Translate this page into:
Phytochemical screening and antimicrobial potentials of Borreria sps (Rubiaceae)
⁎Corresponding author at: Faculty of Agro Based Industry (FIAT), Universiti Malaysia Kelantan, Campus Jeli, Locked Bag-100, 17600 Jeli, Kelantan, Malaysia. Tel.: +60 9 947 7120/+60 1 116124467 (Hp). aurifullah@umk.edu.my (Arifullah Mohammed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Successive hexane, acetone, ethanol and methanolic whole plant extracts of the Borreria sps were investigated for phytochemical screening and assessed for antimicrobial activity. Phytochemical analysis of Borreria sps extracts revealed the presence of phenolics, flavonoids and tannins. Among them, Borreria laevicaulis hexane extracts were found to be most effective showing the largest zone of inhibition against Staphylococcus aureus (22.15 mm) and Candida albicans (25.65 mm). Further studies indicated that the minimum inhibitory concentration of B. laevicaulis hexane extracts was found to be 62.5 μg/ml against S. aureus and 250 μg/ml against C. albicans and the zone of inhibitions was significantly higher than nystatin (positive control). Together, we provide new insights of the B. laevicaulis as a potential candidate for antimicrobial drug discovery using in vitro studies that might be useful to treat human infectious diseases and antibiotic resistant pathogens.
Keywords
Phytochemicals
Rubiaceae
Antibacterial
Antifungal activity
Zone of inhibition
Disk diffusion assay
1 Introduction
Infectious diseases are fatal and life threatening throughout the world. The amplification of diseases is largely due to indiscriminate use of antibiotics (Avila et al., 2008). Recent studies have extensively addressed the dramatic increase of microbial resistance to antibiotics (Triyana, 2009; Kumar et al., 2006) and methods to treat them. Antimicrobial resistance evolved through mutation and genetic exchange systems which render the elimination of diseases becomes ineffective. Hence, there is an urge to continuously search for alternative sources including natural products. Traditional medicinal systems like Ayurvedic, Chinese Medicine, and Unani developed from plant resources have been used to treat various diseases. The isolation of bioactive compounds such as tannins, terpenoids, alkaloids, flavonoids etc. for potential drug discovery has been extensively reported (Choudhury et al., 2012; Taylor, 2013).
Rubiaceae is well known for its medicinal values, used in the treatment of malaria, diarrhea, digestive problems, skin diseases, fever, hemorrhage, urinary and respiratory infections, headache, inflammation of eyes and gums (Conserva and Ferreira, 2012). Most species of Rubiaceae are normally grow as weeds in Malaysia due to their strong habitat adaptation ability. Borreria exilis (L.O. Williams) C.D. Adams is an annual herb distributed throughout the tropical countries, America and Africa (iNaturalist.org, 2013), used for treating headache (Conserva and Ferreira, 2012).
Borreria laevicaulis (Miq.) Ridl. is an annual or perennial herb naturalized along tropical Asia, Africa, Mauritius and East India. Traditionally, it is used as a poultice for headache; wounds healer; plant sap to treat eczema, worms and ringworm (Pravat and Prithwiraj, 2012). Leaf juice is applied for ringworm and eczema while the plant sap is used to treat the wound or lesion (Ebana et al., 1991). Borreria latifolia (Aubl.) K. Schum. is an annual herb that grows as a dominant weed on waste areas or agricultural fields and is normally distributed in India, Southeast Asia and Malaysia. It possesses antimicrobial properties against Bacillus cereus, Bacillus megaterium and Pseudomonas aeruginosa (Choudhury et al., 2012).
Borreria remotifolia DC is an annual herb widely distributed in tropical Asia, Africa, Australia and the Pacific Islands (FOC, 2013). Different plant parts have been used as antidotes to cure venomous stings and bites; roots as a medicinal are used to cure tetanus (Allabi et al., 2011). Richardia brasiliensis Gomes is an annual or perennial herb mainly distributed in the Southeastern United States, Asia, Midwest, South and Southeast of Brazil. It is traditionally used as an expectorant, antiemetic, diaphoretic, anti-inflammatory and in several treatments for hemorrhoids, coughs, bronchitis and headache (Hall et al., 2012). Phytochemical study revealed the presence of coumarin, flavonoids, steroids, terpenoids, alkaloids and resins in aerial parts of the plant (Morais et al., 2013). In the current study, we tested the phytochemical contents and antimicrobial activity of five Borreria sps against different pathogenic bacteria and fungal strains.
2 Materials and methods
2.1 Collection of plant materials
B. exilis, B. latifolia, B. laevicaulis, B. remotifolia and R. brasiliensis were collected from various locations around Kelantan, Malaysia. All the plant samples were identified by a botanist, from the University of Malaysia, Kelantan.
2.2 Preparation of plant extracts
The fresh plant samples (whole plant parts) collected were washed individually under running tap water and dried in an oven at 40 °C for 3 days. The dried plant materials were ground into powder using an electrical blender. About 10 g of dry powdered plant material from each plant was extracted by soxhlation using various solvents like methanol, ethanol, acetone and hexane. Extracts were then concentrated using a rotary evaporator and the concentrated residual extracts were stored at 4 °C in a dry airtight container until further use.
2.3 Microbial culture, inoculum preparation
Pathogenic bacterial and fungal strains were tested for the antimicrobial activity using Borreria sps plant extracts. Tested strains included gram positive bacteria such as Staphylococcus aureus (ATCC 25923), Bacillus subtilis (clinical isolates); Gram negative bacteria such as Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 14028), Klebsiella pneumoniae (clinical isolates), and fungi such as Candida albicans (clinical isolates), Aspergillus niger (clinical isolates). All American Type Culture Collection was obtained from the Veterinary Research Centre at Perak and clinical isolates were obtained from the Hospital University Science of Malaysia, Kelantan. Cultures of bacteria were streaked on nutrient agar (NA) with an incubation hour of 18–24 h at 37 °C and fungi on potato dextrose agar (PDA) and incubated at 28 °C for 72 h.
2.4 Phytochemical screening
Crude plant extracts were subjected to preliminary screening for the presence of active secondary metabolites. Each plant extract was tested individually with specific chemical reagents according to standard procedures (Thamaraiselvi et al., 2012; Wangchuk et al., 2011; Rajesh et al., 2013; Raaman, 2006). Visible color change or precipitate formation was taken into consideration for the presence (+) or absence (–) of particular active constituents.
