Translate this page into:
Phytochemical screening of Bixa orellana and preliminary antidiabetic, antibacterial, antifibrinolytic, anthelmintic, antioxidant, and cytotoxic activity against lung cancer (A549) cell lines
⁎Corresponding authors at: Department of General Science, Ibn Sina National College for Medical Studies, Al Mahajar Street: 31906, Jeddah 21418, Saudi Arabia (S.M. Shakeel Iqubal). iashikh@nu.edu.sa (Ibrahim Ahmed Shaikh), shakeeliqubal@gmail.com (S.M. Shakeel Iqubal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Bixa orellana (B. Orellana) is a frequently utilized plant that has grown in significance in pharmaceutical applications. The leaves extract of B. orellana was used in the current study for preliminary phytochemical analysis in terms of both quantitative and qualitative, which indicates the presence of phenols, alkaloids, and flavonoids. Furthermore, the extract demonstrated antifungal activity at 30 mm zone of clearance against Candida albicans and antibacterial activity at 16 mm zone of clearance against Bacillus nakamuria by well diffusion method against different strains. The plant extract showed a MIC of 20 µg/mL and MBC of 157.11 µg/mL against E. coli by ELISA and broth dilution method, respectively. To evaluate the phytochemicals in the extract, further purification of the extract by TLC, column chromatography, and component analysis by GC–MS, which reported 18 components, and UV spectrum were performed. A number of therapeutic applications for the extract were observed, including antidiabetic activity (anti-glucosidase) of 98.34% inhibition at 100 µg/mL, anti-lipase of 100% inhibition at 100 µg/mL, and anti-amylase activity of 100% inhibition at 100 µg/mL, antioxidant activity (anti-DPPH activity) of 59.74% inhibition at 100 µg/mL, anthelmintic at 1 min by 1000 µg/mL, antifibrinolytic at 20 secs by 1000 µg/mL and cytotoxic activity against A549 lung cell lines, wherein, the cell viability was below 40 % at the highest dose (100 µg/mL), with an IC50 value of 39.9 µg/mL.
Keywords
Bixa Orellana
Phytochemicals
Extracellular Protease
Enzyme activity
Anticancer
Anthelmintic
Antifibrinolytic
Antioxidant
1 Introduction
We all know that plants play an important role in our lives, and that the plant kingdom serves as a treasure house full of useful medicines (Arunkumar, 2009, Edeoga et al, 2005). Roughly 10% of all plant species are of medicinal uses (Schippmann et al, 2007). Medicinal plants refer to any higher plants that have one or more organs (whole plant may be medicinally active) that contains substances that can be used for preparation of valuable drugs and have therapeutic applications (Doughari, 2012, Evans and Evans, 2008). To get a wide variety of drugs, medicinal plants are recommended by the World Health Organization. Herbal medicines, which often contain phytochemicals obtained from medicinal plants, are used by about 80% of people in developed countries (Mohanasundari et al, 2007, Bannerman, 1980). Plants produce phytochemicals, which are either primary or secondary metabolites (found in the plant's bark, leaves, stems, roots, fruits, and flowers) and help them deal with the temporary or permanent threats inherent to their environment. (Wani et al., 1971).
The threat of infectious illnesses to global health exists in both industrialized and developing nations. Even though many pharmaceutical industry have developed class of drugs over three decades but the resistance of microorganisms to these drugs have been increased also antibiotics, may have negative consequences on the host including immunological suppression, allergies, and hypersensitivity and also are less effective, less safe, high cost, difficulty in source availability and have side effects, there is a urgent need of alternative medicine (Badyal et al, 2020, Swamy and Akhtar, 2019; Muddapur et al., 2022). Due to the mentioned problem statement, there is an ever growing interest in the herbal plants due to low cost, less side effects and more effective has led to discovery in novel therapeutic agents (Swamy and Akhtar, 2019), but the extraction of these bioactive compounds is more challenging, the selection of solvent and the appropriate extraction process is paramount to limit the cost (Mojab et al, 2003, Parekh and Nair, 2005).
B. orellana (Family: Bixaceae, common name: achiote, annatto in english, sinduri in sanskrit, rangamala in kannada) belongs to a small sized tree that are grown in the tropical regions of the central and south America which are widely used in food supplements, pharmaceuticals, neutraceuticals and cosmetics (Ongsakul et al, 2009, Parekh and Chanda, 2007). B. orellana is a small sized tree having the height of 3 to 5 m, but in some cases it can reach the height up to 10 m, the truck is also small of about 20 to 30 cm in diameter, the leaves are alternative of about 10 to 20 cm long and 5 to 10 cm wide, their seeds are 0.3–0.5 cm in length and 0.2–0.3 cm in diameter (Kirtikar and Basu, 1987, Aarland et al, 2015). The brilliant red fruit (seedpods) known as annatto is used mostly as a natural colouring additive for food, fabrics, items, the human body (including the hair and skin), and cosmetics. The entire tree has a long history of use as a medicinal herb to cure a variety of ailments, from cancer to fevers (Von Carlowitz Ghori et al, 2015). Two carotenoids are bixin and carotenoids a unique red colour in B. orellana. It is a cheap, safe, inert, and easy to handle product, compared to other products, annatto is naturally occurring and has less side effects and it has 10,650 consumption values (Corrêa et al, 1978). The bioactivity of annatto is majorly found in leaves and seeds, the commercial producers include Kenya, Philippines, Jamaica, Brazil, Mexico, and Peru (Ganju and Ganju, 2014).
Indian Ayurveda practitioners use B. orellana as mild purgative and an astringent because the whole plant is considered as medicinal i.e, capable of producing therapeutic effects. B. orellana is used as antidiabetic, aphrodisiac, antipyretic, antidiarrheal, anti-inflammatory and insect repellent (Voeks and Leony, 2004, Morton, 1981). Also suggested for antimicrobial, antifungal, anticancer activity, and anthelmintic activity (Gordon and David, 2001).
Previous study on B. orellana revealed the presence of flavonoids and terpenoids (Harborne, 1975). And previous pharmacological study estimated the anthelmintic activity (Barrio et al, 2004, Villar et al, 1997), invitro antimicrobial activity (Collier et al, 1968, Irobi et al, 1996), antioxidant activity (Martinez-Tome et al, 2001), antidiabetic (Mukhtar et al, 2004) and anticoagulant (Joshi et al, 2002).
The present study aimed to investigate the phytochemicals in B. orellana by ethyl acetate extract, quantitative analysis of phytochemicals, invitro antibacterial and antifungal activity. To determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), and invitro antidiabetic, antioxidant, anti-inflammatory, anticoagulant anticancer (MTT assay) and anthelmintic activity was determined. Further purification of phytochemicals by thin layer chromatography and column chromatography and characterization by UV spectrum and GC–MS was also undertaken.
