Translate this page into:
Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial efficacies of Pteropyum scoparium (Jaub. & Spach) Sidaf crude extracts
⁎Corresponding author. vijaya.settaluri@utas.edu.om (Vijaya Saradhi Settaluri),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Pteropyrum scoparium Jaub. & Spach locally known as “Sidaf” is a meal known to the ancient Omani people with many health benefits. It is traditionally used in Oman to treat high cholesterol, hypertension, indigestion problems, wound healing, and diabetes. However, these claims are yet to be scientifically proven. Hence, this study aimed to perform phytochemical, antioxidant, and antimicrobial analysis of P. scoparium leaves aqueous and alcoholic extracts to confirm its medicinal potential.
Methods
A detailed phytochemical analysis of ethanol and aqueous extracts of leaves was carried out to confirm the presence of bioactive substances. DPPH (2,2′-diphenyl-1-picrylhydrazyl), agar-well diffusion and disc diffusion methods were used to evaluate antioxidant and antimicrobial potential, respectively. The extracts were tested against four microorganisms viz. E. coli (ATCC 25922), S. aureus (ATCC 23235), Penicillium sp. (ATCC 11597) and Rhizopus stolonifer (ATCC 14037).
Results
The ethanol extract exhibited higher DPPH scavenging activity than aqueous extract that was confirmed with IC50 values of both extracts. However, the aqueous extract was found to be significantly more effective as an antimicrobial agent than the ethanol extract. This could be due to higher coumarins content that is thrice as much as in ethanol extract. One-way repeated measure RM ANOVA showed that there was a statistically significant difference in the antimicrobial susceptibility of all four organisms for the aqueous and ethanol well diffusion extracts (DF = 7; SS = 56.350, MS = 8.050; F = 5.865; P < 0.001). The highest mean zone of inhibition was recorded for S. aureus (12 ± 3.851 mm) well diffusion aqueous extract followed by R. stolonifer (11.750 ± 4.250 mm) well diffusion aqueous extract, and S. aureus (10.625 ± 3.771 mm) well diffusion ethanol extract.
Conclusions
Phytochemical screening of ethanol and aqueous extracts revealed the presence of alkaloids, glycosides, carbohydrates, amino acid, fats & fixed oils, phenolic compounds & tannins, proteins, phytosterols, saponins, gum & mucilage, terpenoids, coumarins and anthocyanins. The findings from this study will be useful in evaluating the phytochemical constituents present in the extract and developing commercial drugs as antioxidant and antimicrobial agents based on this plant.
Keywords
Pteropyrum scoparium
Phytochemical
Antibacterial activity
Antifungal activity
Radical scavenging activity
1 Introduction
The importance of synthetic drugs has been surpassed due to the overwhelming usage of drugs in the treatment of various human diseases. Hence, there has been a development of multidrug resistance among microorganisms. It is one of the critical signs today to cease the excess usage of synthetic drugs. Keeping this into consideration, it is important to explore plant products that have been proved to have more medicinal value since ancient times. The extracts of various plants leaves have been proved to be medicinally active due to presence of various phytochemical constituents such as amino acid, alkaloids, carbohydrates, phenolic compounds proteins, phytosterols, saponins, terpenoids, mucilage and gums, and coumarins (Banu and Cathrine, 2015). Many phytochemicals have been scientifically proven to possess medicinal attributes like anti-inflammatory, antibacterial, antioxidant, & anti-helminthic ones, among others. As a result, numerous parts of the plant have indeed been successfully used to treat a variety of human ailments (Kunle et al., 2003; Roopashree et al., 2008; Kunle and Egharevba, 2009; Begum et al., 2002; Sah et al., 2012; Chandra et al., 2017; Kutama et al., 2018; Kawamura and Muraoka, 2018; Nwozo et al., 2023).
The plant Pteropyrum olivieri Jaub. & Spach (common name in Oman: Sidaf) belongs to the family Polygonaceae and has been reported to treat many common diseases such as diabetes, indigestion problems, liver disease, treating high cholesterol and relieving symptoms of high blood pressure (Al-Qalhati et al., 2021). Oman is one of the Middle Eastern countries where a large percentage of the population uses herbal remedies. P. scoparium is a traditional wild plant with a green color; it grows in plains, hills, valleys, and deserts. This plant is abundant during the rainy and winter season. The height of the plant ranges from 50 cm to 2 m and it has multiple branches, and the leaves are devoid of juice (Al-Abri, 2018).
Many regions of the Sultanate of Oman are blessed with various medicinal plants and herbs that have nutritional and health benefits. From the time immemorial, the Omani people have been consuming the leaves of P. scoparium regularly because of its numerous abovementioned health benefits. On the other hand, this amazing plant is also eaten to get a healthy and slim body free of extra fat and toxins (Al Attabi et al., 2015). P. scoparium is used as a traditional remedy and is used in the manufacturing of many medicines and traditional mixtures for wound healing and treatment of diarrhea and high cholesterol by crushing it and eating it in high concentrations without any additives. As mentioned earlier, this plant is locally known as Sidaf which is a local meal known to the ancient Omani people. This meal is eaten with many toppings to make the food more delicious. Firstly, the leaves of this plant are harvested by striking the shrub to drop the leaves, which are in a soft linear shape, and wash them with water. Secondly, the leaves are crushed with a pestle. Finally, add the rest of the toppings as desired, such as salt, green pepper, lemon, dried fish, and bread (Al-Abri, 2018). Hence, Omani citizens have been taking this plant parts as their local meal for past hundreds of years.
According to existing research, the phytochemical constituents of P. scoparium extracted from its leaf have been seldom evaluated for its antioxidant and antimicrobial properties. Hence, we are prompted to investigate the antimicrobial and antioxidant properties of this plant to further confirm its medicinal value to treat various ailments in the traditional medicinal system of Sultanate of Oman.
2 Materials and methods
2.1 Apparatus
The UV–Visible spectra of DPPH and the antioxidant activity of plant extracts (aqueous & ethanol) with DPPH were performed using UV–Visible spectrophotometer (Evolution 300; Thermo Scientific, UK) with matched quartz cells.
The stirring of the solutions was performed by using an electrical linear plate shaker- SM30 (Edmund Bühler GmbH, Bodelshausen, Germany).
2.2 Reagents and standards
2.2.1 Antioxidant activity:
The stock solution of 0.04 % DPPH (MW = 394.32 g mol−1, Sigma-Aldrich, USA; 400 μg mL−1 or 1.014 × 10-3 M) was prepared by dissolving 0.04 g DPPH in 100 mL methanol (AR Grade, HiMedia Laboratories, India).