2.5 Anti-microbial screening
2.5.1 Zone of inhibition
The zone of inhibition was determined by the disk diffusion method according to the Kirby-Bauer method (Bauer et al., 1966). Pre-loaded sterile disks (500, 800 and 1000 μg/disk) of each extract were dispensed onto the agar surface covered by test microorganisms at equal distance to each other, followed by incubation at 37 °C for bacteria at 16–18 h and 4 days at 28 °C for fungus. DMSO served as a negative control and standard antibiotics chloramphenicol (25 μg/disk) and nystatin (2000 units/disk) were used as positive controls for bacteria and fungus respectively. Antimicrobial activity was measured with a diameter zone of inhibition to the nearest millimeters (mm). All positive control disks were applied individually against each of the seven different test microorganisms (Barbour et al., 2004).
2.5.2 Minimum inhibitory concentration (MIC)
MIC was carried out for each plant extract that displayed antimicrobial activity. Broth dilution method (Andrews, 2001) was applied to determine the lowest concentration of antimicrobial agent which inhibits the visible growth of test microorganisms being investigated. The MIC was performed by sterile Mueller Hinton Broth (MHB) for bacteria and sterile Sabouraud Broth (SB) for fungus respectively. As mentioned above DMSO served as a negative control and standard antibiotics chloramphenicol (25 μg/disk) and nystatin (2000 units/disk) were used as positive controls for bacteria and fungus respectively. The broth was incubated at 37 °C overnight for MHB and 28 °C for 72 h for SB. MIC values were determined by recording the end point of the tube showing no growth of microorganisms.
2.6 Statistical analysis
One way analysis of variance was used in the present study to analyze the data collected. The statistical data were expressed as mean ± standard deviation with p < 0.05 significance.
3 Results
3.1 Phytochemical screening
There has been a growing interest to identify Rubiaceae medicinal plants as therapeutic agents that can effectively interfere with disease causing microbes such as bacteria and fungi. Leading to its previous discoveries with a wide range of biological effects and in an effort to capitalize on its advantages, here we investigated the antimicrobial activity of Borreria sps by in vitro approaches. Phytochemical screening of soxhlet successive crude extracts viz., hexane, ethyl acetate acetone and methanolic extracts from the whole plant of the Borreria sps showed the occurrence of various secondary metabolites such as phenolics, alkaloids, flavonoids, tannins, saponins and terpenoids (Table 1). Among the five species of Borreria, B. latifolia contained phenolics, flavonoids, tannins; B. remotifolia, B. exilis and R. brasiliensis contained phenolics, alkaloids, flavonoids, tannins and terpenoids while B. laevicaulis contained all classes of chemical constituents. Note: +, indicates presence of phytochemicals; −, indicates absence of phytochemical.