2 Materials and methods
2.1 Sample preparation and its extraction.
The leaves samples of B. orellana were collected from University Agricultural Science Dharwad (Fig. 1), the leaves were cleaned properly by using distilled water and was shade dried for over 15 days to get powdered form of leaves (Collier et al, 1968).
Bixa orellana plant.
The leaves of B. orellana were pulverised and used in a Soxhlet device to extract 150 cc of ethyl acetate at 60 °C for 3–6 h. Utilizing a rotary evaporator, the resulting extract was concentrated to remove any remaining solvents. The crude extract stock solution (with a concentration of 94.11 mg/mL) was made by rotary evaporation. The resulting extract was placed in a brown glass bottle and chilled to 4 °C (Ganju and Ganju, 2014, Collier et al, 1968).
2.2 Preliminary phytochemical screening.
We performed preliminary phytochemical profiling to verify that the plant's ethyl acetate extract contains the expected bioactive secondary metabolites. The presence or absence of certain phytochemicals was determined using conventional biochemical testing in accordance with previously published procedures (Anees Ali Jafri et al., 2023; Al Awadh et al., 2022; Dike et al., 2016; Hanif et al., 2023; Muhsinah et al., 2022; Said Nasser Al-Owamri et al., 2023).
Test for saponins, cholesterol, alkaloids, flavonoids, phenols, steroids, triterpenoids, glycosides, anthraquinones, coumarins, diterpenes, catechin, anthocyanosides, resins, volatile oil was carried out using standard biochemical methods (Shilpakar, 2009; De et al., 2010; Harborne, 1998; Parekh and Chanda, 2007; Edeoga et al., 2005; Mace et al., 1976; Kumar et al., 2010; Onwukaeme et al., 2007; Dahiru et al., 2006).
2.3 Quantitative analysis of phytochemicals.
2.3.1 Quantitative analysis of alkaloids (Shilpakar, 2009)
Accurately measure aliquots of atropine standard solution (0.4, 0.6, 0.8, 1, and 1.2 mL) and transfer each to a different separatory funnel. Then, combine 1, 2, 3, and 4 mL of chloroform with 5 mL of pH 4.7 phosphate buffer and 5 mL of BCG solution. The extracts were gathered in a 10 mL volumetric flask and diluted with chloroform to adjust volume. At 470 nm, the absorbance was measured in comparison to a blank that was made in the same manner but without atropine.
2.3.2 Quantitative analysis of flavonoids (Mace et al., 1976)
Determined by referring to the calibration curve using quercetin as a standard.
2.3.3 Quantitative analysis of phenols (Shilpakar, 2009)
The Folin-Ciocalteu colorimetric technique was used to determine the total polyphenol content. It is one of the earliest techniques created to identify the Polyphenols, therapeutic plants. The standard that is most frequently employed in this procedure is gallic acid. Plant extract is used as the standard for every quantitative analysis.
2.4 Antibacterial and antifungal activity of phytochemicals
The bacterial strains used were E. coli, B. Subtilis, S. aureus, P.s aeruginosa, Z. mobilis, B. nakamuria and C. albicans. These strains were procured from were procured from National Collection of Industrial Microorganisms (NCIM), PUNE.
2.4.1 Antibacterial and antifungal activity by well diffusion method
Plates of nutrient agar were made. Bacterial cultures that had been grown for 24 h were analysed in nutrient broth. In each well of the agar plate (which measured around 6–7 mm in diameter), we deposited between 100 and 200 µl of plant extract. Each bacterial culture was diluted to 0.1 mL and smeared across the positive and negative controls. After 24 h of incubation at 37 °C, the clearance zone (in mm) was measured and recorded. (Hammer et al., 1999).
2.4.2 Minimum inhibitory concentration (MIC), and Minimum bactericidal concentration (MBC) of phytochemicals
MIC is the lowest concentration of the plant extract which inhibits the growth of microorganisms in a liquid which can be recognized by the turbidity which is equivalent to that of un inoculated broth (John et al., 2014).
2.4.3 Determination of MIC for bacteria by broth dilution method
The broth dilution method was used to determine the MIC of the plant extract on both gram positive and gram-negative bacteria viz. The concentration range of 20,40,60,80,100,120,140,160 µg/mL was prepared in DMSO solvent with 1 mL of plant extract and it was added to 5 mL nutrient broth then it was inoculated with pathogen, incubated at 37 °C for 24 h and turbidity was measured at 600 nm (John et al., 2014).
2.4.4 Determination of MBC for bacteria
It was then spread out on a plate of nutritional agar to be examined for growth. On the nutrient agar plate, there was no discernible growth, and the MBC of plant extract on that microorganism was assumed (Ganju and Ganju, 2014).
2.4.5 Determination of MIC by ELISA method
Consider a 96 well plate, pour 100 µL of media to each well and add 100 µL of inoculum to all the wells leaving first and last well, the first well acts as positive control (only media) and negative control (media + inoculum + antibiotic), to all the remaining wells the plant extract is serially diluted (20 to 180 µg/mL) added, here the inoculum considered is 0.5 mcf. After the setup, the ELISA plate is read in microplate reader and the reading is taken at 600 nm (Aarland et al, 2015).
2.5 Purification of phytochemicals by thin-layer chromatography and column chromatography
2.5.1 Thin layer chromatography (TLC)
Preparation of TLC plates: The silica gel was prepared by adding 40 g silica gel in 100 mL distilled water. This slurry was then poured on glass plates with the help of an applicator to prepare plates of uniform thickness of 0.5 mm. The plates were dried at temperature of 60 °C for 3–4 h and then kept at 110 °C for 1 h for plate activation (Kagan et al., 2014).
Preparation of developing chamber: The glass chamber was filled with mobile phase as ethyl acetate–methanol-water (100:13.5:10) and covered with a glass slab. Vacuum grease was applied to the glass slab to make the chamber airtight. The set up was left for saturation for 1 h.
Spotting the plate: On the activated silica plate, about 1 cm from the bottom a line is marked with a pencil. The 7–10 µL samples (extracts) and the respective standards were spotted on the plate. The spots were allowed to dry and then kept in a developing chamber. The glass slab was placed back in place. The run was allowed till the solvent reached about 2 cm below the edge of the plate.
Column chromatography carried out (Santosh and Satya, 2010).
The investigation was done to determine how well TLC and column chromatography would purify bioactive compounds. Adsorption of a solution's solutes through a stationary phase creates a separation of the mixture's components. The affinity for the mobile phase and stationary phase is the foundation for this. The molecules with a higher affinity for the stationary phase elute later, while those with a lower affinity elute first.