2.2.2 Plant material collection:
Pteropyrum scoparium Jaub. & Spach plant samples were collected from the wadis in the foothills of Jebel Akhdar Oman (Fig. 1- B & C). The plant species was identified by a botanist Dr. Pankaj Sah, UTAS Muscat. The leaves were thoroughly rinsed with tap water and then with distilled water to remove any traces of dirt or solid residue and particulate matter. These properly cleaned leaves were air-dried for a week in a large stainless-steel sieve. Fifty grams of dried leaves were kept in an oven at 60 °C for 24 h and milled using a blender. The powdered leaves were sieved using a 60-mesh sieve, and the sieved fine powder was then transferred into an amber bottle container. The container was kept in the dark to prevent any decomposition of the biomass material and then used for further studies.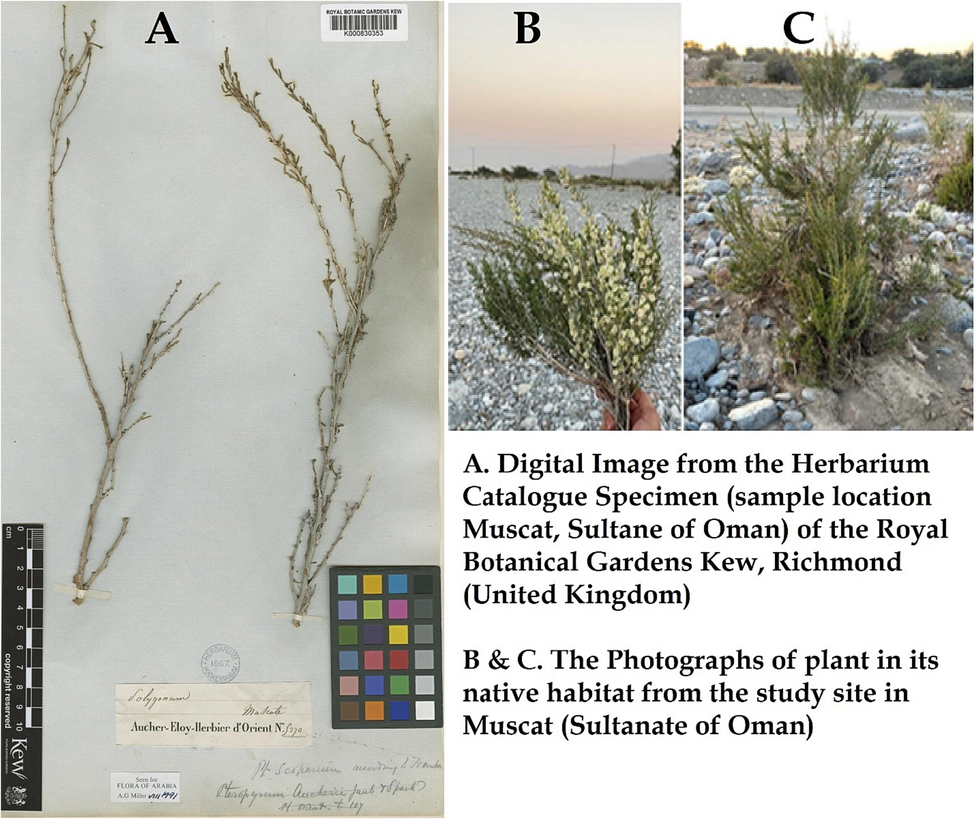
The photograph of plant Pteropyrum scoparium Jaub. & Spach (A) from the Herbarium Catalogue Specimen Royal Botanical Gardens Kew (UK)
http://creativecommons.org/licenses/by/3.0/,
https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:696668-1 and (B) (C) from the current study site in Oman.
Initially, the leaves were separated from the stem and rinsed thoroughly with running water for dust removal and were allowed to dry in the room for 2 days. Dried leaves were grinded using an electric mixer and stockpiled for further usage in an airtight container.
3 Methods
3.1 Extraction of phytochemicals from leaves of Pteropyrum scoparium Jaub. & Spach
A total of 30 g of finely grounded dried leaves powder was transferred into three different conical flasks containing 200 mL of ethanol, glycerol, and water, respectively. Thereafter, 3 solutions in three separate conical flasks were placed on orbital shaker at 37 0C and 110 round per minute (rpm) for a period of 48 h. Each solution was centrifuged three times at 4 0C and 4000 rpm for 15 min and the resulting supernatant was separated from the solid residue. The extracts were filtered using a 0.45 µm polyether sulfone membrane filter (ISOLAB, Wertheim, Germany). The ethanol extract was concentrated by rotary evaporator at a constant pressure and temperature at 78 0C. The aqueous extract was concentrated using evaporating water bath. Finally, 10 % (100 mg ml−1) ethanol, glycerol and aqueous extracts stock solutions were prepared by transferring 6.67 mL of the concentrated extract in 10 mL standard volumetric flasks and diluted up to mark with dimethylsulphoxide (DMSO). The stock solutions of extracts were stored in airtight containers in the refrigerator for further use.
3.2 Screening for phytochemicals
The phytochemical screening was conducted following the published papers Balamurugan et al. (2019), Nortjie et al. (2022), and Saradhi Settaluri, et al., (2023) with some modifications.
-
Test of Alkaloids:
Mayer’s test: One milliliter of plant extract sample was poured in the test tube, and 2 drops of Mayer's reagent was added to tube's walls. The development of a white, creamy textured precipitate suggested the presence of alkaloids.
Test for Amino acid:
Whatman No. 1- filter paper was used to filter the 100 mg of extract, which was already diluted using 10 mL of distilled water then analyzed for amino acids.
3.3 Ninhydrin test:
Two drops of the ninhydrin solution containing 10 mg ninhydrin with 200 mL acetone, were added to 2 mL of the aqueous filtrate. The appearance of purple color indicated the existence of amino acids.
Test for carbohydrates:
3.4 Benedict’s test:
2 mL plant extract was treated with 0.5 mL Benedict’s reagent. This was heated in boiling water bath for two minutes. The appearance of a distinctly colored precipitate confirmed the existence of sugar.
Fixed oils & Fats Test:
3.5 Saponification test:
One milliliter of the extract was mixed with some droplets of alcoholic potassium hydroxide, 0.5 N solution - a mix of 2.8 g KOH in 100 mL ethanol, and one drop of phenolphthalein. In a water bath, this mixture was then heated for two hours. The production of soap or even the partial neutralization of alkali is a sign that there are fixed oils plus fats existing.
