Translate this page into:
Plastics degradation by microbes: A sustainable approach
⁎Corresponding author. rehman.mmg@pu.edu.pk (Abdul Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Plastics play an important part in every sector of economy all over the world due to their extensive use in agriculture, building and construction, health and consumer goods. They are the backbone of many industries because they are used in the manufacturing of different products including defense materials, sanitary wares, tiles, plastic bottles, artificial leather and different other household items. Plastics are also used in packaging of food items, pharmaceuticals, detergents, and cosmetics. Excessive use of plastics poses a serious threat to the ecosystem and human life on the planet. Plastics accumulation on land and sea has aroused interest to degrade these polymers. There is a need to use adequate biodegradable methods in order to reduce plastics burden from the environment. In order to overcome plastics associated environmental problems, understanding of the interaction between microbes and polymers is of prime importance. Many living organisms but predominantly microorganisms have evolved strategies to survive and degrade plastics. The present review focuses on the types of plastics on the basis of thermal and biodegradable nature, degradation and biodegradation types, types of degradable plastics, characterization of biodegradation, and factors affecting biodegradation. Plastic degradation and bioremediation potential make these microorganisms propitious for green chemistry to eliminate harmful plastics from the ecosystem.
Keywords
Plastic types
Toxicity
Degradation
Microorganisms
Bioremediation
1 Introduction
From different hydrocarbons and petroleum derivatives high molecular weight organic polymers are obtained. These polymers are known as plastic (Ahmed et al., 2018). The word “plastic” derived from the Greek word “Plastikos”, that means which can be molded into different shapes. Plastics stated as the polymers which start moving on heating so can be casted into moulds (Kale et al., 2015). Generally, plastic materials are derived from petrochemicals except biodegradable bioplastic (Akmal et al., 2015; Getachew and Woldesenbet, 2016). Plastic consists of chloride, oxygen, hydrogen, carbon, silicon and nitrogen. Polyethylene consists of 64% of total plastic and its general formula is CnH2n (Kale et al., 2015).
For packaging and many other purposes like agricultural films formation, diaper packaging and fishing nets plastics are used. Plastics play an important part in every sector of economy all over the world. In highly growing areas i.e. agriculture, building and construction, health and consumer goods, plastics use ensures that they are in high demand and without plastics no one can do work. Plastics, the backbone of many industries, are used in manufacturing of various products that are used in our daily life i.e. defense materials, sanitary wares, tiles, plastic bottles, artificial leather and different other household items. Plastics are also used in packaging of food items, pharmaceuticals, detergents and cosmetics (Thakur, 2012; Piergiovanni and Limbo, 2016).
One of the rapidly growing fields in global industry is the production of synthetic plastics. Plastics are more superior than other materials due to their unique properties. These properties have been led to increase the plastic production scale to 20 folds since 1964 (Ellen MacArthur Foundation 2016), and production scale exceeds 300 million tons/year (Plastics Europe 2015) in 2015 it reached to 335 million tons (Plastics Europe 2017) (Urbanek et al., 2018). There are advantages and disadvantages of plastics. Plastics are strong, durable, and light weight. On the other hand, they are harmful to the natural environment, resistant to degradation and leading to environmental pollution. On our planet, plastics pose a serious threat by accumulating in large quantities (Ahmed et al., 2018; Yang et al., 2020; Al-Thawadi, 2020).
Plastics can be differentiated into degradable and non-degradable polymers on the basis of their chemical properties (Ghosh et al., 2013). Plastics that are obtained from renewable resources are biodegradable plastics. These are naturally degradable, as a source of cellulose, starch and algal material, an important component in plants, animals and algae. These polymers are also produced by microorganisms. Non-degradable plastics, typically known as synthetic plastics, are derived from petrochemicals and are higher in molecular weight due to the repetitions of small monomer units (Imre and Pukánszky, 2013).
During plastic degradation the generation of plastic particles with a size of < 5 mm are known as microplastics (MPs) which lead to potential ecotoxicological effects (Zhang et al., 2017; Chen et al., 2020a; 2020b; Wong et al., 2020). Fibrous MPs may be inhaled, may persist in the lung, and along with associated contaminants including dyes and plasticizers could lead to health effects like carcinogenicity and mutagenicity (Gasperi et al., 2018; Wong et al., 2020). Generally, it is accepted that plastic waste can permanently be eliminated through incineration. However, unburned material still exists in the bottom ash in the form of a solid residue from incinerators that can produce 360 to 102,000 microplastic particles per metric ton after incineration. This bottom ash is a potential source of MPs released into the environment (Yang et al., 2021). It is reported that plastic fragments in the <100 nm size range, referred to as nanoplastics (NPs), may also be formed in the aquatic environment and may cause potential health effects (Nolte et al., 2017; Revel et al., 2018).
Suman et al. (2020) reported that the histopathology analysis indicated the deformation of epithelial cells in the midgut region after both chronic and acute exposures at 1 and 100 mg/L, respectively to polystyrene microplastics. In another study, Chen et al. (2020a; 2020b) reported that redclaw crayfish (Cherax quadricarinatus) were exposed to different concentrations (0, 0.5, and 5 mg/L) of 200 nm-sized polystyrene microspheres for 21 days and the microplastics were distributed in the intestines and hepatopancreas after ingestion and inhibited the growth of Cherax quadricarinatus. Xiao et al. (2020) reported that freshwater microalgae, Euglena gracilis, exposed to 1 mg/L of polystyrene microplastics (PS-MPs) for 24 h. The vacuoles of microalgae were induced and pigment contents were reduced significantly (p < 0.05).
Plastics can be degraded in the environment by 4 mechanisms i.e. hydrolytic degradation, photodegradation, thermo-oxidative degradation and biodegradation (Webb et al., 2013). Plastics posed serious threats to our environment and their removal from the environment is imperative. The plastics that are degraded by microorganisms are known as biodegradable plastics and microorganisms can degrade them into H2O and CO2 (Nakajima-Kambe et al., 2009). The rate of biodegradation of polymers can be increased by using thermo-oxidant and photo-degrading agents (Mahdiyah and Mukti, 2013).