2.6 Antibacterial activity of TLC and column fractions.
Then the TLC plate was removed and dried for a while. The solvent front is marked on the plate. The plate was subjected for development. The fraction of column chromatography was dried to get rid of solvent then antibacterial assay was performed. The agar well assay for antibacterial activity carried out towards E. coli, B. subtilis, S. aureus, P. aeruginosa, Z. mobilis, B. nakamuria and C. albicans, the zone of clearance was measured (John et al., 2014).
2.7 Characterization of phytochemicals by UV-Spectrum and GC–MS.
2.7.1 UV-spectrum analysis
The collected sample was scanned in the 200–700 nm wavelength range using a (UV 3000+ LABINDIA ANALYTICAL) Double beam UV-Spectrophotometer, and the different peaks were discovered. The spectrum was validated three times via each analysis. From the spectrum the peaks and the wavelength related to these peaks are estimated, to understand the component present in the plant extract (Alam et al., 2015; Parveen et al., 2022; Thite et al., 2013).
2.7.2 GC–MS analysis
The material was examined using GC–MS on a Shimadzu GC–MS with model number QP2010S. A DB 5 MS capillary column (30 m × 0.25 mm (internal diameter) film thickness 0.25 m) was used for the chromatography. At 70 eV, the mass spectrometer was run in electron impact mode. The ion source and transfer line were kept at a constant temperature of 250 °C. The mass spectra of positive ions in the m/z range of 50 to 600 were recorded. A computer or integrator turns each of the signal's peaks into a graph after the detector turns the number of molecules into an electrical signal. Gas chromatography-mass spectrometry (GC–MS), a combined analytical technique used to quantify and identify compounds found in a plant sample, is heavily employed in phytochemical research and chemotaxonomic investigations of medicinal plants containing biologically active components (Kumar et al., 2010).
2.8 Anticancer activity of phytochemicals.
Plant extracts were evaluated for their cytotoxic effects using A549 lung cancer cell lines as a model. The anticancer properties of the Soxhlet extract were examined. Plant-based chemicals have been shown to have anti-cancer effects, such as the ability to stop cancer cell proliferation and even cause cancer cells to commit suicide (a process known as apoptosis) (Kumar et al., 2019; Correa and Haenszel 1978).
Principle of MTT assay: It is now generally recognised that tetrazolium salt reduction is a valid method for analysing cell proliferation. To produce reducing equivalents like NADH and NADPH, metabolically active cells reduce the yellow tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide). Solubilization and spectrophotometric quantification of the resultant intracellular purple formazan are possible. When metabolic events cause cell death, such as apoptosis or necrosis, the assay evaluates the rate at which cell viability decreases (Krishnamurthi et al., 2010; Subapriya, & Nagini, 2005).
2.9 Antifibrinolytic activity of phytochemicals.
Sheep blood was collected and centrifuged to separate plasma and other components, the supernatant was removed and purified, then different concentration of plant extract 100–1000 (µg/mL) was prepared and 1 mL of blood sample of each different concentration of plant extract, then the time required for the clotting the blood was noted down (Fig. 2). Which indicates the effectiveness of plant extract in clotting blood (De et al., 2010).
Antifibrinolytic activity of plant extract.
2.10 Anthelmintic activity of phytochemicals.
Fresh and alive earthworms (Pheretima posthuma) were collected and is placed in sterile Petri plates, the plant extract of different concentration 100–1000 (µg/mL) were added to these 2–3 earthworms, then the time was noted down to kill the earthworms (Fig. 3). This indicates the application in killing earthworms (Prichard, 1994).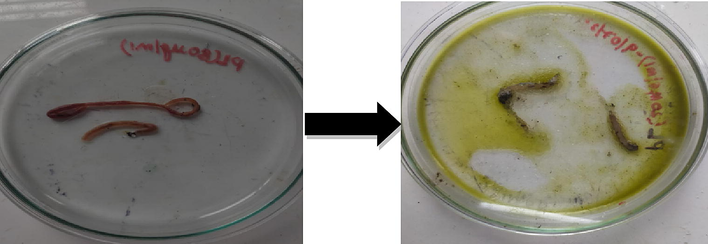
Anthelmintic activity of plant extract against earthworms (P. posthuma).
2.11 Antidiabetic activity of phytochemicals.
The α-amylase and α-glucosidase inhibitory assays were carried out using the procedure of Tafesse et al., 2017. The 50% inhibition of enzyme activity (IC50) of these enzymes was expressed as % inhibition using the expression:
% inhibition = [(Acontrol-ANFE)/Acontrol] × 100, and in-vitro alpha lipase inhibitory assay (Ademiluyi and Oboh, 2013).
2.12 Antioxidant activity of phytochemical
The negative control (blank) was generated using the same method as the positive control (plant extract) but with distilled water instead. All tests were done in triplicate. According to the procedure outlined by Singleton et al., the antioxidant activity of the plant extract was assessed with a variety of solvents based on its scavenging actions on the stable 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical (Singleton et al., 1999; Shrikanth et al., 2015).
3 Results and discussion
3.1 Preliminary phytochemical screening of the leaves of B. orellana
B. orellana's ethyl acetate extract underwent a preliminary screening of plant metabolite chemicals, and the results revealed the presence of several metabolites, including phenolic compounds, steroids, tannins, alkaloids, and flavonoids (Table 1). + phytochemical present. - phytochemical absent.