Phenolic
Alkaloids
Flavonoids
Tannins
Terpenoids
Saponins
FeCl3
Mayer
NaOH
Braymer
Salkowki
Foam test
Borreria exilis
Methanol
+
−
+
+
+
−
Ethanol
+
−
+
+
+
−
Acetone
+
+
+
+
−
−
Hexane
−
+
−
−
−
−
Borreria laevicaulis
Methanol
+
+
+
+
+
+
Ethanol
+
+
+
+
+
+
Acetone
+
+
+
+
−
+
Hexane
+
+
+
+
−
−
Borreria latifolia
Methanol
+
−
+
+
−
−
Ethanol
+
−
+
+
−
−
Acetone
+
−
+
+
−
−
Hexane
+
−
+
+
−
−
Borreria remotifolia
Methanol
+
−
+
+
−
+
Ethanol
+
−
+
+
−
−
Acetone
+
−
+
+
−
−
Hexane
−
+
+
−
−
−
Richardia brasiliensis
Methanol
+
+
+
+
−
+
Ethanol
+
−
+
+
−
−
Acetone
+
−
+
+
−
−
Hexane
−
−
−
−
−
−
3.2 Anti microbial screening
3.2.1 Zone of inhibition
The successive Borreria sps crude extracts viz. hexane, ethyl acetate, acetone and methanol were screened for antibacterial and antifungal activities by employing the disk diffusion method. The activity was recorded as a diameter zone of inhibition using the crude extract concentration ranging from 500 to 1000 μg/disk (Table 2). Hexane extracts of B. remotifolia exhibited activity against S. aureus while B. exilis exhibited activity on both S. aureus and B. subtilis (Fig. 1; Table 3). Hexane extract of B. laevicaulis exhibited significant antibacterial activity against S. typhimurium, S. aureus, B. subtilis (Fig. 2, Fig. 3) as well as antifungal activity against A. niger and C. albicans (Fig. 4). Out of these, maximum antibacterial (22.15 ± 0.07 mm) and antifungal (25.65 ± 0.78 mm) inhibition was observed in the hexane extract of B. laevicaulis at 1000 μg/disk against S. aureus (Figs. 2 and 3) and C. albicans respectively (Fig. 5). Altogether, among the different extracts, acetone and hexane extracts were found to exhibit maximum growth inhibition compared to methanolic and ethanolic extracts (Table 2; Figs. 1–5). These results suggest the antimicrobial activity was found to be stronger with non-polar fractions compared to polar fractions.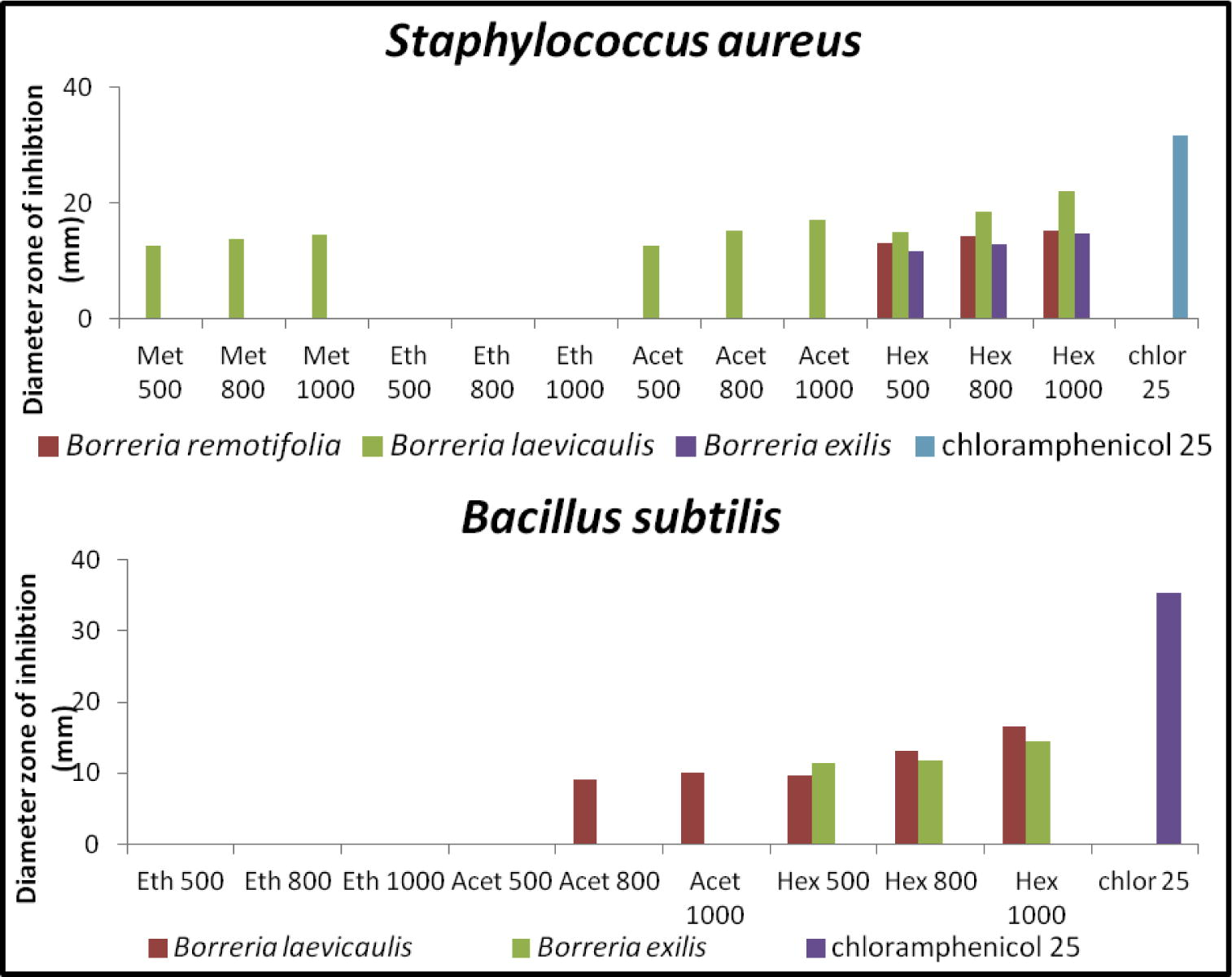
Anti-bacterial activity of Borreria sps crude extracts by disc diffusion assay. Histogram showing quantification of zone of inhibition (mm) displayed against S. aureus and B. subtilis using different concentrations (μg/disk) along with positive control (chloramphenicol).
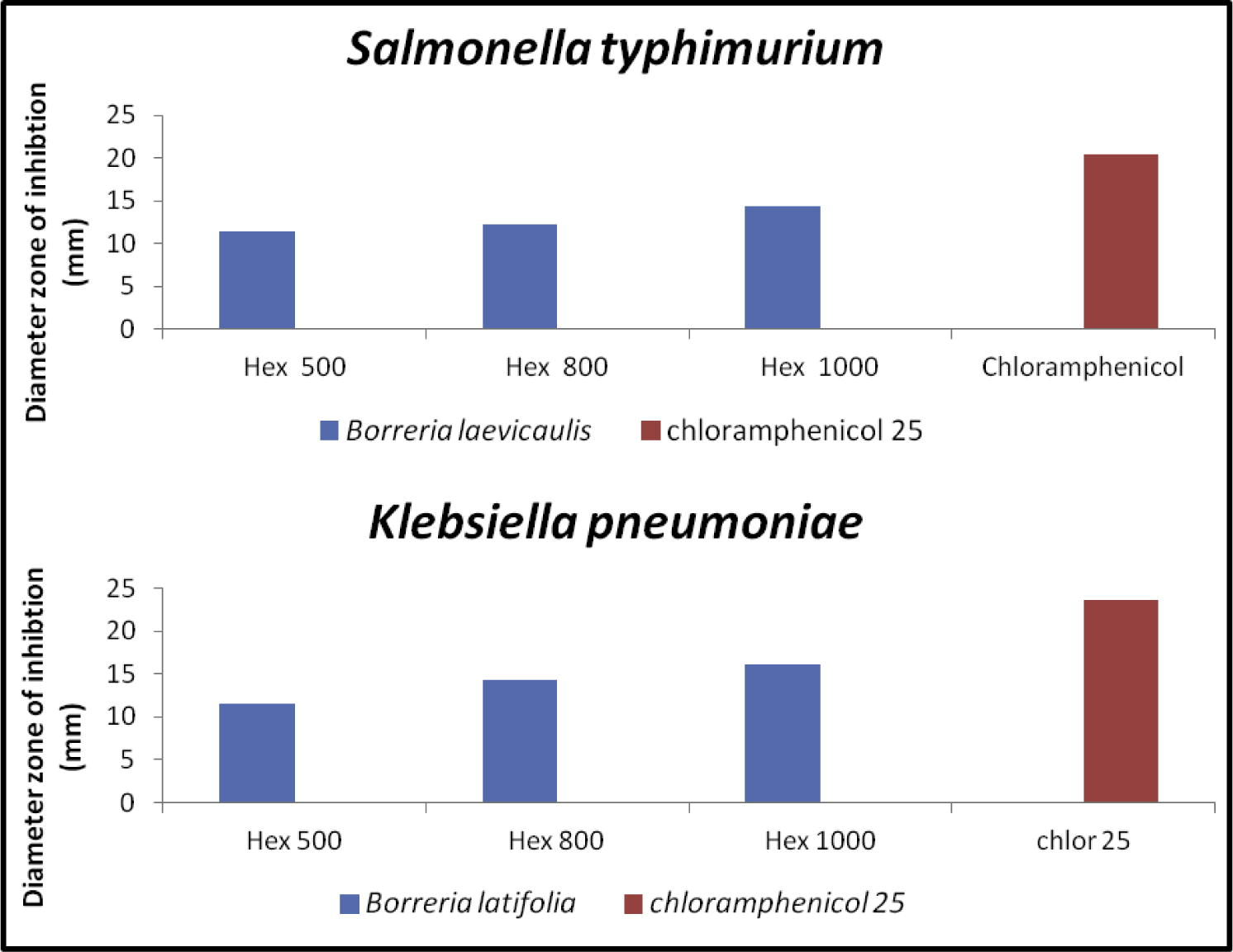
Anti-bacterial activity of hexane extracts of Borreria sps by disc diffusion assay. Histogram showing quantification of zone of inhibition (mm) displayed against S. typhimurium, K. pneumoniae using different concentrations (μg/disk) along with positive control (chloramphenicol).