Free radicals cause rupturing of the chains by oxidizing the polymeric molecules. Many physical and chemical changes occur due to photo oxidation including reduction in polymers molecular weight and production of carbonyl groups. In thermal oxidation high temperature more than melting point is given which decreases the fusion heat and increases level of carbonyl group production. As a result, polymers are more liable to be degraded by microorganisms (Manzur et al., 2004). Phase separation, erosion, discoloration treatment types, cracking and types of polymers are the various factors that are responsible for the biodegradation and source of pollution in the environment (Thomas et al., 2015).
As most of the reviews are on fates of plastics/microplastics including transportation, toxicity, and risk assessment to humans with reference to terrestrial and marine ecosystems (Nolte et al., 2017; Revel et al., 2018; Chen et al., 2020a; 2020b; Wong et al., 2020). The present review describes plastic classification, plastic types, degradation types, biodegradation and mechanism of biodegradation. This review focuses on microbial degradation of plastic/microplastics which has received less attention as compared to the toxicity of plastic/microplastics in terrestrial and marine environments to eradicate plastic pollution from the ecosystem Geyer et al. (2017).
2 Plastic classification on the basis of thermal properties
On the basis of thermal properties plastics are divided into two classes, i.e. thermoplastics and thermosetting polymers. By the polymerization of small molecules, plastics can be synthesized.
2.1 Thermo-plastics
It is a type of plastic that can be molded for several times but on heating it cannot undergo any chemical change in its composition. Examples of thermo-plastics are polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), polystyrene (PS) and polytetrafluoroethylene (PTFE). These plastics are ranges from 20,000 to 500,000 amu (atomic mass unit) in molecular weight and are generally known as common plastics. Macromolecules are arranged in linear manner in the form of chain in which atoms and molecules are attached end to end in carbon chains. By opening of double bond that is required to form linear macromolecules and the reaction is proceeded by free radical mechanism. This type of polymerization is known as addition polymerization and examples include are polypropylene and polyethylene.
2.2 Thermosetting polymers
Another type of plastic is the thermosetting plastic in which plastic once melt and casted into a certain shape, after solidification it cannot be melted and modified again. All chemical changes, irreversible, are not examples of thermosetting polymers (Ghosh et al., 2013). Polyurethanes and phenol–formaldehyde under favorable conditions are formed by step growth polymerization. At each step, H2O and HCl are released as a by products and allowing the condensation of bi-functional molecules inter molecularly. In thermosetting plastic, monomers convert themselves into an infusible mass by undergo small chemical changes on heating (Singh and Sharma, 2008).
3 Types of degradable plastic
Plastics that are easily degradable can be divided into four types: Photodegradable bioplastics, bio-based bioplastics, compostable bioplastics and biodegradable bioplastics.
3.1 Photodegradable bioplastics
In this type of plastic, the groups that are connected to the polymer backbone are light sensitive. By giving long time exposure of UV radiation, the polymeric structure can be disintegrated. When radiation supply stopped then degradation is not possible. Landfills lack sunlight so plastics in landfill are not degradable (Arikan and Ozsoy, 2014). Artificial photo-degradation can lead to the release of toxic volatile organic compounds (VOCs) which are potentially hazardous and associated with the environmental weathering of plastic debris (Lomonaco et al., 2020).
3.2 Bio-based bioplastics
Types of plastics in which 100% of carbon is obtained from renewable resources, like forestry and agricultural resources are known as bio-based plastics. Starch, corn, soybean and cellulose are the examples of these resources (Getachew and Woldesenbet, 2016; Marichelvam et al., 2019; Maraveas, 2020).
3.3 Compostable bioplastics
In composting process, requires a specific setting in order to break down whereas biodegradable products break down naturally, the plastics are decomposed biologically without leaving any toxic material (Meereboer et al., 2020). The rate of composting of this plastic is similar to the other compostable material. Plastic is designated as bio-compostable, by taking into account its total biodegradability, ecological toxicology and its disintegration degree by standardized testing.
3.4 Biodegradable plastics
Plastics that are degraded by action of microorganisms are known as biodegradable plastics. Biodegradable is a term that is used for the materials that are disintegrated into biogases and biomass by the action of microorganisms (Jain et al., 2010).
4 Types of degradation
4.1 Photo-oxidative degradation
The primary source of polymers damage is light. This process is started by light absorption and examples of this degradation process are photodegradation and photo-oxidation (Rånby, 1989). Synthetic polymers are prone to be degraded by processes that are initiated by ultraviolet (UV) radiations. The lifetime of polymeric material, used for various applications, is determined by UV radiations ranging from 290 to 400 nm and sunlight is the source of such radiations (Jensen and Kops, 1980). Photo-irradiation produces ester, aldehyde, propyl and format groups at the soft segments of polymers where degradation occurs. The C–C bonds are easily cleaved by UV radiations (Nagai et al., 2005).
4.2 Thermal degradation
Normally, thermal and photochemical degradation are considered as similar processes and both are classified as oxidative processes or oxidative degradation. The first difference is in the sequence of initial steps while the second difference is in the site of reaction. In thermal degradation, reactions occur on the whole part of the polymer while in photochemical degradation reactions occur only on the polymer surface (Tyler, 2004). Thermal degradation takes place by accidental or depolymerization reaction. For its initiation, temperature and UV light are required (Teare et al., 2000). Due to imperfections the bonds (peroxide /ether link) present in the chain become weak and depolymerization usually starts at such weak bonds. At high temperature, a large amount of polymers is depolymerized e.g. PE is decomposed at high temperature and produces small monomers. Polymethylmethacrylate (PMMA) can also be converted quantitatively back to monomers (Ramis et al., 2004).
4.3 Ozone degradation
Ozone normally present in the atmosphere causes polymeric degradation. Polymers are lasting for a longer time when oxidative processes are not active (Teare et al., 2000). Ozone in the atmosphere is present in very small amount but has a markedly great effect on polymers. Ozone degrades polymeric materials by the formation of reactive oxygen species (ROS) (Kefeli et al., 1971). These ROS are formed by the reduction in molecular weight, by change in electrical and mechanical properties of polymers (Andrady et al., 1998). When polymers are exposed to ozone then it results different types of carbonyl and unsaturated carbonyl products are formed. These products are based on ketones, lactones, esters and aromatic carbonyl. These all are further associated with another phase known as styrene phase (Allen et al., 2003). Chains in polymer that contain C–C bonds and others saturated hydrocarbon links, aromatic ring ozone reactions occur. During these reactions, intermediates (bipolar ions/peroxy radicals) are formed that are unstable and cause the degradation of large molecules or polymers.