Tests
Result
Remarks
Alkaloids
Mayer test
+
Alkaloids are present
Wagner test
+
Hager test
+
Tannic acid test
+
Dragendroff test
+
Flavonoids
Shinoda test
–
Flavonoids are present
Alkaline reagent test
+
Ammonium test
+
Lead acetate test
+
Saponins
Emulsion test
+
Saponins are present
Frothing test
+
Steroids and triterpenoids
Salkowoski test
+
Steroids and triterpenoids are present
Tannins and phenols
Ferric Chloride test
+
Tannins and phenols are present
Gelatin test
+
Braemer test
+
Glycosides
Legal test
–
Glycosides are absent
Kellar-Killiani test
–
Baljet’s test
–
Bomtrager’s test
–
Carbohydrates
Benedict’s test
–
Carbohydrates are absent
Anthraquinones
Borntrager’s test
+
Anthraquinones are present
KOH test
+
Coumarins
Coumarins test
+
Coumarins are present
Diterpenes
Copper acetate test
+
Diterpenes are present
Catechins
Matchstick test
–
Catechins are absent
Anthocyanosides
Anthocyanosides test
–
Anthocyanosides are absent
Resins
Acetone water test
+
Aesins are present
Oils and fats
Volatile oil test
–
Oils and fats are absent
3.2 Quantitative analysis of the phytochemicals
The below graphs are standards of alkaloids, flavonoids, and phenols (Fig. 4). And Equation of straight line is used for calculating the concentration.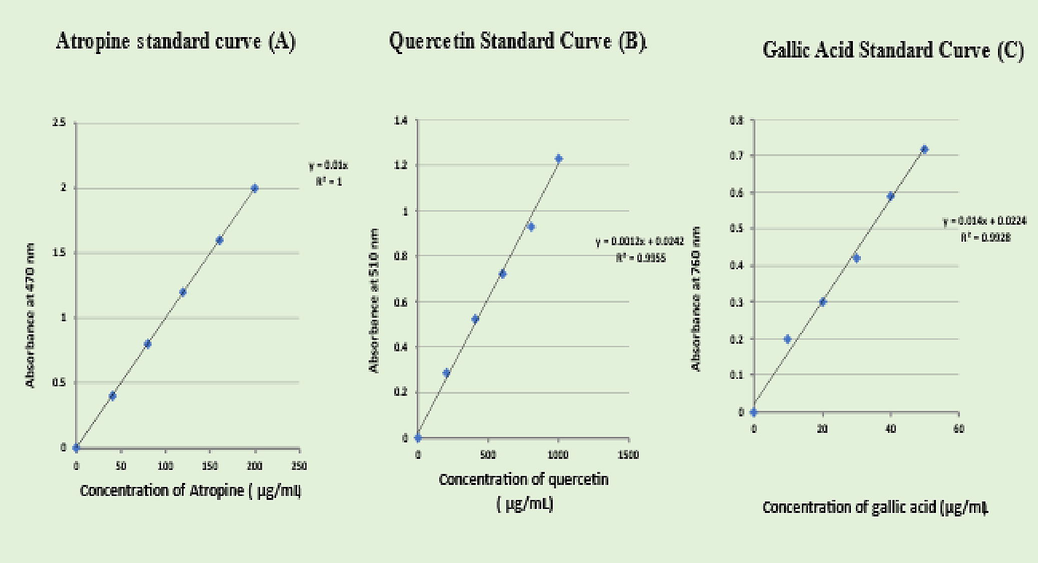
Standard graphs of alkaloids, flavonoids, and phenols.
3.2.1 Quantitative analysis of alkaloids, flavonoids, and phenols
See Fig. 5.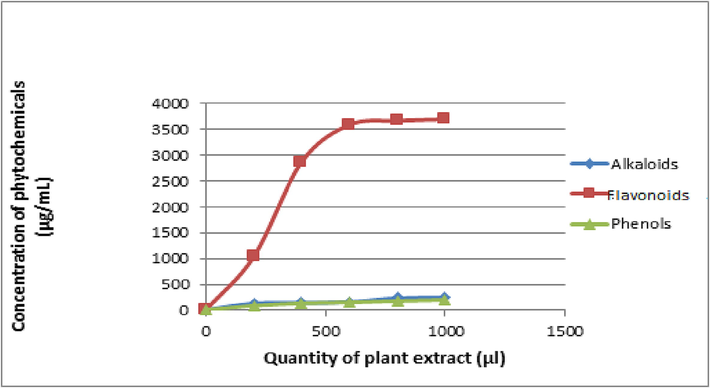
Quantitative analysis of phytochemicals.
3.2.2 Concentration of alkaloids, flavonoids, and phenols
The total phenol content was determined using the Folin-Ciocalteu reagent. The ability of phenolic compounds to chelate metals, inhibit lipoxygenase, and scavenge free radicals may be related to their bioactivities, which function as free radical terminators (Akanksha et al., 2015). The standard curve equation was used to convert the total phenols into mg/g equivalents of gallic acid, which was the standard compound used in the experiment. The Quercetin reagent's use revealed that the ethyl acetate extract had a phenolic component at a maximum concentration of 192.4 µg per mL. Total flavonoid content was determined using this information. The standard curve equation was used to compute the total flavonoid concentration, with quercetin acting as the reference substance (Fig. 5). The flavonoid concentration in the ethyl acetate extract was the highest, coming in at 3699 µg per mL. Similarly, a concentration of 256.0 (g/mL) of alkaloids was determined to be present in the ethyl acetate extract. Results showed the highest levels of flavonoid and phenolic, which can be utilized to test potential novel medications (Akanksha et al., 2015).
3.3 Antibacterial and antifungal activity of phytochemicals.
There is evidence that some herbs used in Indian medicine have antibacterial properties (Shrikath et al., 2015; Shaikh et al., 2022). Two gram-negative (E. coli, Z. mobilies), three gram-positive (Bacilli, S. aureus, B. nakamuria), and one fungal (Candida albicans) bacteria and one ethyl acetate extract of B. orellana were used in the experiment, with the ethyl acetate extract showing promising results (Fig. 6).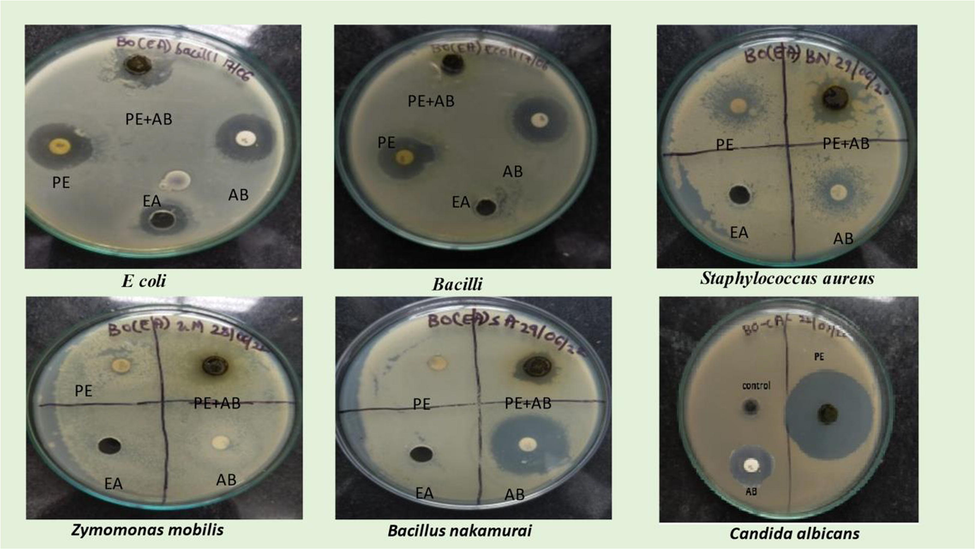
Antibacterial and antifungal activity of phytochemicals.
3.4 MIC, MBC, and minimum fungicidal concentration (MFC).
3.4.1 Broth dilution method.
Broth dilution method (abs at 600 nm) minimum concentration of PE to inhibit the microbial growth of E. coli, Bacilli, P. aeruginosa, S. aureus, Z. Mobilis, B. nakamuria, C. albicans is 80, 100, 80, 120, 100, 120, 140 (µg/mL) respectively (Figs. 7-10).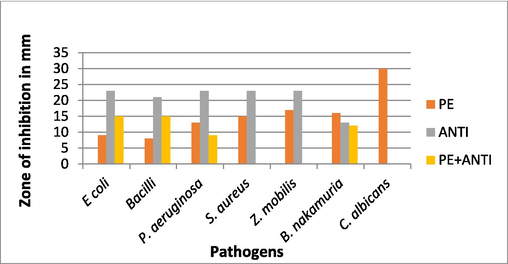
Zone of inhibition against bacteria and fungi.