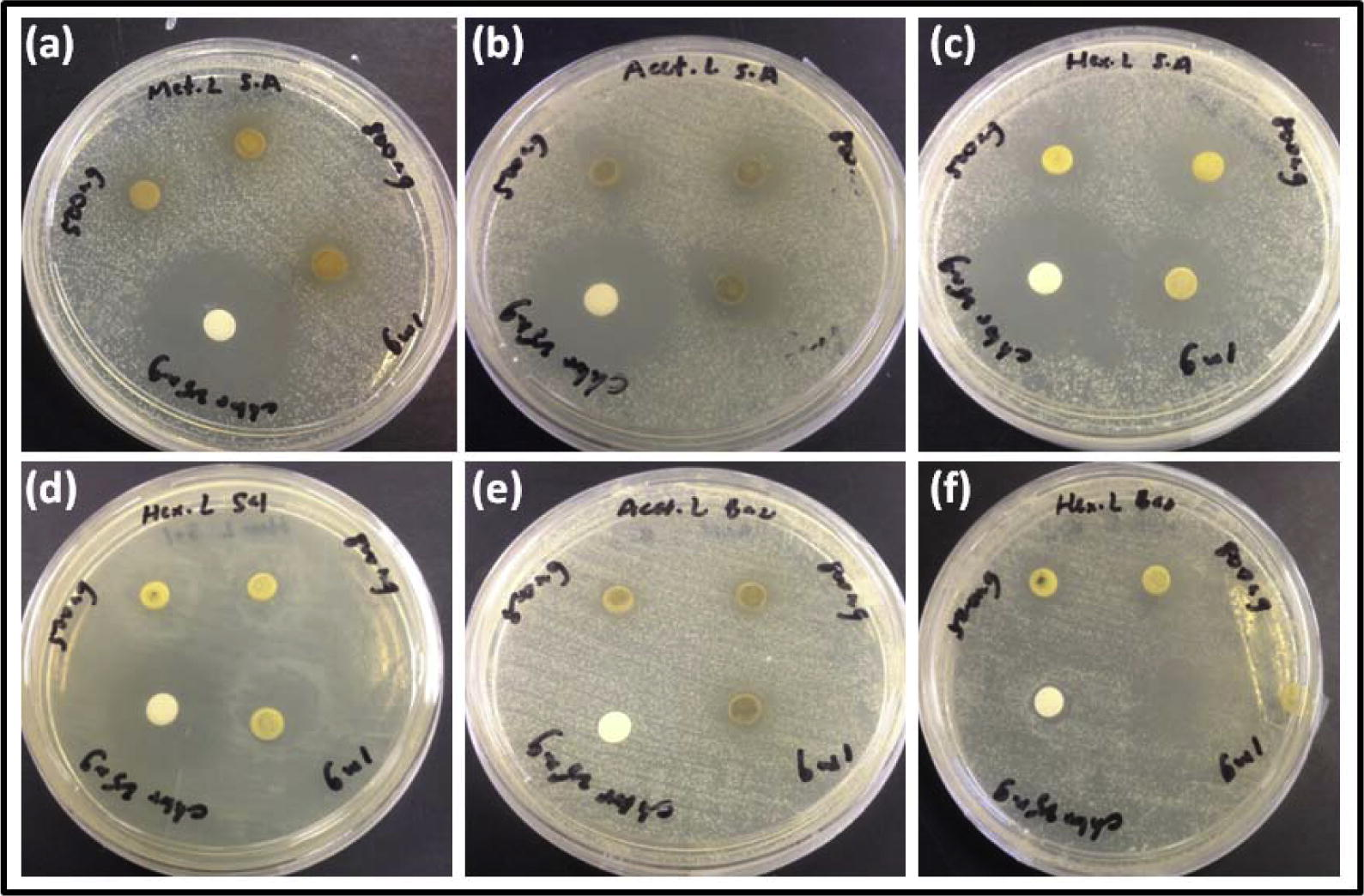
Anti-bacterial activity of Borreria laevicaulis by the disc diffusion assay. Zone of inhibition displayed on Staphylococcus aureus by (a) methanolic extracts (b) acetone extracts and (c) hexane extracts. Zone of inhibition displayed by (d) hexane extracts on Salmonella typhimurium (e) acetone extracts on Bacillus subtilis and (f) hexane extracts on Bacillus subtilis.