4.4 Mechanochemical degradation
It involves polymer chains breakdown under the mechanical stress and ultrasonic irradiations (Gol'Dberg and Zaikov, 1987; Li et al., 2005). Due to chain-side radical reaction, branches in long chains are increased in numbers. The width of weight distribution function of molecules is decreased (correlation between crosslinks and ruptures), double bond concentration is also changed (Striegel, 2003). Nitroxide molecules work as chain terminating agents in mechanochemical degradation of polymethylmethacrylate (PMMA) and produces radicals that are known as macro radicals. These radicals are used in polymerization reaction (which is free radical polymerization reaction) (Schmidt-Naake et al., 2002). In air, the molecular weight of polyvinyl chloride is reduced by mechanochemical dichlorination with different oxide powders e.g., SiO2, CaO, Al2O3 and Fe2O3 (Inoue et al., 2004).
4.5 Catalytic degradation
Catalytic waste polymers transformation into hydrocarbons is a field of great interest. Catalytically degraded polyolefins produce oils and gases. By using this degradation method, not only quality of obtained products (obtained after pyrolysis of plastics) has been improved but it also provides an opportunity to achieve the desired products. Different types of catalysts used for polymers degradation have been reported e.g. Pt-Mo, Pt-Co maintained by SiO2 (Gimouhopoulos et al., 2000), transition metal catalysts (chromium, nickel, molybdenum, cobalt and ferrous) with provision of Al2O3 and SiO2 (Williams and Bagri, 2004), zeolite catalysts and non-zeolite catalysts (Lin and Yen, 2005), zeolite (Kim et al., 2004). The degradation mechanism for polypropylene (PP) is a free radical mechanism, in which Fe/activated carbon used as a catalyst (Sekine and Fujimoto, 2003). In catalytic degradation, when polymers are heated above 38 °C, their depolymerization take place, and they are degraded by free radical chain reactions (Wall et al., 1954).
5 Biodegradation
In a material, any physical and chemical change that is caused by the action of microorganisms is known as biodegradation. Natural and synthetic plastics are degraded by the action of microorganisms including bacteria, actinomycetes, and fungi (Ishigaki et al., 2004; Alshehrei, 2017).
5.1 Aerobic biodegradation (aerobic respiration)
In this type of degradation, microorganisms break down large organic compounds into smaller compounds by using oxygen as an electron acceptor (Fig. 1). By-products of this process are carbon dioxide and water (Müller, 2005; Priyanka and Archana, 2011).
Carbon plastic + Oxygen → carbon dioxide + water + Carbon residual
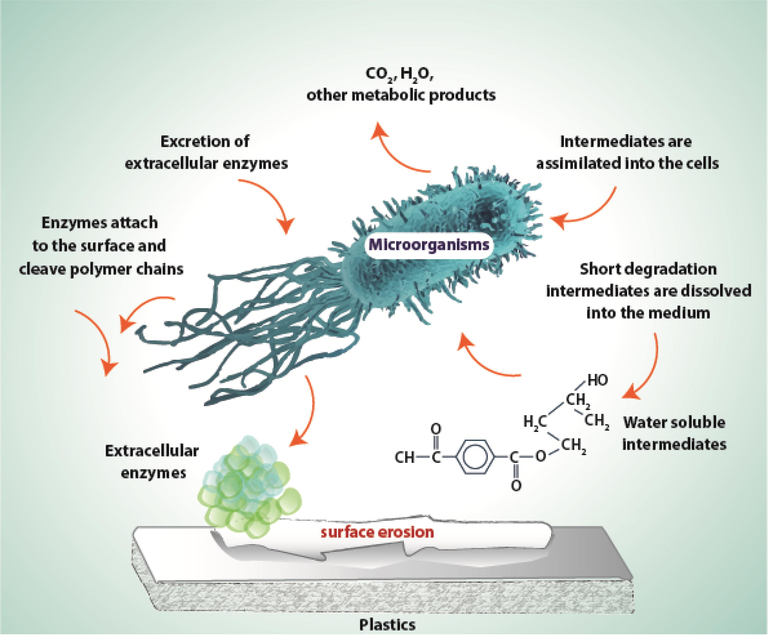
General mechanism of plastic degradation under aerobic conditions (Müller, 2005).
5.2 Anaerobic biodegradation
In anaerobic biodegradation, oxygen is not necessary for the breakdown of compounds by the action of microorganisms. Oxygen is an important component for the natural attenuation of contaminants at sites of hazardous waste. Anaerobic bacteria use nitrate, iron, sulphate, manganese and CO2 as an electron acceptor in place of oxygen to break down large organic compounds into smaller compounds. Carbon (plastic) → methane + carbon dioxide + water + Carbon residual
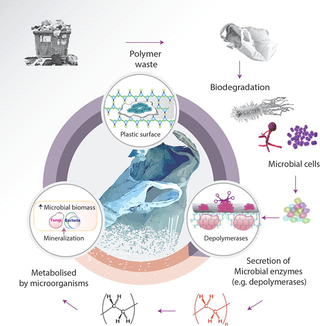
All polymers are not directly transported into the cells of microorganisms through their cell walls because they are large in their size and are not water soluble. Microorganisms can use these polymers as a source of energy by secreting extracellular enzymes. Polymers are depolymerized by these enzymes outside the bacterial cells. Enzymes play their role in polymers biodegradation both by intra-cellularly and extra-cellularly. Depolymerization and mineralization are the two processes that are involved in biological degradation of plastic polymers.
Exoenzymes, extra-cellularly secreted enzymes, break down the large polymers and produce small molecules that are small enough and water soluble. These molecules can pass semipermeable bacterial membrane and utilized as source of energy. The process in which large polymers are broken down is known as depolymerization while the process in which the end products are inorganic species like H2O, CH4, CO2 is known as mineralization (Gu, 2003). In case of aerobic environment, only production of H2O, CO2, and microbial mass as an end products was recorded, whereas under anaerobic/methanogenic and sulfidogenic conditions, in addition to these three key components, CH4 and H2S were recorded as the extra end products (Fig. S1) of the polythene (Shahnawaz et al., 2016).