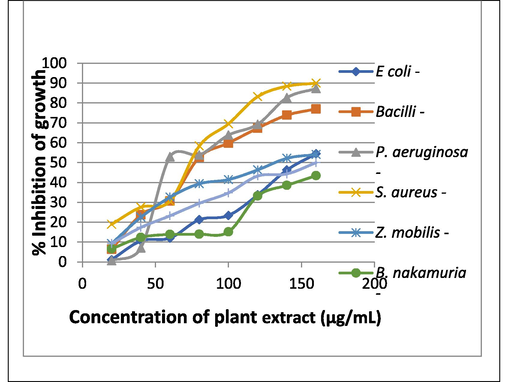
% Inhibition of growth of various microbial strains.
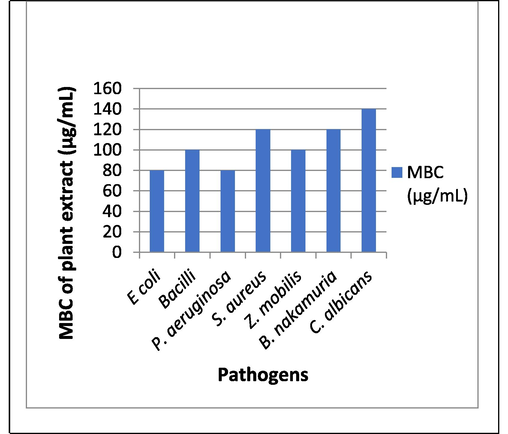
MBC of plant extract.
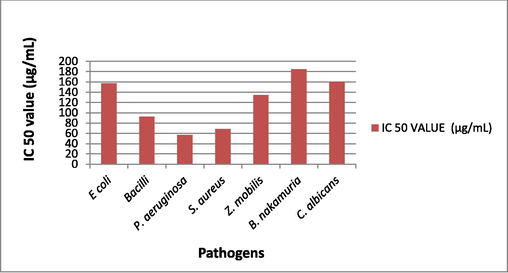
IC50 value of plant extract against various microbial strains.
3.5 Purification of plant extract.
3.5.1 Purification by chromatography
After verifying the components in the plant's crude extract using thin layer chromatography, the extract was purified using silica gel column chromatography. Fractions were then gathered in a variety of solvents, including ethyl acetate, chloroform, methanol, and petroleum ether, and they were then used in analytical studies (Fig. 11).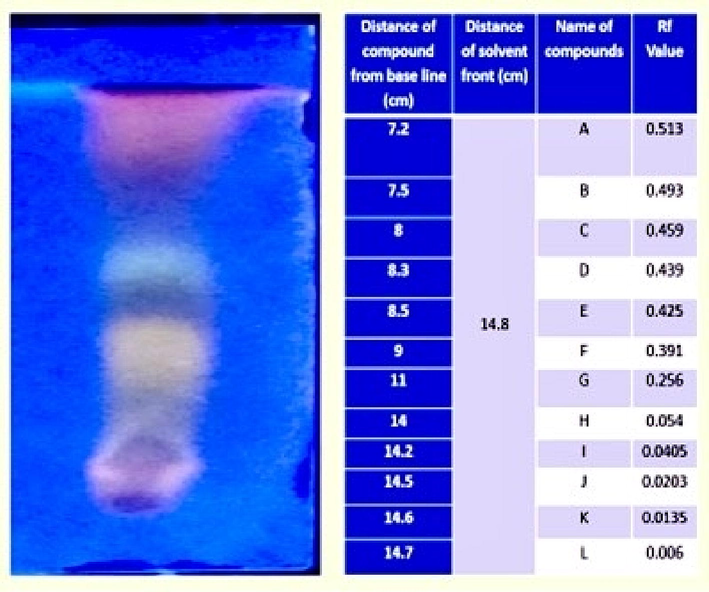
TLC of purified plant extract.
3.6 Characterization of plant extract
3.6.1 UV spectrum analysis of plant extract
UV–Vis spectrophotometry measured for the identification of chromophoric groups and aromatic rings (Fig. 12). Alkaloids, flavonoids, phenolic acids, and tannins (Lava et al., 2021) were found to be present in plant extracts, as indicated by their characteristic UV–Vis absorption bands. Gallic acid, a pure reference chemical, displayed many absorption bands at 265 nm; quercetin, two at 250 nm and 370 nm; rutin, two at 250 nm and 355 nm; and tannic acid, one at 275 nm. Like the first absorption band between 270 and 280 nm, the second between 350 and 500 nm indicates the presence of tannins and flavonoids, while the absorption band between 400 and 450 nm is attributed to the presence of carotenoids(shekh et al., 2022). As a result, the chromophoric groups included in molecules isolated from various plant sources were successfully identified using UV–Vis spectroscopy.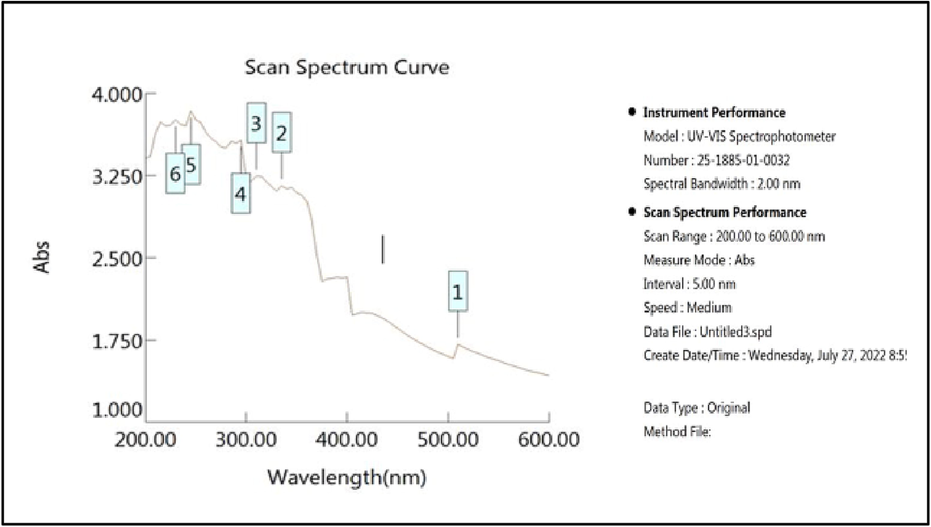
UV spectrum of the plant extract.