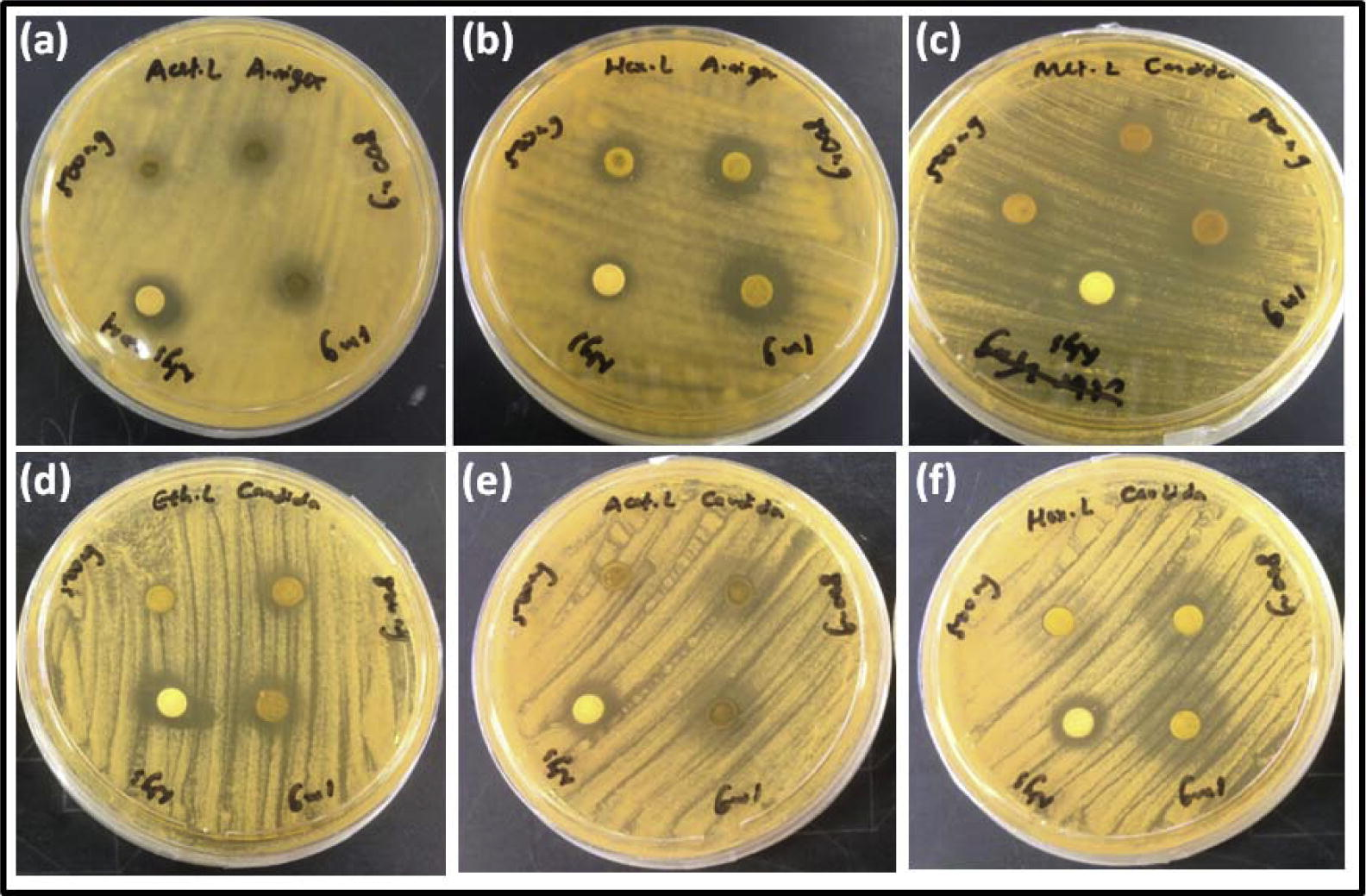
Anti-fungal activity of Borreria laevicaulis by the disc diffusion assay. Zone of inhibition displayed by (a) acetone extract (b) hexane extract on Aspergillus niger. Zone of inhibition displayed by (c) methanolic extracts (d) ethanolic extracts (e) acetone extract and (f) hexane extract on Candida albicans.
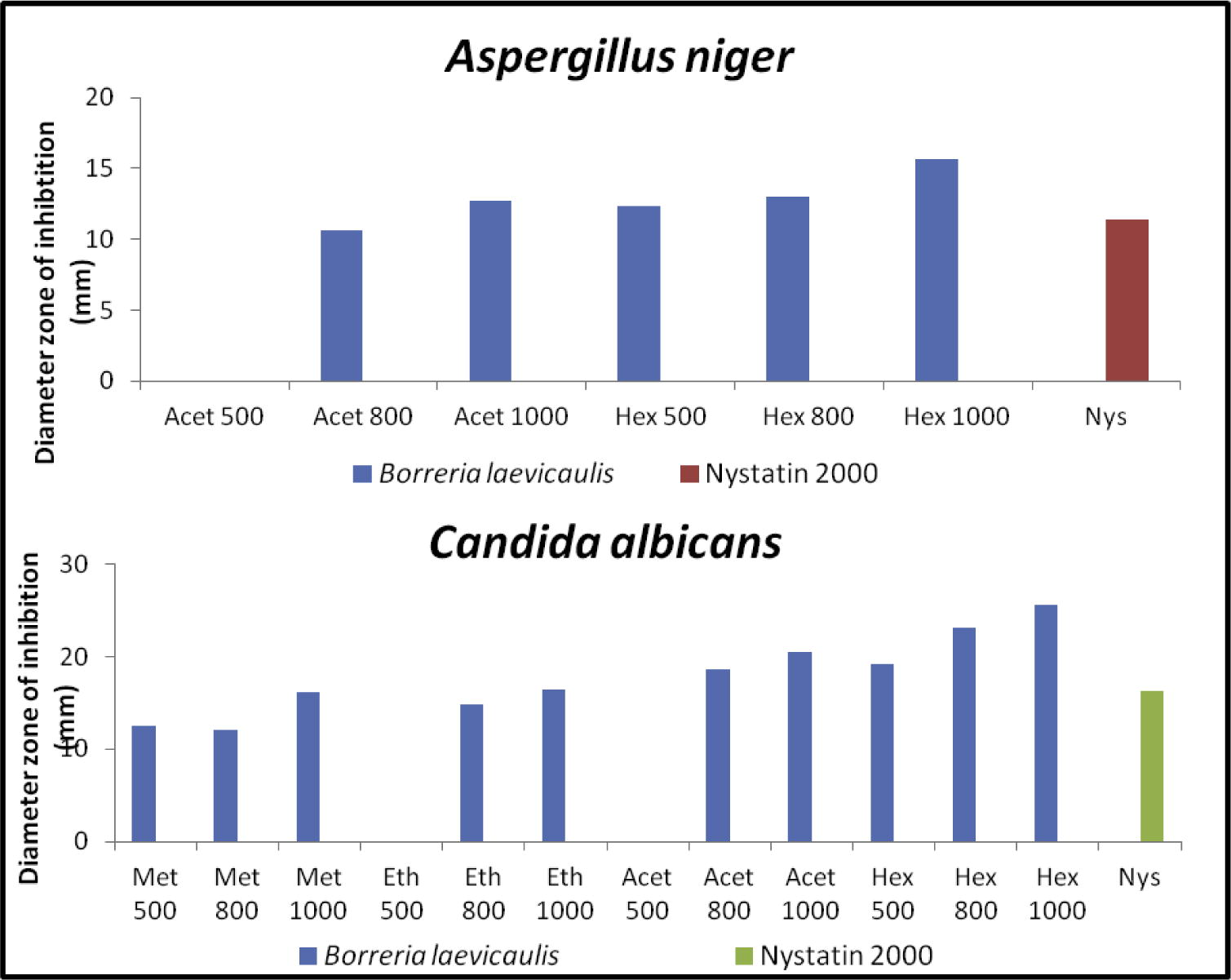
Anti-fungal activity of Borreria sps by disc diffusion assay. Histogram showing quantification of zone of inhibition displayed against A. niger, C. albicans using different concentrations (μg/disk) along with nystatin (2000 units/disk), positive control.