Test for Glycosides:
After 50 mg extract was hydrolyzed for two hours in a water bath containing powerful hydrochloric acid, it was filtered and tested in the following methods.
3.6 Borntrager’s test:
To 2 mL extract, acetic acid and chloroform each 2 mL were added, in that order. The solution was then allowed to sit for 5 min before being added with conc. sulfuric acid drops. The existence of glycoside was recognized by green / reddish brown tint.
3.7 Legal’s test:
0.5 mL extract was diluted with 2 mL of pyridine, 2 g of sodium nitroprusside solution was added to 100 mL of distilled water, and 10 % NaOH was added to the solution to make it alkaline. The existence of glycoside was shown by the pink tint.
3.8 Test for phenolic compounds and tannins:
3.8.1 Ferric chloride test:
For this test, 50 mg of extract was dissolved in 5 mL pure water. This was then mixed with some neutral ferric chloride solution drops with 5 % ferric chloride. The dark green tint served as a sign that phenolic chemicals were present.
3.8.2 Gelatin test:
Before combining the 50 mg extract in 2 mL of 1 % Gelatin solution with 10 % NaCl, the same was diluted in 5 mL of distilled water. White precipitate's presence indicated the existence of phenolic chemicals.
3.8.3 Alkaline reagent test:
10 percent ammonium hydroxide solutions in water were used to treat 2 mL of extract. Yellow fluorescence indicated the existence of flavonoids.
Test for phytosterols:
3.8.4 Libermann-burchard’s test:
In this test, 50 mg of extract was treated in 2 mL of acetic anhydride. 1 or 2 drops of con. Sulphuric acid was carefully added along the test tube's walls. Different color-changing behaviors suggested the existence of phytosterols.
Test for proteins:
A total of 100 mg extract was assayed for proteins after being filtered using Whatmann No. 1 filter paper and diluted in 10 mL of distilled water.
3.8.5 Millon’s test:
With 2 mL of filtrate, some of Millon's reagent drops were added. White precipitate formed verified the existence of proteins.
3.8.6 Biuret test:
To 2 mL filtrate, 1 drop 2 % copper sulphate solution had been put. This was succeeded with an oversupply of potassium hydroxide granules and 1 cc of 95 % ethanol. The protein existence was suggested by the pink color of the ethanolic layer that was detected.
3.8.7 Test for saponins:
A total of 50 mg of extract was diluted with filtered water to make 20 mL. The suspension subsequently had been shacked for about 15 min in graded cylinder. Foam for about two-centimeter layer was allowed for the discovery of saponins.
3.8.8 Test for gum and mucilages:
For this test 100 mg extract was diluted with 10 mL of distilled water, then 2 mL of 100 % alcohol was added with the mixture being constantly stirred. Gums and mucilage were indicated by white as well as foggy precipitation (Banu and Cathrine, 2015).
3.8.9 Test for coumarins:
To 2 mL of the extract, 3 mL of 10 % aqueous NaOH solution was added. The content of coumarins was detected by the generation of yellow color (Ismail et al., 2017).
3.8.10 Test for terpenoids:
1 mL extract was combined with 1.5 mL strong sulfuric acid plus 1 mL of chloroform. The existence of terpenoids was suggested by the reddish-brown tint (Prabhavathi et al., 2016).
3.8.11 Antioxidant activity:
1.5 mL of 0.04 % DPPH was transferred in a 10 mL volumetric flask and diluted up to mark with methanol. The UV–Visible spectrophotometric spectrum was scanned in the wavelength range of 340–800 nm against methanol. The λmax of DPPH was selected and the absorbance was recorded. The antioxidant activity was performed using the same amount of DPPH with varied volumes (0.1–0.5 mL) of plant extracts to analyze IC50 value of DPPH. The antioxidant property of the plant extracts was assessed based on IC50 value at the lowest concentration of plant extracts.
3.8.12 Antimicrobial analysis:
To evaluate the antibacterial activity of plant extract chosen, conventional well diffusion & disc diffusion methods were employed (Bauer et al., 1996). All the experiments for each treatment were performed in triplicates and the mean zone of inhibition was recorded as Mean ± SE (mm).
3.8.13 Antibacterial activity:
Nutrient agar preparation: 8.4 g of nutrient agar was weighed and dissolved in 300 mL of distilled water. It was then dissolved upon heating on hot plate at 70 0C and sterilized in autoclave at a pressure of 15 lbs at 121.5 0C about 15 to 20 min. The sterilized nutrient agar was further cooled at room temperature and then approximately 20 aliquots of medium were transferred into each sterile Petri plate for the study.
E. coli (ATCC 25922) (gram negative) and S. aureus (ATCC 23235) (gram positive) were collected from the Microbiology laboratory in the Department of Applied Science, UTAS-Muscat. The slants were maintained under sterile conditions storing at 4 0C till future use. According to previously published research, the plant extract's antibacterial activity was tested employing both the disc diffusion as well as agar-well diffusion methods (Boyanova et al., 2005). To make the plant extract's initial stock, the powdered extract of 50 g was solvated in 300 mL of methanol and were kept in shaker incubator at 150 rpm for 48 h at 37 0C. A 10 % (100 mg ml−1) stock solution was prepared by transferring 6 mL of the concentrated extract in 10 mL standard volumetric flask and diluted up to mark with dimethylsulphoxide (DMSO). Aliquots (0.1, 0.2, 0.3 and 0.4 mL) of 10 % stock solution were further taken in 10 mL volumetric flask and diluted up to mark with DMSO. The final concentration of 1.0, 2.0, 3.0 and 4.0 mg mL−1 extracts were prepared.
3.9 Inoculation of the bacteria
3.9.1 Well diffusion Method
Four seeded nutrient agar plates were used for well diffusion method: 2 for E. coli bacteria and other two for S. aureus. Under aseptic conditions, 4 wells of 6 mm size were made by using sterile cork pores, after that 60 µL of each solvent extract i.e., ethanol and H2O extracts poured into each well with the help of a micro pipette.
On the other hand, amoxicillin medication of equal volume and concentration was chosen as positive control, while ordinary distill water & ethanol had all been added individually as negative controls.