3.6.2 GC–MS analysis of plant extract.
Table 2 displays the results of GC–MS spectral analysis of the ethyl acetate fractions of plant leaves extract, demonstrating the peaks of the various chemicals found in the GC. The GCMS revealed the presence of 18 different bioactive compounds namely, Octadecane, Delta-elemene, (-)-Alpha.-copaene, (-)-Elema-1,3,11(13)-trien-12-ol, Beta.-elemen, (-)-.Beta.-caryophyllene, Alpha.-bulnensene, Alpha.-caryophyllene, Alpha-guaiene, (-)-Germacrene d, Gamma.-elemene, (-)-Alpha-panasinsen, 5,6:7,8-Diepoxycholestan-3.beta.-ol, (-)-Spathulenol, (-)-Guaiol, 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester, Aromadendrene, Retinal respectively.
Peak#
R. Time
Area
Area%
Height
Height%
Name
Base m/z
1
13.036
122,971
0.48
43,479
0.69
Octadecane
57.10
2
14.756
359,871
1.40
137,675
2.17
Delta-elemene
121.15
3
15.824
144,061
0.56
49,400
0.78
(-)-.Alpha.-copaene
105.10
4
16.049
61,171
0.24
23,347
0.37
(-)-Elema-1,3,11(13)-trien-12-ol
79.10
5
16.239
1,129,517
4.38
354,138
5.59
.Beta.-elemen
93.10
6
16.999
469,428
1.82
156,314
2.47
(-)-.Beta.-caryophyllene
93.10
7
17.605
463,733
1.80
163,032
2.57
Alpha.-bulnensene
108.10
8
17.874
423,902
1.64
86,311
1.36
Alpha.-caryophyllene
93.10
9
18.157
9,091,289
35.27
3,049,746
48.11
Alpha-guaiene
105.10
10
18.575
1,987,111
7.71
588,792
9.29
(-)-Germacrene d
161.15
11
18.963
2,486,897
9.65
416,856
6.58
.Gamma.-elemene
121.15
12
19.508
355,912
1.38
123,239
1.94
(-)-Alpha-panasinsen
122.15
13
20.801
253,257
0.98
72,351
1.14
5,6:7,8-Diepoxycholestan-3.beta.-ol
152.10
14
20.996
219,529
0.85
64,680
1.02
(-)-Spathulenol
93.10
15
21.424
1,306,114
5.07
272,803
4.30
(-)-Guaiol
161.15
16
27.251
5,911,368
22.93
425,470
6.71
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester
149.00
17
29.609
669,983
2.60
232,129
3.66
Aromadendrene
107.10
18
31.652
319,140
1.24
79,724
1.26
Retinal
69.10
25,775,254
100.00
6,339,486
100.00
3.7 Anticancer activity (MTT assay)
The MTT assay was used to assess the cytotoxicity of PE on A549 cells. According to the results of the MTT assay, cell viability was below 40% at the highest dose (100 µg/mL). Plant Extract shows similar cell viability property compared to doxorubicin; which revealed a cell viability is 29.5% at 100 µg/mL of PE (Fig. 13).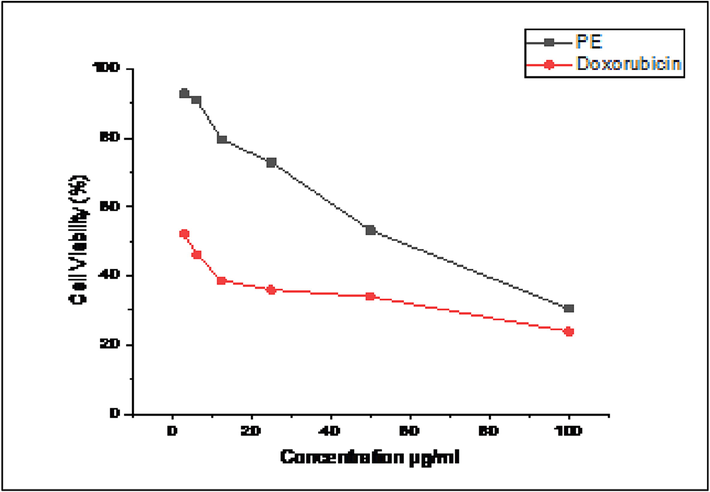
% Cell viability of lung cancer (A549 cell lines) against the plant extract.
3.8 Invitro antidiabetic activity.
PE shows Anti--diabetic property of 100% (Anti--amylase activity) at conc of 100 µg/mL and Anti--lipase activity, hence the PE contains chemical components that can be further explored for diabetic treatment and management (Table 3).
Concentration (µg/mL)
In vitro alpha-amylase inhibitory activity
In vitro alpha-lipase inhibitory activity
In vitro alpha-glucosidase inhibitory activity
Absorbance at 540 nm
% Inhibition of amylase
Absorbance at 405 nm
% Inhibition of lipase
Absorbance at 405 nm
% Inhibition of glucosidase
Control
0.895
–
0.08
–
0.725
–
20
0.129
85.59
0.035
56.25
0.225
68.96
40
0.076
91.51
0.025
68.75
0.113
84.41
60
−0.034
100
0.020
75.00
0.095
86.89
80
−0.049
100
0.017
78.75
0.043
94.06
100
−0.057
100
−0.020
100
0.012
98.34
3.9 Invitro antioxidant activity.
To put it simply, antioxidants are bioactive substances that either prevent or significantly slow the oxidation of molecules (Halliwell et al., 1995). Natural antioxidants are those that occur in nature, while synthetic antioxidants are created in a lab. The antioxidants BHT, BHA, propyl gallate, and tertbutylhydroquinine are all examples of synthetic antioxidants (Figs. 14, 15). Since it has recently been shown that synthetic antioxidants are hazardous and carcinogenic, leading many scientists to worry about their safety, especially in light of the fact that they have been linked to health problems such liver damage. Plants have long been utilized as a reliable source of traditional remedies for the treatment of a wide range of illnesses; as a result, there has been an uptick in the research and development of safer antioxidants from natural sources. Antioxidant-rich phytochemicals are found in abundance in several of these therapeutic plants. Tamarind, cardamom, lemon grass, and galangal basil are some examples of frequent ingredients that have been employed in ethnic foods. Scientists have discovered antioxidants in certain spices and herbs (Javanmardi, 2003). As a result, there has been an uptick in interest in testing plants for antioxidant and antibacterial capabilities. About 20% of plant species have had their safety and benefits verified by pharmacological and biological uses (Suffredini et al., 2004; Shaikh et al., 2023). At 100 g/ml, plant extract has antioxidant activity of 59.74%.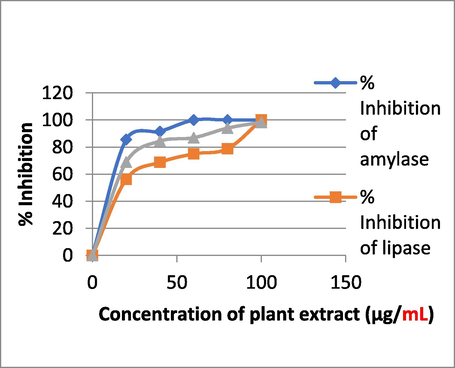
Antidiabetic activity of plant extract.
