Translate this page into:
Phytochemical investigation and evaluation of in vitro anti-inflammatory activity of Euphorbia hirta ethanol leaf and root extracts: A comparative study
⁎Corresponding author. drkkdsd@gmail.com (Kuntal Das),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Inflammation is an unpleasant complex biological condition that is with macrophages, leucocytes, and even mast cells. Many marketed effective chemical anti-inflammatory drugs are available but due to many disadvantages people are relying on the herbals with low health risk and toxicity.
Objective
The research was performed to carryout the phytochemical investigation and explore the in vitro anti-inflammatory activities of leaf and roots extracts of Euphorbia hirta (EH).
Methodology
Dried EH parts (viz. leaf and roots) were extracted with ethanol solvent using Soxhlet method and preliminary screened for presence of various phytochemicals. The extracts were also further tested for total alkaloids, flavonoids, and phenolics. After that, anti-inflammatory activity was measured in vitro using albumin denaturation, anti-proteinase, and anti-lipoxygenase activities against conventional anti-inflammatory agent, aspirin (100 g/ml). Finally, a correlation study was generated from the results for presence of the amount of flavonoid and phenolics in connection to the activity.
Results
The result revealed the yield was higher in ethanol root extract (48.2 g) than leaf extract. Bioactive compounds alkaloids, glycosides, flavonoids, tannins, and phenolics were reported in leaf and root extracts. Further, the number of total flavonoids and phenolics showed higher in ethanolic leaf extracts (87.53 mg QE/g and 143.20 mg GAE/g). Total alkaloids content was higher in ethanolic root extracts (13.01 mg atropine/g). Ethanol EH leaf extract exhibited significant anti-inflammatory activity than root by inhibiting albumin denaturation, proteinase, and the lipoxygenase activities with 87.51 %, and 51.2 % followed by 97.30 %, and 54.21 % followed by 94.43 % and 48.21 % respectively at 100 µg/ml concentration.
Conclusion
The results affirmed that ethanol EH leaf extract showed better anti-inflammatory activity than root extracts when evaluated with three different in vitro models and the dose dependency activity was recorded.
Keywords
Euphorbia hirta
Phytochemicals
Albumin denaturation
Anti-proteinase
Anti-lipoxygenase
1 Introduction
Since ancient times, various parts of multiple medicinal plants have been used to treat distinct afflictions, especially in India. India's indigenous medical systems, such as Ayurvedic, Siddha, and Unani, have been practiced for generations. In modern medicines, herbs occupy significant importance as herbalism (therapeutic use of herbal plants). Synthetic medications are useful in combating a variety of disorders, yet they remain out of reach for not only do they number in the millions, but they also cause many serious adverse effects. On the other way, the use of herbalism as modern medicamentis gaining importance with their negligible toxicity and reachable to all people with low cost. Therefore, the role of herbs in our daily life cannot be ignored. The present research endowed an anti-inflammatory activity of EH plant. Inflammation is a distressing and complicated biological disease (Babu et al., 2009). Arachidonic acid metabolites change the permeability of vasculature making it easier for leukocytes to migrate to the inflammatory area (Sarkhel, 2015; Yasmen et al., 2018). At large doses or for long periods of usage of commercially available anti-inflammatory treatments including steroidal, nonsteroidal drugs can cause unwanted and dangerous side effects such as osteoporosis, ulcer aggravation, and major infections (Da Silvaet al., 2014).
Of late, Euphorbia hirta (EH) (family Euphorbiaceae) is one of the common plant in the subtropical region and, considered as a weed that is distributed to the hotter parts in tropical and subtropical countries, especially in India (Tripathi et al., 2021). Traditionally, the EH was applied for diseases, cough, bronchitis, asthma, diarrhea, jaundice, acne, gonorrhea, digestive issues, tumors, and measles (Tripathi, et al., 2021) and grows well in roadside, opened grassland in temperature ranges from 10 °C to 20 °C. Antimicrobial, anti-inflammatory, anti-amoebic, antifertility, anti-malarial, antioxidant, sedative, cytotoxic, aflatoxin inhibition, larvicidal, immunomodulatory, and other properties were reported of EH (Verma, 2017). Recently, the plant was studied for its possible role against SARS-CoV-2 (Khursheed et al., 2022). The activities are mostly attributable with plant secondary metabolites in EH, particularly flavonoids (quercetin, quercitrin, quercitol) (Yan et al., 2011), terpenoids (triterpenes: α-amyrin, β-amyrin, friedelin, taraxerol) (Baslas and Agarwal, 1980), phenols, tannins (especially terchebin, the monomerichydrolysable tannins) (Ogunlesiet al., 2009), and essential oil. It is also evident that plant constituents are varied with the geographical location, climatic conditions, soil nature and many other factors (Kumar et al., 2017; Bisht et al., 2018; Das et al., 2019). Not only that, but the bioactivity of plant elements differs depending on geographic location (Mangoale and Afolayan, 2020; Vilkickyte and Raudone, 2021). Herbs are relatively safe, therapeutically effective, economical, and dependable natural supply of pharmaceuticals in all countries, as well as being less poisonous and more accessible. There are no published reports on comparative investigation of roots and leaf for anti-inflammatory activity through in vitro models. Bioactive chemicals such as alkaloids, flavonoids, saponins, glycosides, coumarins, and terpenoids have been suggested as a good starting point for discovering and developing new anti-inflammatory medications (Meshram et al., 2015). Therefore, this study was carried out to evaluate the phytochemical constituents of leaf and root extracts of Euphorbia hirta and correlate it with their in vitro anti-inflammatory activities.