3.2.2 Minimum inhibitory concentration (MIC)
Next, we carried out MIC for each plant extract that only displayed antimicrobial activity. We observed minimum antibacterial (9.10 ± 0.14 mm) and antifungal (10.65 ± 0.50 mm) inhibition with an acetone extract of B. laevicaulis (800 μg/disk) against B. subtilis and A. niger (Table.2; Fig. 6). Lowest MIC for antibacterial activity was 62.5 μg/ml, on S. aureus of hexane extract of B. laevicaulis. Moreover, hexane extract of B. laevicaulis possessed a much lower MIC value of 250 μg/ml against C. albicans compared to others of 500 μg/ml (Fig. 6). Highest MIC value of 1 mg/ml was recorded on the hexane extract of B. laevicaulis against S. typhimurium. –, represents no activity. Values are the mean of triplicates ± SE. Statistical analysis was found to be significant at p < 0.05. Zone of inhibition including the diameter of the disc. Chloramphenicol for bacteria; nystatin for fungi.
Test sample
Extracts
Concentration (μg/disc)
Diameter zone of inhibition (mm)
Microorganisms
Escherichia coli
Salmonella typhimurium
Klebsiella penumonia
Staphylococcus aureus
Bacillus subtilis
Aspergillus niger
Candida albicans
Chloramphenicol
25
29.45
20.38a
23.20a
31.85a
35.33a
±0.53
±0.03
±0.28
±0.64
±0.18
Nystatin
2000 units/disc
11.42bc
16.25cd
±0.11
±0.07
DMSO
–
–
–
–
–
–
–
Borreria exilis
Hexane
500
–
–
–
11.75i
11.50de
–
–
±0.64
±0.14
800
–
–
–
13.00gh
11.70cd
–
–
±0.14
±0.85
1000
–
–
–
14.83ef
14.51b
–
–
±0.89
±0.69
Borreria laevicaulis
Methanol
500
–
–
–
12.70ghi
–
–
12.55fg
±0.78
±0.57
800
–
–
–
13.79fg
–
–
12.10g
±0.69
±0.00
1000
–
–
–
14.68ef
–
–
16.15cd
±0.68
±0.35
800
–
–
–
–
–
–
14.84def
±0.65
1000
–
–
–
–
–
–
16.45cd
±0.07
Acetone
500
–
–
–
12.61hi
–
–
–
±0.69
800
–
–
–
15.21e
9.10f
10.65c
18.64bc
±0.07
±0.14
±0.50
±0.76
1000
–
–
–
17.15d
10.08f
12.70bc
20.45b
±0.07
±0.03
±0.99
±0.07
Hexane
500
–
11.35d
–
15.13e
9.60f
12.35bc
19.15b
±0.01
±0.18
±0.71
±0.21
±0.07
800
–
12.20c
–
18.64c
13.10cd
13.00b
23.21a
±0.00
±0.76
±0.00
±0.00
±1.68
1000
–
14.40b
–
22.15b
16.60b
15.63a
25.65a
±0.28
±0.07
±0.71
±0.66
±0.78
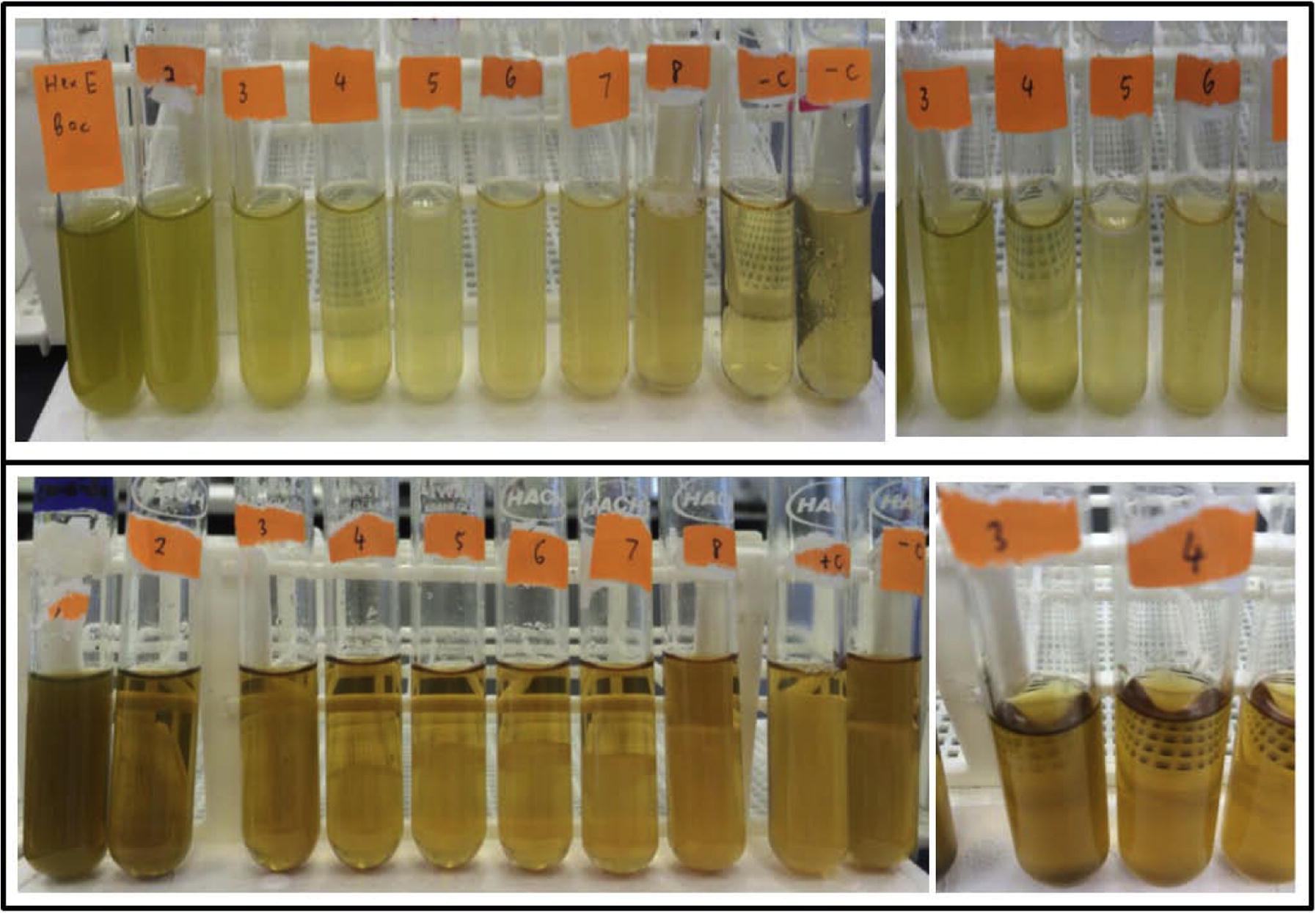
Minimum inhibitory concentration by hexane extracts of the Borreria sps. Top panel represents MIC displayed by B. exilis. Tube 4 showing the end point of clear solution and observed with turbid tube 5 onward against B. subtilisin Mueller Hinton Broth. Bottom panel represents MIC displayed by B. laevicaulis. Tube 3 showing the end point of a clear solution and observed with growing fungus in tube 5 onward against C. albicans in Sabouraud Broth.
Among the different species of Borreria, almost all tested plants except R. brasiliensis displayed growth inhibition against at least one strain of microorganism tested indicating the broad spectrum pharmacological activity of Rubiaceae (Table 3). –, represents no activity. Values are the mean of triplicates ± SE. Statistical analysis was found to be significant at p < 0.05. Zone of inhibition including the diameter of the disc. Chloramphenicol for bacteria; nystatin for fungi.
Test sample
Extracts
Concentration (μg/disc)
Diameter zone of inhibition (mm)
Microorganisms
Escherichia coli
Salmonella typhimurium
Klebsiella pneumonia
Staphylococcus aureus
Bacillus subtilis
Aspergillus niger
Candida albicans
Chloramphenicol
25
29.45
20.38a
23.20a
31.85a
