Agriculture and Technology; 2005.
- Regional patterns of gene flow and its consequence for GM oilseed rape. Monogr.-British Crop Protect. Council 1999:95-100.
- [Google Scholar]
- Dissection of the genetic architecture of three seed-quality traits and consequences for breeding in Brassica napus. Plant Biotechnol. J.. 2018;16(7):1336-1348.
- [Google Scholar]
- Effects of foraging activity of honeybees (Apis mellifera L.) on onion (Allium cepa) seed production and quality. Pak. J. Biol. Sci.. 2005;8(1):123-126.
- [Google Scholar]