6 Mechanism of biodegradation
Biodegradation of polymers consists of three steps; (a) microorganism attachment on the surface of polymer, (b) utilization of polymer as a source of carbon, and (c) polymer degradation. Microorganisms attach to the surface of polymers and degrade these polymers by secreting enzymes in order to obtain energy for their growth (Danso et al., 2018). Large polymers degraded into monomers and oligomers that are low molecular weight molecules. Some oligomers may be assimilated in the internal environment of microorganisms after diffusing inside them (Fig. 2).
Mechanism of enzymatic biodegradation of polymers (Alshehrei, 2017).
7 Analytical procedures for biodegradation
A wide variety of methods is currently available for measuring the biodegradability of polymeric materials (Yang et al., 2005). Several test methods to assess the potential biodegradability of plastics have been developed by International Standard Organization (ISO) and American Society for Testing and Materials (ASTM) (Piergiovanni and Limbo, 2016) including gas chromatography/mass spectrometry (GC–MS), stereomicroscopy and micro-Fourier transform infrared spectroscopy (µ-FTIR) (Lomonaco et al., 2020; Corami et al., 2020). Biodegradation can be characterized with loss of weight, change in tensile strength, change in dimensions, change in chemical and physical properties, carbon dioxide production, bacterial activity in soil and change in molecular weight distribution (Kathiresan, 2003; Sivan, 2011; Kumar and Maiti, 2016; Chen et al., 2020a; 2020b).
8 Polymers degradation by microorganisms
Microbial polymers degradation includes biodeterioration, bio-fragmentation, mineralization, and assimilation.
8.1 Biodeterioration
It is the process that affects the surface of plastics and changes their chemical, physical and mechanical properties. All chemical and structural changes depend upon the structure and composition of polymers. Environmental conditions also influence change in polymers properties. Substrate formation inside the plastic and biofilm formation both are due to the process of deterioration (Vivi et al., 2019).
8.2 Bio-fragmentation
After biodeterioration, the next step is bio-fragmentation that involves the enzymatic action on plastic polymers. Oxygenases, mostly contained enzymes in bacteria, have the ability to break oxygen molecules are added in the carbon chains and as a result, alcohol and peroxyl products are formed that are less harmful (Pathak, 2017; Dussud and Ghiglione, 2014). Furthermore, the transformation process of carboxylic groups is catalyzed by lipases and esterases or by endopeptidases for amide groups.
8.3 Mineralization
Plastic polymers that are formed in the bio-fragmentation process enter in the microbial cells through cell membranes. Monomers that are large in size cannot enter inside the cells and stay outside. The small monomers that moved inside the cells are oxidized and used for the energy production. This energy eventually is utilized for biomass production (Lucas et al., 2008; Kale et al., 2015).
8.4 Assimilation
In the assimilation process, atoms are integrated in the microbial cells for complete degradation. Secondary metabolites are transported outside the cells or transfer to other microbes that further perform degradation and use these metabolites (Fig. 3). The oxidized products i.e. CO2, N2, H2O and CH4 are released during metabolites degradation (Krzan et al., 2006; Lucas et al., 2008).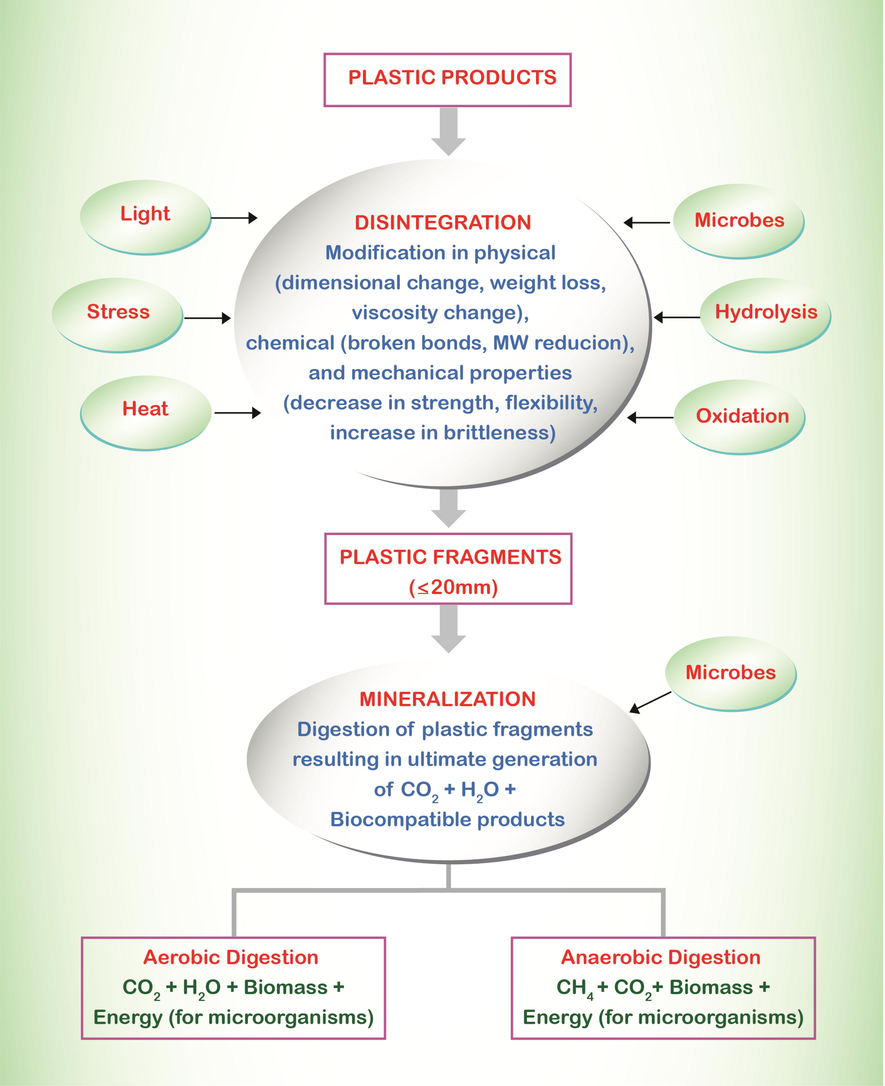
Schematic representation of plastic degradation (Krzan et al., 2006).