35.33a
±0.53
±0.03
±0.28
±0.64
±0.18
Nystatin
2000 units/disc
11.42bc
16.25cd
±0.11
±0.07
DMSO
0
0
0
0
0
0
0
Borreria latifolia
Methanol/Ethanol/Acetone
500
–
–
–
–
–
–
–
800
–
–
–
–
–
–
–
1000
–
–
–
–
–
–
–
Hexane
500
–
–
11.60d
–
–
–
–
±0.57
800
–
–
14.24d
–
–
–
–
±1.05
1000
–
–
16.10bc
–
–
–
–
±1.55
Borreria remotifolia
Methanol/Ethanol/Acetone
500
–
–
–
–
–
–
–
800
–
–
–
–
–
–
–
1000
–
–
–
–
–
–
–
Hexane
500
–
–
–
13.20gh
–
–
–
±0.14
800
–
–
–
14.30ef
–
–
–
±0.00
1000
–
–
–
15.20e
–
–
–
±0.07
Richardia brasiliensis
Methanol/Ethanol/Acetone
500
–
–
–
–
–
–
–
800
–
–
–
–
–
–
–
1000
–
–
–
–
–
–
–
4 Discussion
Firstly, we show phytochemical screening of different species of Borreria crude extracts showing the occurrence of various secondary metabolites. Secondly, we demonstrate acetone and hexane extracts exhibit maximum growth inhibition compared to methanolic and ethanolic extracts suggesting that antimicrobial activity was found to be stronger with non-polar fractions compared to polar fractions. Third, we display B. laevicaulis as a potential candidate for antimicrobial drug discovery using in vitro studies to treat infectious diseases and antibiotic resistant pathogens. Conclusions drawn are based on several different analyses as described in our paper.
Hexane extracts induced highest zone of inhibition suggesting its antibacterial and antifungal activities. This can be explained with the active compounds which are responsible for antibacterial and antifungal activities of the extract reside in the non-polar fractions in relatively higher concentrations (Tadeg, 2004). Our observations are evidenced as shown by Onawumi et al., 2012; Nino et al., 2012. The cell wall of gram negative bacteria is more complex than gram positive bacteria which makes it more susceptible and impermeable (Kumar et al., 2006; Zaidan et al., 2005). Therefore, this explains why the gram negative bacteria were more resistant to antimicrobial compounds with its effective diffusion barrier. However, few plant species extracts still possess some degree of inhibition toward gram-negative bacteria.
It is not surprising to note that E. coli in our study was the most resistant microorganism among all bacteria strains, despite the fact that E. coli developed multi drug resistance toward different kinds of antimicrobial agents (List and Schmidt, 1989; Sader et al., 2002). On the other hand, S. aureus was the most susceptible bacteria of all the bacterial strains tested. Several reports suggest that S. aureus is the most common pathogen to cause skin infections (Satima et al., 1999; Jones et al., 2003). Thus, the fact that all selected plant species showing activity except R. brasiliensis and B. latifolia can be used for the treatment of skin infections. Although we observed no activity with R. brasiliensis, it does not mean that the plant lacks bioactive compounds. Doses employed to exhibit antimicrobial activity might be in low quantities (Mendonça-Filho, 2006; Stapleton et al., 2004; Ikigai et al., 1993; Brantner and Grein, 1994). This also suggests that usage of larger doses can overcome this problem Ogwal-Okeng et al., 2003; Rajeh et al., 2010. Further the dose employed and the types of bacterial and fungal strains used can be factors which influence its inhibitory effect (Tahiya et al., 2014).