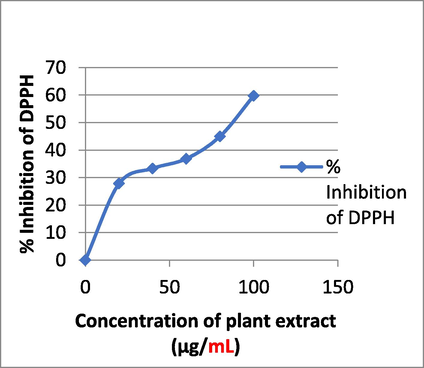
Antioxidant activity of plant extract.
3.10 The antifibrinolytic activity and anthelmintic activity of plant extract
When applied at a concentration of 1000 µg/mL, PE has strong anti-fibrinolytic activity, causing blood to clot in just 20 s, which is a significant difference over the control. PE also demonstrates anti-helminthic activity, as 1000 µg/ml of PE was found to diminish earthworm (P. posthuma) activity compared to control within 1 min. (Figs. 16, 17).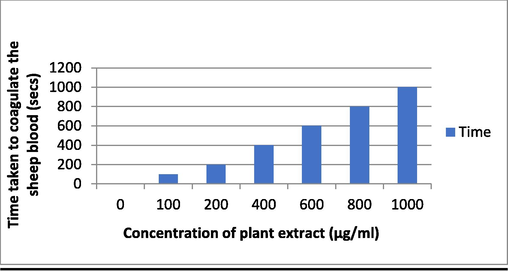
Antifibrinolytic activity of the plant extract.
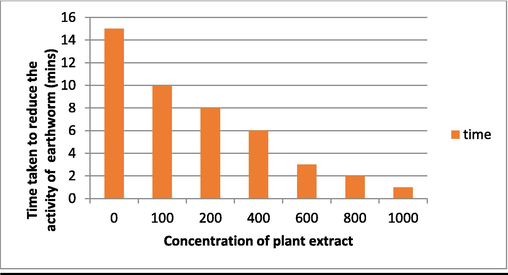
Anthelmintic activity of the plant extract.
4 Conclusion
Preliminary phytochemical screening was carried out using an ethyl acetate extract of B. orellana leaves, revealing the presence of alkaloids, flavonoids, phenols, glycosides, and carbohydrates. Alkaloids, flavonoids, and phenols were found in satisfactory quantities during the quantitative analysis. Antibacterial and antifungal properties were also observed. Component extracted material showed antibacterial activity of 22 mm zone of clearance against B. nakamuria, In addition, GC–MS analysis revealed several phytochemicals in the crude extract. Results from tests for anticancer, anti-diabetic, anti-glucosidase, antilipase, antioxidant, and anthelmintic activities revealed significant activity. Pure compounds can be isolated and studied further to evaluate their medicinal potential.
Funding
The authors thank the Deanship of Scientific Research at Najran University for funding this research project through the Research Centers Researchers Funding Program and code for Project No. NU/RCP/MRC/12/4.
Acknowledgement
The authors thank the Deanship of Scientific Research at Najran University for funding this research project through the Research Centers Researchers Funding Program and code for Project No. NU/RCP/MRC/12/4; KLE Technological University, BVB Campus, Hubballi-580031, Karnataka, India and ISNC Jeddah, Saudi Arabia
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A pharmacological and phytochemical study of medicinal plants used in Mexican folk medicine. Indian J. Tradit. Knowl. 2015;4:550-557.
- [Google Scholar]
- Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol.. 2013;65:305-309.
- [CrossRef] [Google Scholar]
- Sustainable synthesis and characterization of zinc Oxide Nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals (Basel). 2022;12:1142.
- [CrossRef] [Google Scholar]
- DFT, Hirshfeld surfaces, spectral and in vivo cytotoxic studies of 7a-Aza-B-homostigmast-5-eno [7a,7-d] tetrazole. J. Mol. Struct.. 2015;1099:588-600.
- [CrossRef] [Google Scholar]
- Evaluation of some essential traditional medicinal plants for their potential free scavenging and antioxidant properties. J. King Saud Univ. Sci.. 2023;35:102562.
- [CrossRef] [Google Scholar]
- Analysis of phytochemical constituents and antimicrobial activities of aloevera L. against clinical pathogens. World J. Agril. Sc. 2009;5:572-576.
- [Google Scholar]
- Traditional medicine in modern health care services. Int. relat.. 1980;6:731-748.
- [CrossRef] [Google Scholar]
- In vitro antiparasitic activity of plant extracts from Panama. Pharm. Biol.. 2004;42:332-337.
- [Google Scholar]
- The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother.. 1968;32:295-310.
- [CrossRef] [Google Scholar]
- Diciońario das Plantas Úteis Do Brasil e das Exóticas Cultivadas. Rio de Janeiro, Brazil: Ministério da Agricultura/IBDF; 1978.
- Phytochemical screening and antiulcerogenic effect of Moringa oleifera aqueous leaf extract. Afr. J. Tradit. Complement. Altern. Med.. 2006;3
- [CrossRef] [Google Scholar]
- Phytochemical investigation and chromatographic evaluation of the different extracts of tuber of Amorphaphallus paeoniifolius (Araceae) Int. J. Pharm. Biol. Res.. 2010;1:150-157.
- [Google Scholar]
- Phytochemical and proximate analysis of foliage and seed of Bixa orellana linn. Int. J. Pharm. Sci. Rev. Res. 2016;36:247-251.
- [Google Scholar]
- Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents. Rijeka, Croatia: INTECH Open Access Publisher; 2012.
- Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotech.. 2005;4:685-688.
- [Google Scholar]
- Evans, W.C., Evans, 2008. Trease and Evans’ Pharmacognosy. WB Saunders Company Ltd.
- Phytochemical Analysis of Seeds of Bixa orellana Linn. J. Medical Pharm. Innovation. 2014;1:21-24.
- [Google Scholar]
- Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit. Rev. Food Sci. Nutr.. 1995;35:7-20.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol.. 1999;86:985-990.
- [CrossRef] [Google Scholar]
- Nepeta paulsenii Briq. inhibits hepatic toxicity in albino rats: Phytochemical analysis and chemical profiling. J. King Saud Univ. Sci.. 2023;35:102542
- [CrossRef] [Google Scholar]
- Flavonoid bisulfates and their co-occurrences with ellagic acid in the Bixaceae, Frankeniaceae, and related families. Phytochemistry. 1975;14:1331-1337.