3.9.2 Disc diffusion method
For disc diffusion method also 4 petri plates were placed: 2 for E. coli bacteria and other two for S. aureus. Filter paper discs of 5 mm size were impregnated in ethanol and H2O extracts in above specified concentrations (60 µL per each disc). After a few minutes, filter paper discs were taken and allowed them to dry in an oven. The sterile discs were placed at a distance with the help of sterile forceps on the seeded nutrient agar plates aseptically. Here, water and ethanol of same concentration were also taken as negative control and Amoxicillin antibiotic disc as positive control, respectively. Finally, all Petri plates were incubated in an incubator for 24 h.
3.9.3 Antifungal activity:
Fungal Strains: Penicillium sp. (ATCC 11597) and Rhizopus stolonifer (ATCC 14037) used in this study were obtained from the Microbiology laboratory in the Department of Applied Science, UTAS-Muscat.
3.9.4 Potato dextrose agar preparation:
7.4 gm of potato dextrose was dissolved in 200 mL distilled water and kept on hot plate until it became transparent. After that sterilization was completed by steam sterilizing with autoclave under a pressure of 15 lbs at 121.5 0C about 15 to 20 min, cooled at room temperature and then approximately 20 aliquots of medium were transferred into each sterile Petri plate for the study.
3.10 Inoculation of the fungus
3.10.1 Well diffusion method:
120 µL of water and ethanol extracts were loaded separately in the wells of seeded PDA plates. In this study 4 PDA plates were used for well diffusion method: two plates each for Penicillium sp., and two for Rhizopus stolonifer. The voriconazole antibiotic at same concentration was taken as positive control and plain water and ethanol of same concentration as negative control.
3.10.2 Disc diffusion method:
For disc diffusion method also 4 PDA plates were used: 2 for Penicillium and other two for Rhizopus. Each Filter paper disc of 5 mm size was impregnated in 120 µL of ethanol and H2O extract. After a few minutes, filter paper discs were allowed to dry in an oven. The sterile discs were placed at a distance with the help of sterile forceps on the seeded PDA plates aseptically. Here also water and ethanol of same concentration were taken as negative and voriconazole antibiotic disc as positive control. Finally, all Petri plates were incubated in an incubator for 48 hrs.
3.11 Statistical analysis
A detailed data analysis on the antimicrobial efficacy of P. scoparium aqeous and ethanol extracts was performed using inferential statistical studies. The data was analyzed for comparative statistics e.g., mean ± standard error, One-way Repeated Measure Analysis of Variance RM ANOVA. The data was also analyzed for associative statistics using Pearson bivariate correlation. Values of P < 0.05 were considered as significant. All statistical analyses were performed using Sigma Plot (Systat Software, San Jose, California, USA), IBM SPSS Statistics for Windows, Version 20.0 Armonk, NY: IBM Corp), and JASP (Version 0.171) University of Amsterdam, Netherlands.
4 Results
4.1 Phytochemical analysis of Pteropyrum scoparium Jaub. & Spach
The preliminary screening of phytoconstituents of P. scoparium leaf extracts showed the presence of amino acid, alkaloids, carbohydrates, glycosides, fixed oils and fats, phenolic compounds & tannins, proteins, phytosterols, saponins, gum & mucilages, terpenoids, coumarins, and anthocyanins in ethanol extract. It was observed that except glycosides and Gum & Mucilage’s rest of all phytoconstituents were found to be present in aqueous extract. In addition to this coumarins content was three times higher in aqueous extract (Table 1).
Test
Aqueous Extract
Ethanol Extract
Amino Acid
+
+
Alkaloids
++
+++
Phenolic compounds
++
++
Carbohydrate (Benedict test)
+
+
Glycosides (Borntrager,s test, Legal test)
–
+
Gum and Mucilages
–
+
Saponins
+
++
Fixed oils and Fats (saponification test)
+
++
Phenolic Compounds and Tannins (Ferric chloride)
+++
+++
Phytosterols (Liebermann-Buchard test)
+
+
Protein (Biuret test, Millon’s test)
+
+
Coumarins
+++
+
Terpenoids
+++
+++
Anthocyanin
+
++
4.2 Antioxidant analysis
UV–visible spectrum of DPPH and its scavenging activity in the presence P. scoparium extracts:
Methanolic solution of DPPH (1.521 × 10-4) was scanned in the wavelength range of 300 to 700 nm and peaked at 517 nm (absorbance of 1.25). Aqueous and ethanolic extracts of P. scoparium extracts were tested for DPPH scavenging potential, a free radical that is frequently used to measure the antioxidant activity of substances. The results are shown in Fig. 2. As can be observed from the figure, the ethanolic extract was more effective in providing antioxidant activity. IC50 % inhibition was calculated using the following formula (Sulastri et al., 2018, Saradhi Settaluri,et al., 2023).
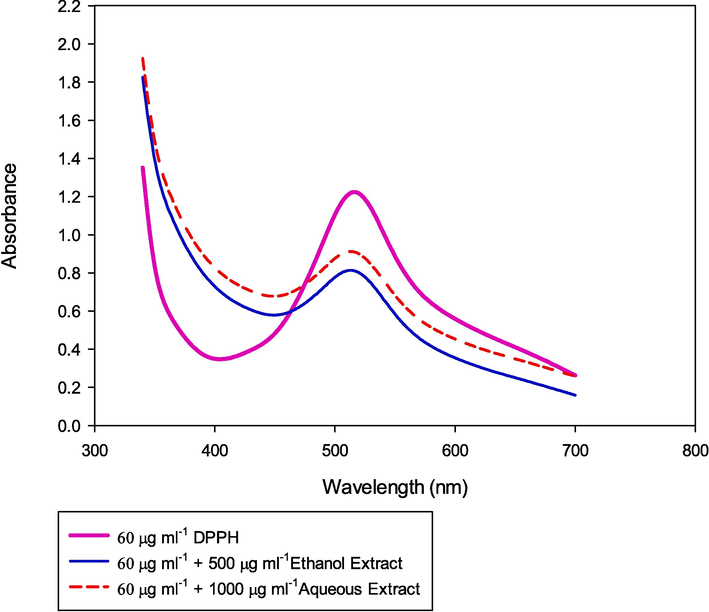
UV–visible spectrum of DPPH and its scavenging activity in the presence of ethanolic and aqueous extract of P. scoparium.
IC50% inhibition was achieved with 500 μg mL−1 ethanolic extract whereas the same IC50 % inhibition was at 1000 μg mL−1 aqueous extract of P. scoparium. The aqueous extract provided higher antioxidant activity when compared with ethanolic extract because of the presence of more alkaloids, oils, glycosides and anthocyanin.