9 Enzymatic degradation of plastics
Due to the absence of hydrolysable groups in the carbon–carbon backbone, the degradation of plastic by microbial enzymes is a very difficult task. The reduction of molecular weight is the first step that is achieved by the action of both biotic and abiotic factors. By UV light exposure, the carbonyl group of polymer is easily attacked by microbial enzymes (Leja and Lewandowicz, 2010; Novotný et al., 2018). For polymers degradation different enzymes are used e.g. laccase, manganese-dependent enzymes (lignin degrading enzymes), urease, lipase, and protease. Thermostable laccase can degrade the polyethylene (PE) in 48 h of incubation at 37 °C (Jaiswal et al., 2019).
9.1 Characterization of plastic biodegradation
All polymers are not dissolved in water but water soluble polymers are easily degraded and converted into alcohols, ketones, and acids. There are some points by which plastics biodegradation can be monitored (Bhardwaj et al., 2013).
-
Plastic surface properties are changed.
-
Physical and mechanical properties of the plastic are changed.
-
Products are analyzed on the basis of chemical composition.
-
Oxygen consumption rate
-
Carbon dioxide evolution rate
-
Production of biomass which shows the microorganisms utilizes the plastics as the carbon source for their growth.
9.2 Factors affecting biodegradation
Plastic biodegradability can be determined by following physical and chemical properties. There are following factors that affect plastic degradation by microbes.
-
Functional groups availability by which hydrophobicity is increased (Wang et al., 2020).
-
Complexity in structure i.e. linear/branched (Tokiwa et al., 2009)
-
Bond type bonds are easily breakable like amide bonds and ester bonds. Coupling in chain (ester > ether > amide > urethane) (Shams et al., 2020).
-
Composition on the base of molecules (Shams et al., 2020).
-
Form of polymer its nature and physical appearance example pellet, films, powder (Kawai, 1995)
-
Polymer density and its molecular weight (Tokiwa et al., 2009)
-
TM morphology: amount of region amorphous region and crystalline region (Wang et al., 2021).
-
Toughness polymers which are soft degrade faster than those that are hard or tough ones (Swift, 1993)
Degradation ability of microorganisms is reduced when solubility of polymers is decreased. Plastics are less vulnerable to microbial attack by decreasing their solubility. They are adapted to microbes through their cell membrane (Siracusa et al., 2008). Amorphous nature of polymers is more vulnerable to microbial enzymes attack than the crystalline nature. So, increase in crystallinity decreases polymer degradation (Slor et al., 2018). In a hydrophobic environment, plastics can restrict microbial activity by inhibiting the process of water absorption.
10 Polyethylene degradation
Plastic is the most used material for food wrapping and is basically made of PE material (Agustien et al., 2016). Shopping bags are PE bags. These bags are composed of PE and 10% of the municipal waste, all over the world, is due to the excessive use of plastic material which is mainly PE (Begum et al., 2015). Usage of PE bags all around the globe is about 500 billion to one trillion annually. The plastic accumulation in the terrestrial environment or in the sea coast is about 25 million tons every year (Madhu et al., 2014).
PE is chemically inert and hydrophobic in nature and microorganisms have no appropriate mechanism to digest these synthetic plastics (Yoon et al., 2012). PE polymers are used by microorganisms as a substrate for their growth. Erosion, discoloration, cracking and phase separation are the indicators of PE degradation (Trivedi et al., 2016; Agustien et al., 2016).
PE degradation is further classified into two classes: abiotic and biotic. In abiotic degradation all natural factors like temperature, ultraviolet rays cause degradation of PE while in case of biotic degradation microorganisms are involved that consume the plastics by changing their properties (Sen and Raut, 2015). As PE is safe, cheap, harmless and stable in the environment, and is easy to proceed, it is one of the polymers that are mostly seen all over the world. The two possible ways by which PE usefulness is maintained in nature are to use microbes in order to degrade polymers or PE. The second is to make polymers artificially that are prone to degradation by microorganisms (Okoh and Atuanya, 2014).
Polyolefins, low density PE, are unreactive in their chemical nature. For a shorter period 95 °C is used while for a longer time it may be used at 80 °C (Billmeyer, 1984). Due to incomplete crystallinity that ranges in 50–60%, there are several properties of low-density polyethylene (LDPE) such as rigidity, tensile strength, flexibility and tear strength (Ferreira et al., 2005). The carbonyl group, generated in polyethylene oxidation, is used by microorganisms for its degradation (Cornell et al., 1984; Awasthi et al., 2017).
The oxidative degradation mechanism, used for non-hydrolysable polymers e.g. polyethylene and polypropylene, leads to loss in molecular weight of polymers. Several oxidative enzymes are involved in oxidation of ethylenic groups; these enzymes are monooxygenase, peroxidase, manganese, peroxidase, dehydrogenase and oxidase. By the action of extracellular and intracellular enzymes, polymers convert into oligomers and monomers that are utilized by microorganisms for a source of energy (Arkatkar et al., 2009). β-oxidation of fatty acids that occurs in animals and humans shows similarities with β-oxidation of polyethylene (Albertsson et al., 1987).
Microorganisms that are capable of degrading polymers have been investigated and isolated from the natural environment. Polymer materials that are used for microbial degradation e.g., polyethylene and polypropylene (Park and Kim, 2019). Polymer degrading microbial species that are associated with degradation were identified as Streptococcus, Klebsiella, Micrococcus Staphylococcus, Pseudomonas (Das and Kumar, 2015). Biodegradability of polyethylene is enhanced by blending polyethylene with different additives, by adding these additives auto-oxidation of polyethylene enhances, by which molecular weight of polymer reduces and microorganisms then easily degrade these low molecular weight polymers (Fig. 4).