- [Google Scholar]
- Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall Ltd; 1998.
- Antimicrobial Activity of Annatto (Bixa orellana) Extract. Int. j. pharmacogn.. 1996;34:87-90.
- [CrossRef] [Google Scholar]
- Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci.. 2014;6:406-408.
- [Google Scholar]
- Changes in certain haematological parameters in siluroid catfish, Clarias batrachusLinn., exposed to cadmium chloride. Pollut. Res.. 2002;21:129-131.
- [Google Scholar]
- Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem.. 2003;83:547-550.
- [CrossRef] [Google Scholar]
- Thin-layer chromatographic (TLC) separations and bioassays of plant extracts to identify antimicrobial compounds. J. Vis. Exp. 2014
- [CrossRef] [Google Scholar]
- Indian Medicinal Plants. Dehra Dun, India: International Book Distributors; 1987.
- Krishnamurthi, K., 2010. 17-Screening of Natural PRODUCTS FOR Anicancer and Antidiabetic properties. Health Administrator XX 69–75.
- Anticancer activity of plant leaves extract collected from a tribal region of India. 3 Biotech. 2019;9
- [CrossRef] [Google Scholar]
- Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biomed. Res.. 2010;4:191-195.
- [Google Scholar]
- More VS. Characterization, anticancer, antibacterial, anti-diabetic and anti-inflammatory activities of green synthesized silver nanoparticles using Justica wynaadensis leaves extract. Mater. Today: Proc.. 2021;46:5942-5947.
- [Google Scholar]
- Histochemistry and identification of disease-induced terpenoid aldehydes in Verticillium-wilt-resistant and-susceptible cottons. Can. J. Bot.. 1976;54:2095-2099.
- [Google Scholar]
- Antioxidant properties of Mediterranean spices compared with common food additives. J. Food Prot.. 2001;64:1412-1419.
- [CrossRef] [Google Scholar]
- Antibacterial properties of Passiflora foetida L.- a common exotic medicinal plant. Afr. J. Biotechnol.. 2007;6:2650-2653.
- [Google Scholar]
- Phytochemicals screening of some species of Iranian plants. Iran. J. Pharm. Res. 2003;3:77-82.
- [Google Scholar]
- Atlas of medical plants of Middle America: Bahamas to Yucatan. Springfield, Charles C: Thomas; 1981.
- Molecular modelling and simulation techniques to investigate the effects of fungal metabolites on the SARS-CoV-2 RdRp protein inhibition. J. King Saud Univ. Sci.. 2022;34:102147
- [CrossRef] [Google Scholar]
- Antibacterial activity of Illicium verum essential oil against MRSA clinical isolates and determination of its phyto-chemical components. J. King Saud Univ. Sci.. 2022;34:101800
- [CrossRef] [Google Scholar]
- Effect of water extract of Psidium guajava leaves on alloxan induced diabetic rats. Pharmazie. 2004;9:734-735.
- [Google Scholar]
- Antibacterial effect of crude alcoholic and aqueous extracts of six medicinal plants against Staphylococcus aureus and Escherichia coli. J. Health Res.. 2009;23:153-156.
- [Google Scholar]
- Evaluation of phytochemical constituents, antibacterial activities and effect of exudate of Pycanthus Angolensis Weld Warb (Myristicaceae) on corneal ulcers in rabbits. Trop. J. Pharm. Res.. 2007;6:725-730.
- [Google Scholar]
- In Vitro Antimicrobial Activity and Phytochemical Analysis of Some Indian Medicinal Plants. In Vitro Antimicrobial Activity and Phytochemical Analysis of Some Indian Medicinal Plants. Turk. J. Biol.. 2007;31:53-58.
- [Google Scholar]
- Preliminary screening of some folklore medicinal plants from western India for potential antimicrobial activity. Indian J Pharmacol.. 2005;37:408-409.
- [Google Scholar]
- Isolation, Identification, Spectral Studies and X-ray Crystal Structures of Two Compounds from Bixa Orellana, DFT Calculations and DNA Binding Studies. Crystals 2022:12.
- [Google Scholar]
- Phytochemical, Antioxidant, hair growth and wound healing property of Juniperus excelsa, Olea oleaster and Olea europaea. J. King Saud Univ. Sci.. 2023;35
- [CrossRef] [Google Scholar]
- Herbal Remedies of Wetlands Macrophytes in India. Int. J. Pharma Biosci.. 2010;1:21-24.
- [Google Scholar]
- A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In: Medicinal and Aromatic Plants. Netherlands, Dordrecht: Springer; 2007. p. :75-95.
- [Google Scholar]
- Characterization of Bioactive Compounds from Acacia concinna and Citrus limon, Silver Nanoparticles’ Production by A. concinna Extract, and Their Biological Properties. Molecules. 2022;27:2715.
- [CrossRef] [Google Scholar]
- Extracellular protease production, optimization, and partial purification from Bacillus nakamurai PL4 and its Applications. J. King Saud Univ. Sci.. 2023;35:102429
- [CrossRef] [Google Scholar]
- Phytochemical screening and analysis of antibacterial and antioxidant activity of Ficus auriculata. Pokhara, Nepal: Lour. Stem bark; 2009.
- Antimicrobial and antioxidant activity of methanolic root extract of Tabernaemontana alternifolia L. Int. J. Pharm. Pharm. Sci. 2015;7:66-69.
- [Google Scholar]
- [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Oxidants and Antioxidants Part A. Elsevier 1999:152-178.
- [Google Scholar]
- Current medicinal chemistry- anticancer agent; medicinal properties of neem leaves. Asian Pac. J. Cancer Prev.. 2005;5:149-155.
- [Google Scholar]
- Screening of antibacterial extracts from plants native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res.. 2004;37:379-384.
- [CrossRef] [Google Scholar]
- Chemistry, Pharmacology and Health Care Practices. Springer Nature Singapore Pte Ltd; 2019.
- Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement. Altern. Med.. 2017;17
- [CrossRef] [Google Scholar]
- Preliminary Phytochemical Screening of Some Medicinal Plants”. IJPCBS. 2013;3:87-90.
- [Google Scholar]
- Screening of 17 Guatemalan medicinal plants for platelet antiaggregant activity. Phytother. Res.. 1997;11:441-445.
- [CrossRef] [Google Scholar]
- Forgetting the forest: assessing medicinal plant erosion in Eastern Brasil. Econ. Bot.. 2004;58:S294-S324.
- [Google Scholar]
- Voluntary control of corticomuscular coherence through neurofeedback: a proof-of-principle study in healthy subjects. Neuroscience. 2015;290:243-254.
- [CrossRef] [Google Scholar]
- Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc.. 1971;93:2325-2327.
- [CrossRef] [Google Scholar]