4.3 Antimicrobial analysis
The antibacterial and antifungal efficacies of various methods and extracts of P. scoparium were recorded by measuring the zone of inhibition (mm). The results of the experiments were documented and presented as photographic plates (Figs. 3 and 4).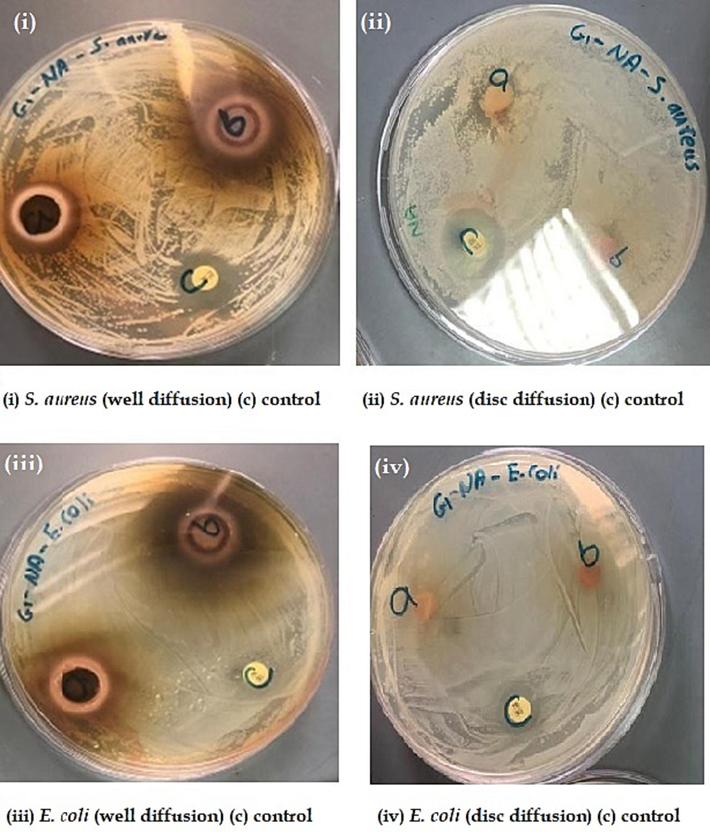
Antibacterial activities for well diffusion, disc diffusion, and control.
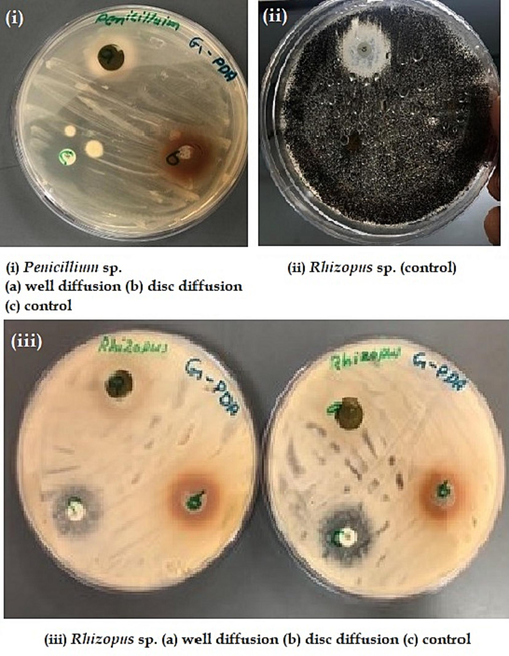
Antifungal activities for well diffusion, disc diffusion, and control.
In addition to this detailed data analysis on the antimicrobial efficacy of P. scoparium was also performed using inferential statistical studies. The Pearson correlation exhibited a very strong and significant correlation between the plant extract gradient and the zone of inhibition across all the studied microbes (i.e., gram + ve, gram -ve, and the fungal species) (Fig. 5) in the well diffusion method of ethanolic extract of P. scoparium. This clearly underlines the possibility of a broad-spectrum antimicrobial agent of P. scoparium ethanol extract. As per the correlation analysis, the highest antimicrobial impact was on the gram negative (-ve) E. coli. (r = 0.991; P < 0.01) (Fig. 5).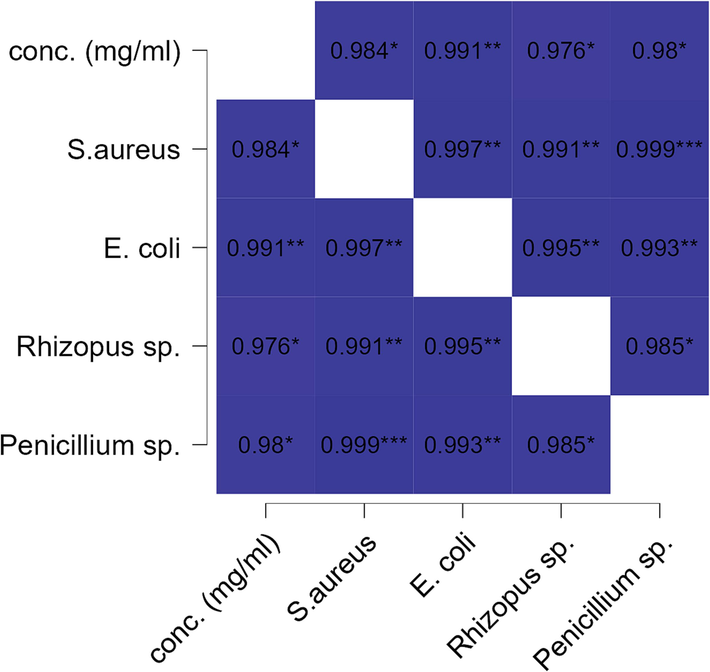
Heat map of Pearson bivariate correlation between ethanol extract concentration (mg/ml) and microbial organisms ZI (mm) in well diffusion assay. The r values with * shows the significance level of each correlation (* p <.05, ** p <.01, *** p <.001).