Biodegradation of polyethylene. (1) Everyday use of plastic bags generates huge amounts of polymeric waste material. Shopping bags are made up of polymers of ethylene i.e., PE. (2) Certain microorganisms such as bacteria (e.g., Micrococcus sp., Staphylococcus sp.) and fungi (e.g., Mucor sp., Rhizopus sp.), produce extracellular PE degrading enzymes. (3) Depolymerases are one type of the polyethylene degrading enzymes, that can split the PE chain into macromere fragments (i.e., oligomers, dimers) which subsequently get converted into monomers i.e., ethylene. (4) Microorganisms could metabolize theses monomers (ethylene) through aerobic or anaerobic pathways and utilize them as a carbon and energy source. (5) Microorganisms utilize this energy to reproduce which results in an increase in microbial biomass.
Skariyachan et al. (2017) reported that Bacillus vallismortis bt-dsce01 was able to degrade LDPE up to 75% after 120 d of incubation. Similarly, Muhonja et al. (2018) described that both Aspergillus oryzae strain A5 and B. cereus strain A5 were able to degrade LDPE 36.4 and 35.72%, respectively after 112 d of incubation. Taghavi et al. (2021) reported that fungus, Aspergillus flavus, was capable of breakdown 5.5% HDPE within 100 days. Maroof et al. (2021) described a new bacterial strain, B. siamensis, which has the ability to degrade 8.46% LDPE after 90 d of incubation (Table 1).
Sr. #
Organism
Plastic type
Degradation time (Days)
Biodegradation efficiency (%)
Reference
1
Pseudomonas fluorescens
PE
270
18.0
Thomas et al. (2015)
2
Bacillus vallismortis bt-dsce01
LDPE
120
75.0
Skariyachan et al. (2017)
3
Klebsiella pneumoniae CH001
HDPE
60
18.4
Awasthi et al. (2017)
4
Aspergillus oryzae strain A5
LDPE
112
36.4
Muhonja et al. (2018)
5
Bacillus cereus strain A5
LDPE
112
35.72
Muhonja et al. (2018)
6
Trichoderma viride RH03
LDPE
45
5.13
Munir et al. (2018)
7
Aspergillus nomius RH06
LDPE
45
6.63
Munir et al. (2018)
8
Bacillus sp. & Paenibacillus sp.
PE
60
14.7
Park and Kim (2019)
9
Aspergillus flavus
HDPE
100
5.5
Taghavi et al. (2021)
10
Bacillus siamensis
LDPE
90
8.46
Maroof et al. (2021)
Various products are produced when polyethylene is subjected to thermo-photo oxidation. These products are ketone, aldehydes, carboxylic acids, alkanes alcohols, lactones, dicarboxylic acids and esters. Despite all these attempts, the microbial degradation of PE is still slow (Tokiwa et al., 2009; Jaiswal et al., 2020). High molecular weight of PE limitized its use as a substrate for many enzymatic reactions. There are two important reactions in the biodegradation process of PE; the first one is the loss in molecular weight and the second is oxidation.
There are two reasons behind molecular weight loss. The first reason is to make possible the molecule transport from outside into the cell through cell membrane and the second reason is to provide opportunity to the microbial enzymes to attack on the certain molecules in the cell carrying molecular weight usually in the range of 10–50 carbons. The enzymatic activity up to 2000 carbons has been reported in such degradation processes (Yoon et al., 2012). By the loss of molecular weight the size of molecule is reduced and after this oxidation is required to convert hydrocarbons into carboxylic acid.
Carboxylic acid is metabolized through β-oxidation and Krebs cycle (Restrepo-Flórez et al., 2014). The first one is to observe the microbial growth on the sample in order to check the adverse effect of polymer on microorganisms. The second one is to monitor the organism activity on polymer that causes loss in polymer integrity. Loss of integrity includes mechanical strength loss or may be reduction in average weight of molecules. In the abiotic system, there is no effect on polymers so only one system by which degradation is possible is the microbial degradation (Sudhakar et al., 2007). Polymers degradation by microbes can be assessed by using different methods. The measurement of weight reduction, tensile strength, film surface analysis by scanning electron microscopy (SEM), and carbonyl index can be ascertained through these methods. Microorganisms produce biosurfactants that help microbes to adhere to the surface of polymers and also help in the degradation of different products that are formed during oxidation (Das and Mukherjee, 2005).
Raaman et al. (2012) reported that fungal strains including Aspergillus niger and Aspergillus japonicus were evaluated for their ability to degrade LDPE and both strains were found able to degrade 8–12% PE after one month incubation. Munir et al. (2018) reported that out of 9 fungal isolates, two strains i.e., Trichoderma viride and Aspergillus nomius were used for PE film degradation. Both fungal strains were able to reduce the weight of LDPE film by 5.13% and 6.63% after 45 days of cultivation. The tensile strength of treated film was reduced significantly up to 58% and 40% by T. viride and A. nomius, respectively.
11 Biodegradable plastics types based on the mode of degradation pathway
The degradable plastics are further classified into two types based on the mode of degradation pathway. (a) Oxo-biodegradable plastics and (b) Hydro-biodegradable plastics (Mukamto et al., 2015; Vázquez-Morillas et al., 2016). The hydro-biodegradable plastics are those that are degraded through hydrolytic mechanisms (Nampoothiri et al., 2010). The examples of these plastics are cellulose, most general polyesters like polyhydroxyalkanoate (PHA), and starch. Oxo-degradation consists of two stages: the abiotic oxidation and biotic degradation (Fig. S2). In the first stage, the carbon backbone of polyolefins is oxidized abiotically into small fragments. This oxidative degradation is accelerated by thermal degradation and UV radiations but the degradation rate of the entire process is not determined exactly. In the second stage, degradation of polyolefin is taken by microbes (Liu et al, 2014; Nikolić et al., 2017; Montazer et al., 2018).
The PE polymer is changed due to addition of a carbonyl group in the polymer backbone and this change is achieved by abiotic photooxidation. Polyethylene molecule carrying carbonyl group is transformed to an alcohol in the presence of monooxygenase. The alcohol is oxidized to an aldehyde through alcohol dehydrogenase. Subsequently, aldehyde is converted into fatty acid by the action of aldehyde dehydrogenase. Finally, the fatty acid is metabolized through β-oxidation pathway (Fig. S3) (Gautam et al., 2007).