5 Conclusion
Here, we show phytochemical screening of hexane, ethanol, acetone and methanolic extracts showed the occurrence of phenolics, alkaloids, flavonoids, tannins, saponins and terpenoids. We demonstrate acetone and hexane extracts exhibit maximum growth inhibition compared to methanolic and ethanolic extracts. Further we identify B. laevicaulis as a potential candidate for antimicrobial drug discovery based on our in vitro studies to treat infectious diseases and antibiotic resistant pathogens. Conclusions drawn are based on several different analyses as described in our paper. The present study is important as the search for new pharmacologically active compounds from plant extracts has led to the discovery of many clinically useful drugs. Nonetheless, the efficacy of these plant extracts needs to be validated in vivo. Importantly, as these extracts contain many compounds along with the active compounds they may cause side or toxic effects. Hence future directions should be focused on the isolation and identification of active compounds with antimicrobial activity rather than simply screening the plant crude extracts.
Acknowledgements
We would like to acknowledge Universiti Malaysia Kelantan for providing the facilities and laboratory assistance throughout the completion of this work. Sincere gratitude also goes to microbiology laboratory of Veterinary Research Centre and hospital Universiti Sains Malaysia for providing some microorganisms which were very helpful for this study. The authors are highly grateful to the UMK for their logistical support under Grant No. R/SGJP/A07.00/00710A/001/2012/000081).
References
- The use of medicinal plants in self-care in the Agonlin region of Benin. J. Ethnopharmacol.. 2011;133:234-243.
- [Google Scholar]
- Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother.. 2001;48:5-16.
- [Google Scholar]
- Screening of selected indigenous plants of Lebanon for antimicrobial activity. J. Ethnopharmacol.. 2004;93:1-7.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45:493-496.
- [Google Scholar]
- Antibacterial activity of plant extracts used externally in traditional medicine. J. Ethnopharmacol.. 1994;1:35-40.
- [Google Scholar]
- Antibacterial activity of some plants belonging to the family Rubiaceae: a review. WJPPS. 2012;1:1179-1194.
- [Google Scholar]
- Borreria and Spermacoce species (Rubiaceae): a review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacol. Rev.. 2012;6:46-55.
- [Google Scholar]
- Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Borreria ocymoides, Kola nitida and Citrus aurantifolia. J. Appl. Bacteriol.. 1991;71:398-401.
- [Google Scholar]
- eFloras.org, FOC [internet], 2013. Mitracarpushirtus (Linnaeus) Candolle, Prodr. 4.572.1830. [Updated 2010; cited 12 Dec 2013]. Available from <http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242332831>.
- Hall, D.W., Vandiver, V.V., Sellers, B.A., 2012. Brazil Pusley, Richardia brasiliensis (Moq.). Institute of food and agricultural sciences, University of Florida. Available from http://edis.ifas.ufl.edu/pdffiles/FW/FW03300.pdf.
- iNaturalist.org, 2013. California Academy of Science. Available from <http://www.inaturalist.org/taxa/287503-Borreria-exilis>.
- Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta. 1993;1147(1):132-136.
- [Google Scholar]
- Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int. J. Antimicrob. Agents. 2003;22:406-419.
- [Google Scholar]
- Search for antibacterial and antifungal agents from selected Indian medicinal plants. J. Ethnopharmacol.. 2006;107:182-188.
- [Google Scholar]
- Pharmaceutical technology. London: Heyden & Son; 1989. p. :51-56.
- Bioactive phytocompounds: new approaches in the phytosciences. In: Ahmad I., Aqil F., Owais M., eds. Modern Phytomedicine: Turning Medicinal Plants into Drugs. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2006. doi: 10.1002/9783527609987.ch1
- [Google Scholar]
- Evaluation of antimicrobial and modulatory activity of the extract of Richardia brasiliensis gomes. Indian J. Tradit. Knowledge. 2013;12(4):619-622.
- [Google Scholar]
- Antibacterial and antifungal activities of crude plant extracts from Colombian biodiversity. Int. J. Trop. Biol.. 2012;60:1535-1542.
- [Google Scholar]
- Acute toxicity effects of the methanolic extract of Fagara zanthoxyloides (Lam.) root-bark. Afr. Health Sci.. 2003;3:124-126.
- [Google Scholar]
- Preliminary studies on phytochemical and antimicrobial investigation of plants (Irawo-ile) Mitracarpus villosus, Euphorbia hirta and Spermacoce ocymoides. IJRRAS. 2012;10:78-81.
- [Google Scholar]
- Pharmacognostical profile of Spermacoe ocymoides (Burm. F) DC – a study on a medicinal botanical. Der Pharma. Lettre. 2012;4:1414-1425.
- [Google Scholar]
- Raaman, N., 2006. Qualitative phytochemical screening. Phytochemical techniques, New India Publishing Agency, pp. 19–22.
- Assessment of Euphorbia hirta L. & leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules. 2010;15(9):6008-6018.
- [Google Scholar]
- Phytochemical analysis of methanolic extract of Curcuma longa Linn. Int. J. Univ. Pharm. Bio Sci.. 2013;2(2):39-45.
- [Google Scholar]
- Skin and soft tissue infections in Latin American Medical Centers. Four year assessment of the pathogen frequency and antimicrobial susceptibility patterns. Diagn. Microb. Infect. Dis.. 2002;44:281-288.
- [Google Scholar]
- Prevalence of skin disease in rural Tanzania and factors influencing the choice of health care, modern or traditional. Arch. Dermatol.. 1999;134:1050-1055.
- [Google Scholar]
- Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents. 2004;5:462-467.
- [Google Scholar]
- Tadeg, H., 2004. Phytopharmaceutical studies of some selected medicinal plants locally used in the treatment of skin disorders. (Master’s thesis), Pharmaceutics, school of Pharmacy, Addis Ababa University, p. 148.
- Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci.. 2014;26:237-243.
- [Google Scholar]
- Alternative natural sources for a new generation of antibacterial agents. Int. J. Antimicrob. Agents. 2013;42:195-201.
- [Google Scholar]
- Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Eichhornia crassipes. Asian J. Plant Sci.. 2012;2:115-122.
- [Google Scholar]
- Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J. Ethnopharmacol.. 2011;137:730-742.
- [Google Scholar]
- In vitro screening of five local medicinal plants for antibacterial activity using disk diffusion method. Trop. Biomed.. 2005;22:165-170.
- [Google Scholar]