While performing the Pearson correlation for the well method of plant aqueous extract, it also exhibited a very strong and significant correlation across all the studied microbes (i.e., gram + ve, gram -ve, fungal species) (Fig. 6). This further confirms the possibility of a broad-spectrum antimicrobial activity of P. scoparium in aqueous extract also. However, it was found that the highest antimicrobial impact was against the fungus Penicillium. spp. (r = 0.993; P < 0.01) (Fig. 6).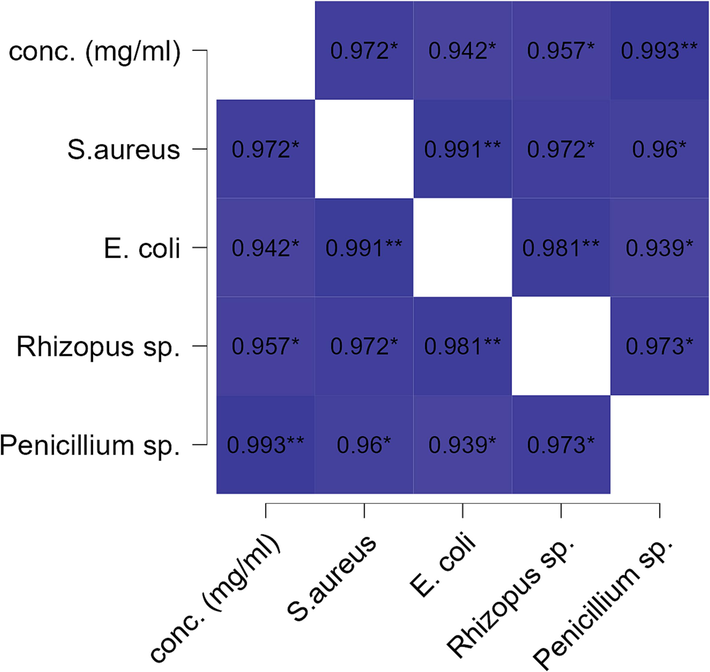
Heat map of Pearson bivariate correlation for aqueous extract (mg/ml) vs microbial zone of inhibition (mm) in well diffusion assay. The r values with * shows the significance level of each correlation (* p <.05, ** p <.01, *** p <.001).
The one-way RM ANOVA confirms a statistically significant difference among all the treatments (P < 0.001) (Table 2). The highest mean zone of inhibition was observed in the aqueous well diffusion against S. aureus (Mean 12.00 ± 3.851 mm) followed by Rhizopus sp. (aqueous well diffusion assay) (Mean 11.750 ± 4.250 mm). The highest inhibition zone in ethanol extract well diffusion assay was reported against S. aureus (Mean 10.625 ± 3.111 mm) followed by Rhizopus sp. (Mean 9.750 ± 3.637 mm). One-way RM ANOVA for all well extracts (WE = Well Ethanol; WA = Well Aqueous).
Treatment Name
N
Missing
Mean Zone of Inhibition ZI (mm)
Std Dev
SEM
S. aureus (WE)
4
0
10.625
7.543
3.771
E. coli (WE)
4
0
8.775
6.890
3.445
Rhizopus sp. (WE)
4
0
9.750
7.274
3.637
Penicillium sp. (WE)
4
0
8.000
6.325
3.162
S. aureus (WA)
4
0
12.000
7.703
3.851
E. coli (WA)
4
0
9.500
6.856
3.428
Rhizopus sp. (WA)
4
0
11.750
8.500
4.250
Penicillium sp. (WA)
4
0
9.075
7.503
3.751
Source of Variation
DF
SS
MS
F
P
Between Subjects
3
1267.638
422.546
Between Treatments
7
56.350
8.050
5.865
<0.001***
Residual
21
28.824
1.373
Total
31
1352.812
The zone of inhibition values (mm) for ethanol and aqueous well diffusion assays were plotted in box and whisker graph (Fig. 7). The results revealed that both extracts were very effective in antimicrobial activities. However, the median values and the inter-quartile ranges (IQR) were higher in aqueous extract for all the species. This explains that the aqueous well diffusion had slightly better antimicrobial results than the ethanol well diffusion assay.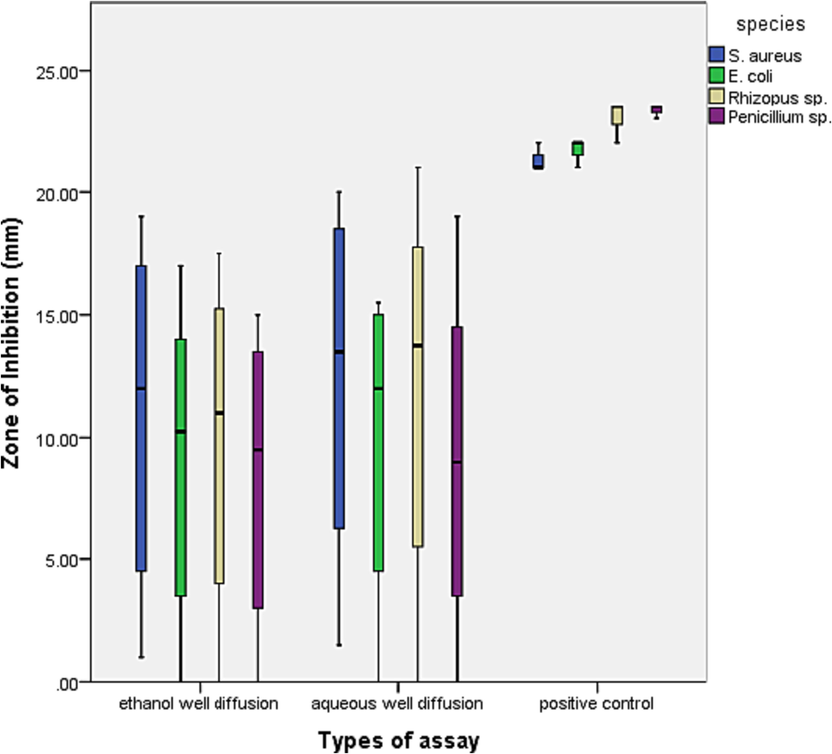
The box and whisker plot shows zones of inhibition values for different species in ethanol and aqueous well diffusion assay along with positive controls.