Microbes, colonized on the surface of PE, cause change in mechanical properties by increasing the roughness of PE surface, by increasing its fragility and by reduction in its molecular weight (Palmisano and Pettigrew, 1992; Watanabe et al., 2009; Nowak et al., 2011; Park and Kim, 2019). The hydrophobic surface of PE is turned to hydrophilic by the attachment of microorganisms (Sudhakar et al., 2008). The change in carbonyl index was ascertained through FTIR and ketone and aldehyde groups were formed. The CO₂ and H₂O were produced as a result of β-oxidation (Novotný et al., 2018). Gram negative bacteria including Klebsiella pneumoniae play an important role in wastewater treatment (Maal et al., 2014). K. pneumoniae secretes lipase (Peil et al., 2016), tyrosinase, peroxidase, and laccase which can be involved in PE degradation (Dhanve et al., 2008). Surfactants, released by K. pneumoniae extracellularly, play their role in hydrophilic and hydrophobic phase exchange. These phase exchanges help microbes to penetrate easily into the PE and degrade it.
11.1 Recommendations/suggestions
As plastics, due to their massive use and release outside directly, are now reaching alarming concentration levels in our environment. Various physicochemical approaches i.e. photo-oxidative, thermal, Ozone, mechanochemical and catalytic are used, although such methods are costly and are not suitable to use at low plastic concentration, to exterminate plastics from the environment to save living organisms. For plastics degradation, microbial use is now considered as an eco-friendly method as compared to the conventional methods. Some suggestions are given below to consider during microbial use against plastic degradation.
-
To degrade plastic potential of microbes including bacteria, fungi, and algae should be investigated.
-
To maintain optimum conditions of microbes for efficient plastic extermination.
-
Use of appropriate consortium of aerobic and anaerobic bacteria for more efficient plastic degradation.
-
Successive use of microbes (bacteria, fungi, and algae) can also be effective for plastic degradation
-
Use of microbial enzymes e.g. laccase, lignin degrading enzymes, urease, lipase, and protease can also be exploited to degrade plastic under aerobic and anaerobic conditions.
So the microbial consortia, their processes, and enzymes can make an effective sustainable strategy for plastic degradation.
12 Conclusions
Plastics are petroleum-derived polymers and are used for various purposes. PE bags are used all over the world at large levels. The availability of micro- and nanoplastics in aquatic environment has been increased many folds due to biodegradation, thermo-oxidative degradation, photodegradation, thermal and hydrolysis processes in the ecosystem and poses serious threat to the aquatic life (fresh and marine) and human life through food web. There is a need to use adequate biodegradable methods to eradicate these polymers from the ecosystem. Due to the hydrophobic and inert nature, it is difficult to remove or degrade polymers. Besides physical and chemical methods, microbes have shown promising potential to degrade these polymers.
The potential use of microbes for polymers removal needs to be further evaluated using original polymers contaminated wastewater. The removal of microplastics/nanoplastics, their toxicity and the utilization of microbes remain to be addressed. The transfer of plastic polymers from the waste into the aquatic ecosystem including rivers and oceans through different processes and the strategy to shift these polymers from the wastewater to a suitable place for deposition/incineration should properly be advocated. Long-term coordinated cleanup operations are needed to evaluate the progressive ecosystem effects.
Funding
No funding was received for this work from any organization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Microplastics and nanoplastics in aquatic environments: challenges and threats to aquatic organisms. Arab. J. Sci. Eng.. 2020;45(6):4419-4440.
- [Google Scholar]
- Screening polyethylene synthetic plastic degrading-bacteria from soil. Der Pharm. Lett.. 2016;8(7):183-187.
- [Google Scholar]
- Biosynthesis of copolymer poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from palm oil and n-pentanol in a 10L bioreactor. Rasayan J. Chem.. 2015;8:389-395.
- [Google Scholar]
- Influence of ozone on styrene–ethylene–butylene–styrene (SEBS) copolymer. Polym. degrad. Stabil.. 2003;79(2):297-307.
- [Google Scholar]
- Biodegradation of synthetic and natural plastic by microorganisms. J. Appl. Environ. Microbiol.. 2017;5(1):8-19.
- [Google Scholar]
- Effects of increased solar ultraviolet radiation on materials. J. Photochem. Photobiol. B. 1998;46(1–3):96-103.
- [Google Scholar]
- Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech. 2017;7(5):332.
- [Google Scholar]
- Biodegradation of polythene bag using bacteria isolated from soil. Int. J. Curr. Microbiol. Appl. Sci.. 2015;4(11):674-680.
- [Google Scholar]
- Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol.. 2020;224:105297
- [Google Scholar]
- Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health.. 2020;18:14-19.
- [Google Scholar]
- A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere. 2020;238:124564
- [Google Scholar]
- Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol.. 2018;85(19):e01095-19.
- [Google Scholar]
- Characterization of biochemical properties and biological activities of biosurfactants produced by Pseudomonas aeruginosa mucoid and non-mucoid strains isolated from hydrocarbon-contaminated soil samples. Appl. Microbiol. Biotechnol.. 2005;69(2):192-199.
- [Google Scholar]
- An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech. 2015;5(1):81-86.
- [Google Scholar]
- Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health.. 2018;1:1-5.
- [Google Scholar]
- A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotechnol.. 2007;141(1):85-108.
- [Google Scholar]
- Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes. 2016;9(1):509.
- [Google Scholar]
- Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res.. 2013;20(7):4339-4355.
- [Google Scholar]
- Optimization studies of waste plastics-lignite catalytic coliquefaction. Waste Manag. Res.. 2000;18(4):352-357.
- [Google Scholar]
- Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int. Biodeter. Biodegr.. 2003;52(2):69-91.
- [Google Scholar]
- Mechanochemical dechlorination of polyvinyl chloride by co-grinding with various metal oxides. Adv. Powder Technol.. 2004;15(2):215-225.
- [Google Scholar]
- Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov.. 2020;17:100567
- [Google Scholar]
- Microbial degradation of plastic: a review. J. Biochem. Technol.. 2015;6(2):952-961.
- [Google Scholar]
- Calytic Degradation of mixed plastics using natural clinoptilolite catalyst. React. Kinet. Catal. Lett.. 2004;81(1):73-81.
- [Google Scholar]
- Standradization and certification in the area of enivironmentaly degradable plastics. Polym. Degrad. Stabil.. 2006;91(12):2819-2833.