Similarly, the zone of inhibition values (mm) for ethanol and aqueous disc diffusion assays were also plotted in box and whisker graph (Fig. 8). The results exhibited reduced antimicrobial efficacies of disc diffusion assay than well diffusion assay (Figs. 7 and 8). It was further observed that both types of disc diffusion assays did not inhibit the growth of E. coli. There was almost equal effect on S. aureus in both assays. However, the median values and the inter-quartile ranges (IQR) were higher in aqueous disc diffusion assay than ethanol disc diffusion for both the fungal species. This further explains that the aqueous disc diffusion also had slightly better antifungal results than the ethanol disc diffusion assay.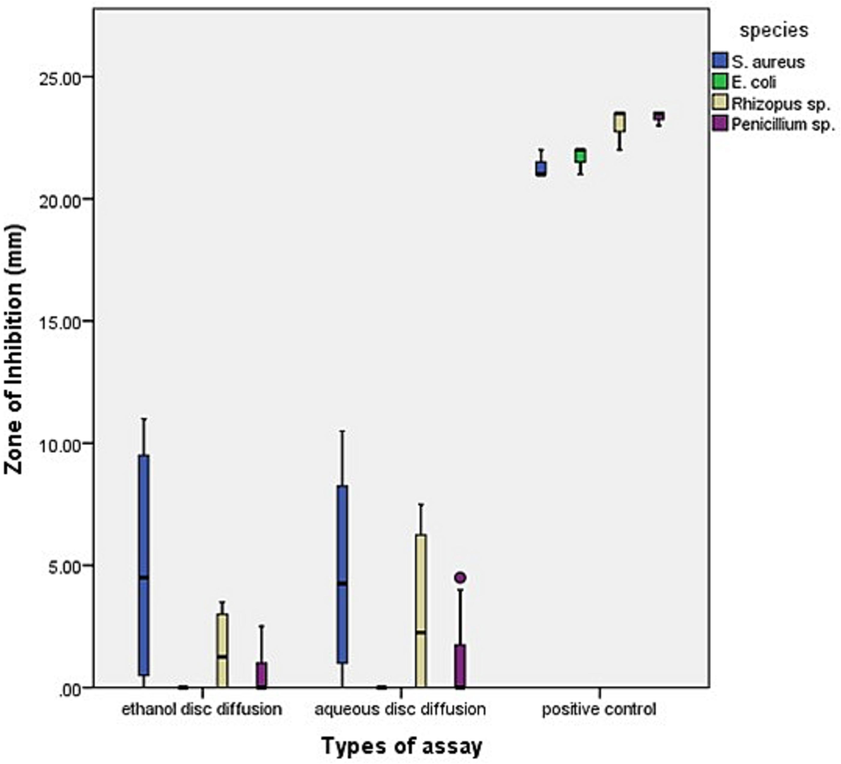
The box and whisker plot shows zones of inhibition values for different species in ethanol and aqueous disc diffusion assay along with positive controls.
5 Discussion
As per WHO (2002), the medicinal plants that have been reported to be a significant source of secondary metabolites possessing various pharmacological properties can be employed in the treatment of a variety of infections (Iwu et al., 1999). In this study, preliminary screening confirmed the presence of various important phytoconstituents such as amino acid, alkaloids, carbohydrates, fixed oils & fats, phenolic compounds & tannins, glycosides, phytosterols, saponins, proteins, gum & mucilage’s, terpenoids, coumarins, and anthocyanins were also confirmed in P. scoparium leaves extracts. All these phytochemicals have earlier been reported to arrest the bacterial growth as well as protect the plants from fungal infections (Halliwel, 1991).
Medicinal plants are highly rich in antioxidants. Antioxidants significantly delay or prevent oxidation of oxidizable substrates when present at lower concentrations than the substrate. These are the special phytoconstituents which can trap the free radicals and delay the oxidative damage (Yamagishi and Matsui, 2011). Hence, they are good sources of natural products for the treatment of age-related sicknesses (Wu et al., 2011). According to a study carried out in 2009, it was reported that antioxidants significantly delay or prevent oxidation of oxidizable substrates when present at lower concentrations than the substrate (Chand and Dave, 2009).
In this study, it was found out that P. scoparium leaf ethanol and aqueous extracts had an antioxidant activity of around 83.1 % and 78.3 %, respectively. Antioxidants have the potential to scavenge free radicals in humans and have been linked to plant-based meals' protection against illnesses including cancer, cardiovascular disease (CVD), and type 2 diabetes. In plants, polyphenols are plant-based antioxidants that are found in all plant parts with antioxidant properties. In addition, plants produce antioxidants to protect themselves from ultraviolet radiation and oxygen gas that is produced from photosynthesis process. Finally, the static headspace gas chromatography (HS-GC), 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method, and beta-carotene bleaching test (BCBT), all frequently employed in antioxidant activity evaluation, have been assessed regarding their use in the assessment of plant extracts (Santos-Sánchez et al., 2019). Plants also contain secondary metabolites that are reported to exert antimicrobial activity, hence serving as a bio-factory to produce antimicrobial agents (Medina et al., 2005; Romero et al., 2005). Antimicrobial agents can inhibit the growth of microorganisms, especially disease-causing organisms, such as bacteria, fungi, etc. Furthermore, antimicrobial agents are the drugs used in treating and preventing infections in humans, animals, and plants. Microorganisms show resistance to traditional antibiotics, and their rapid progression has sparked major concern in the treatment of infectious diseases. Therefore, many studies are being conducted to identify possible solutions to these problems. Hence, the present study on P. scoparium displaying antimicrobial activity further confirms its traditional role in the treatment of various infections. Hence, polar extracts of P. scoparium leaves could provide lead for the discovery of safe antimicrobial agents.
Our study has revealed that both aqueous and ethanol extract of P. scoparium can kill all the microbes very effectively, thus it strongly supports the age-old traditional practice of using this plant in Oman to treat several diseases. We further found that although the ethanol extract had more antioxidant activity, the aqueous extract had higher antimicrobial activity (Fig. 2). This could be attributed to the presence of three times higher coumarins content in aqueous extract than the ethanol extract (Table 1). Scientists have observed that coumarins are regarded as a promising class of bioactive heterocyclic compounds that have a wide range of biological activities like anti-microbial, anti-viral, anti-diabetic, anti-cancer activity, anti-parasitic, anti-helmintic, anti-convulsant, anti-inflammatory and anti-hypertensive activities etc., (Al-Majedy et al., 2017). It has also been reported that the coumarins are highly antimicrobial and showed significant antibacterial effects against both Gram -ve and Gram + ve bacteria (Basile et al., 2009).
Scientists have found that due to their characteristic cell wall structure, the Gram-negative bacteria are more resistant than Gram-positive bacteria, and thus cause significant morbidity and mortality worldwide (Breijyeh et al., 2020). The importance of coumarins against gram -negative bacteria is also significant as scientists have observed that the antibacterial activities of coumarins such as aegelinol and agasyllin were significantly higher against Gram -ve than Gram + ve bacteria as reported previously from other plants (Basile et al., 1997 & 1999). Thus, the present study can lead towards the establishment of broad-spectrum antimicrobial products.
In this study though oils have not been explored, further studies can be considered by isolating essential oils containing aromatic plants from P. scoparium which are generally considered as the most significant natural sources of antimicrobial agents and are frequently used in traditional medicine to study the possible prevention of infectious diseases. Plants containing natural products are also an important source for development of potential drugs. Usage of plant derived antimicrobial agents has also been reported to replace the harmful effects of antibiotics (Nemanja et al., 2016).
6 Conclusion
This study provides information on the therapeutic values of P. scoparium to use it as a medicinal plant. The presence of phytochemicals such as amino acids, alkaloids, phenolic compound, coumarins, terpenoids and anthocyanin, its antioxidant and antimicrobial nature vividly justifies that this plant could be useful in treating many common diseases such as diabetes, indigestion, liver disease, high cholesterol, and hypertension. The results of this study again assure us that herbal plants have the potential to become effective medicines to replace synthetic medicines. However, further study is still required to confirm its efficacy as a safe herbal remedy.
Acknowledgements
We are extremely grateful to the Hon’ble Vice Chancellor and Deanship of UTAS Muscat for their encouragement and motivation. We would also like to express our sincere thanks to the Dean of College of Applied Sciences and Pharmacy, the Heads of the Applied Sciences Department, and Applied Biology for their continuous support and guidance. The authors would like to acknowledge the funding support by the Researchers Supporting Project Number (RSP2023R371), King Saud University, Riyadh, Saudi Arabia. Thanks, are also due to all laboratory technicians for the smooth running of laboratory.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Coumarins: The Antimicrobial agents. Systematic Reviews in Pharmacy. 2017;8(1):62-70.
- [Google Scholar]
- Anti-diabetic Potential Properties of Two Edible Omani Wild Plants. Journal of Agricultural and Marine Sciences. 2021;26(2):56-63.
- [Google Scholar]
- Antioxidant potential properties of three wild Omani plants against hydrogen peroxide-induced oxidative stress. Canadian Journal of Clinical Nutrition. 2015;3(2):16-22.
- [Google Scholar]
- A guide to phytochemical analysis. International Journal of Advance Research and Innovative Ideas in Education. 2019;5(1):236-245.
- [Google Scholar]
- General techniques involved in phytochemical analysis. International Journal of Advanced Research in Chemical Science. 2015;2(4):25-32.
- [Google Scholar]
- Antibacterial activity in Actinidia chinensis, Feijoa sellowiana and Aberia caffra. International J. Antimicrob. Agents. 1997;8(3):199-203.
- [Google Scholar]
- Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry. 1999;52:1479-1482.
- [Google Scholar]
- Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae) Molecules. 2009;14(3):939-952.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol.. 1996;45:493-496.
- [Google Scholar]
- Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J. Med. Microbiol.. 2005;54(5):481-483.
- [Google Scholar]
- Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340.
- [Google Scholar]
- In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr. J. Microbiol. Res.. 2009;3:981-996.
- [Google Scholar]
- Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—a review. Plants. 2017;6(2):16.
- [Google Scholar]
- Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med.. 1991;91:14S-22S.
- [Google Scholar]
- Qualitative analysis for phytochemicals of selected medicinal plants from Gilgit-Baltistan, Pakistan. Asian Journal of Chemistry. 2017;29(9):1929-1932.
- [Google Scholar]
- New antimicrobials of plant origin in. Perspectives on new crops and new uses. In: Janick J., ed. Plant Breeding Reviews. Alexandria, Virginia: ASHS Press; 1999.
- [Google Scholar]
- Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants. 2018;7(9):119.
- [Google Scholar]
- Preliminary studies on Vernonia ambigua: Phytochemical and antimicrobial screening of the whole plant. Ethnobotanical Leaflets. 2009;3:1216-1221.
- [Google Scholar]
- Antimicrobial activity of various extracts and carvacrol from Lippia multiflora leaf extract. Journal of Phytomedicine. 2003;10(1):59-61.
- [Google Scholar]
- Phytochemical compositions in some Nigerian medicinal plants and their pharmacological properties: A review. International Journal of Anesthesia and Clinical Medicine. 2018;6(1):15.
- [Google Scholar]
- Composition and antimicrobial activity of Anemopsis californica leaf oil. J. Agric. Food Chem.. 2005;53:8694-8698.
- [Google Scholar]
- Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS - Wageningen Journal of Life Sciences. 2016;78:21-28.
- [Google Scholar]
- Extraction methods, quantitative and qualitative phytochemical screening of medicinal plants for antimicrobial textiles: a review. Plants. 2022;11(15):2011.
- [Google Scholar]
- Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int. J. Food Prop.. 2023;26(1):359-388.
- [Google Scholar]
- Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Pelagia Research Library. 2016;7(4):11-17.
- [Google Scholar]
- Antibacterial properties of common herbal remedies of the southwest. J. Ethnopharmacol.. 2005;99:253-257.
- [Google Scholar]
- Antibacterial activity of antipsoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. International Journal of Applied Research in Natural Products. 2008;3:20-28.
- [Google Scholar]
- Effect of temperature on antibiotic properties of garlic (Allium sativum L.) and ginger (Zingiber officinale Rosc.) Afr. J. Biotechnol.. 2012;11(95):16192-16195.
- [Google Scholar]
- Santos-Sánchez, N.F., Salas-Coronado, R., Villanueva-Cañongo, C., Hernández-Carlos, B. 2019. Antioxidant compounds and their antioxidant mechanism. In (Ed.), Antioxidants. Intech.
- Comparative studies on phenolic, anti-oxidative, biochemical and GC–MS analysis of crude and refined edible oils. Journal of King Saud University-Science. 2023;35(1):102432.
- [Google Scholar]
- Sulastri, E., Zubair, M.S., Anas, N.I., Abidin, S., Hardani, R., Yulianti, R., Aliyah 2018. Total phenolic, total flavonoid, quercetin content and antioxidant activity of standardized extract of Moringa oleifera leaf from regions with different elevation. Pharmacognosy Journal 10(6) Suppl: s104-s108.
- Evaluation of the antioxidant effects of four main aflavin derivatives through chemiluminescence and DNA damage analyses. J. Zhejiang Univ. Sci. B. 2011;12:744-751.
- [Google Scholar]
- Nitric oxide, a Janus-faced therapeutic target for diabetic micro angiopathy-friend or foe? Pharmacology Research. 2011;64(3):187-194.
- [Google Scholar]