- [Google Scholar]
- Controlled biodegradation of polymers using nanoparticles and its application. RSC Adv.. 2016;6(72):67449-67480.
- [Google Scholar]
- Degradation kinetics of polystyrene and EPDM melts under ultrasonic irradiation. Polym. Degrad. Stabil.. 2005;89(1):6-14.
- [Google Scholar]
- Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym. Degrad. Stabil.. 2005;89(1):101-108.
- [Google Scholar]
- Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: A neglected source of environmental pollution. J Hazard. Mater.. 2020;394:122596
- [Google Scholar]
- Polymer biodegradation: mechanisms and estimation techniques. Chemosphere. 2008;73:429-442.
- [Google Scholar]
- Production of sustainable and biodegradable polymers from agricultural waste. Polymers. 2020;12(5):1127.
- [CrossRef] [Google Scholar]
- Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers. 2019;7:32.
- [Google Scholar]
- Identification and characterization of low density polyethylene-degrading bacteria isolated from soils of waste disposal sites. Environ. Eng. Res.. 2021;26(3):200167
- [Google Scholar]
- Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem.. 2020;22:5519.
- [Google Scholar]
- Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic dump soil. J. Polym. Environ.. 2018;26:3613-3625.
- [Google Scholar]
- Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE. 2018;13(7):e0198446
- [Google Scholar]
- Isolation of oxo-degradable polyethylene degrading-bacteria of Benowo landfill soil Surabaya. Microbiology. 2015;9(1):9-16.
- [Google Scholar]
- Müller R.J., 2005. Biodegradability of Polymers: Regulations and Methods for Testing. In Biopolymers Online, pp. 365–374. doi: 10.1002/3527600035.bpola012.
- Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Series: Earth and Environmental. Science. 2018;126:012145
- [Google Scholar]
- The challenges in lifetime prediction of oxodegradable polyolefin and biodegradable polymer films. Polym. Degrad. Stabil.. 2017;145:102-119.
- [Google Scholar]
- The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat Toxicol.. 2017;183:11-20.
- [Google Scholar]
- Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by Bacillus amyloliquefaciens. Int. Biodeter. Biodegr.. 2018;132:259-267.
- [Google Scholar]
- Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019;222:527-533.
- [Google Scholar]
- Piergiovanni, L., Limbo, S., 2016. Plastic packaging materials. Food Packaging Materials, pp. 33-49, Springer.
- Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around chennai. J. Acad. Indus Res.. 2012;1(6):313-316.
- [Google Scholar]
- Photodegradation and photo-oxidation of synthetic polymers. J. Anal. Appl. Pyrolysis. 1989;15:237-247.
- [Google Scholar]
- Micro(nano)plastics: a threat to human health? Curr. Opin. Environ. Sci. Health. 2018;1:17-23.
- [Google Scholar]
- Combination of mechanochemical degradation of polymers with controlled free-radical polymerization. Macromol. Chem. Phys.. 2002;203(15):2232-2238.
- [Google Scholar]
- Catalytic degradation of PP with an Fe/activated carbon catalyst. J. Mater. Cycles Waste Manag.. 2003;5(2):107-112.
- [Google Scholar]
- Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng.. 2015;3(1):462-473.
- [Google Scholar]
- Aggregation and stability of nanoscale plastics in aquatic environment. Water Res.. 2020;171:115401
- [Google Scholar]
- New perspectives in plastic biodegradation. Curr. Opin. Biotechnol.. 2011;22(3):422-426.
- [Google Scholar]
- Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ. Sci. Pollut. Res.. 2017;24:8443-8457.
- [Google Scholar]
- Influence of chain architecture on the mechanochemical degradation of macromolecules. J. Biochem. Bioph. Meth.. 2003;56(1–3):117-139.
- [Google Scholar]
- Biofouling and biodegradation of polyolefins in ocean waters. Polym. Degrad. Stability. 2007;92(9):1743-1752.
- [Google Scholar]
- Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater.. 2020;400:123220
- [Google Scholar]
- Directions for environmentally biodegradable polymer research. Acc. Chem. Res.. 1993;26(3):105-110.
- [Google Scholar]
- Degradation of plastic waste using stimulated and naturally occurring microbial strains. Chemosphere. 2021;263:127975
- [Google Scholar]
- Cellular attachment to ultraviolet ozone modified polystyrene surfaces. Langmuir. 2000;16(6):2818-2824.
- [Google Scholar]
- Degradation of plastic and polythene materials by some selected microorganisms isolated from soil. World Appl. Sci. J.. 2015;33(12):1888-1891.
- [Google Scholar]
- Role of microbes in degradation of synthetic plastics and manufacture of bioplastics. J. Chem. Pharm. Res.. 2016;8(3):211-216.
- [Google Scholar]
- Mechanistic aspects of the effects of stress on the rates of photochemical degradation reactions in polymers. J. Macromol. Sci.. 2004;44(4):351-388.
- [Google Scholar]
- Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol.. 2018;102:7669-7678.
- [Google Scholar]
- Biodegradation and ecotoxicity of polyethylene films containing pro-oxidant additive. J. Polym. Environ.. 2016;24(3):221-229.
- [Google Scholar]
- The depolymerization of polymethylene and polyethylene. J. Am. Chem. Soc.. 1954;76(13):3430-3437.
- [Google Scholar]
- Wang, J., Zhao, X., Wu, A., Tang, Z., Niu, L., Wu, F., Wang, F., Zhao, T., Fu, Z., Aggregation and stability of sulfate-modified polystyrene nanoplastics in synthetic and natural waters. Environ. Pollut. 114240. 10.1016/j.envpol.2020.114240.
- A review of microplastics aggregation in aquatic environment: influence factors, analytical methods, and environmental implications. J. Hazard. Mater.. 2021;402:123496
- [Google Scholar]
- Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ.. 2020;719:137512
- [Google Scholar]
- Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere. 2020;255:126914
- [Google Scholar]
- Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol.. 2020;104:6501-6511.
- [Google Scholar]
- Is incineration the terminator of plastics and microplastics? J. Hazard. Mater.. 2021;491:123429
- [Google Scholar]
- Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut.. 2017;220(Pt B):1282-1288.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101538.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