2 Materials and method
2.1 Collection of plant material
The whole EH plants were collected (before flowering stage) from field side of IIHR, Bengaluru, Karnataka. The EH plant was authenticated by registered plant taxonomist from IIHR, Bengaluru and the whole dried plant was preserved with the voucher specimen (KCP-PCOG/EH/330) in our department’s herbarium. Thereafter, the separated said parts were used for further investigation (Fig. 1).
2.2 Preparation of plant crude extracts
The EH plant parts (leaf and roots) were cleaned with running tap water and air dried in shade for 2 weeks and then coarse powdered made separately and finally stored into the plastic sealed cover with proper labeling to prevent any contamination until it is used for the research activities.
The ethanol was used as solvent for the extract preparation. Extraction was performed using 250 g of powdered drugs by Soxhlet method at temperature of 40 °C for 4 hrs. Filtered the extract with No 42 Whatman filter and thickened to produced viscos consistency using rotary evaporator at 50 °C.
2.3 Phytochemical screening
The presence of a group of bioactive compounds in the EH plant extracts was performed separately to determine the presence of bioactive constituents namely, alkaloids, glycosides, tannins, saponins, terpenoids, flavonoids, and sterols as per standard methods (Das et al., 2020).
2.4 Total phenolics
2.4.1 Standard gallic acid preparation for calibration curve
Folin–Ciocalteu colorimetric technique with modification was used to evaluate total phenolic content in ethanolic extracts from EH's leaf and roots. The absorbance of the resulting solution was recorded (650 nm) based on the details described elsewhere (Siddiqui et al., 2017).
2.5 Preparation of samples for total phenolic content
1 mg/ml of sample was mixed in Folin-phenol Ciocalteu's reagent (1 mL). The extracts' TPC was measured in mg gallic acid equivalents (GAE) per gram of dry weight (mg/g).
2.6 Total flavonoid content
2.6.1 Standard quercetin for calibration curve
The TFC of the extracts was determined using an aluminium chloride colorimetric assay. Based on the calibration curve, the TFC was represented as quercetin equivalents.
2.6.2 Preparation of samples for total flavonoid content
TFC was calculated using the manner previously stated (Shamsa et al., 2008). The TFC was determined as quercetin equivalent based on the standard calibration curve (mg QE/g).
2.7 Determination of total alkaloids content
2.7.1 The calibration curve of atropine
Bromocresol green solution was made by heating 69.8 mg bromocresol green with 3 mL NaOH 2 N and 5 mL distilled water. The detail of the method was described by Shamsa et al., 2008.
2.7.2 Sample preparation for total alkaloid content
After being dissolved in HCl 2 N, a part of this residue was filtered. The extracts were placed in a 10 mL volumetric flask and diluted with chloroform to make up the volume. In chloroform, the complex's absorbance was measured at 470 nm.
2.8 In vitro anti-inflammatory efficacy
2.8.1 Albumin denaturation inhibition
The anti-inflammatory efficacy of ethanolic EH roots and leaf extract were tested using a modified form of the standard method (Rajesh et al., 2019). The protocol was followed for inhibiting albumin denaturation. Any anti-inflammatory drugs help in prevention of the protein or albumin denaturation, which acts as antigens and prompts autoimmune diseases and hence the present study was undertaken. The reaction mixture is made up of an equal volume of varying concentrations of test extracts (20–100 g/ml) and 1 % aqueous bovine albumin. A little amount of 1 N HCl was used to alter the pH. The sample was incubated at 37 °C for 20 min before being heated to 51 °C for another 20 min. Finally, % inhibition of protein denaturation was calculated.
2.8.2 Anti proteinase activity
The effectiveness was determined as per earlier method with some modifications (Sakat et al., 2010). In the present study in vitro, antiinflammatory activity was performed by proteases inhibition activity because protease enzymes have correlation with inflammatory activity. They can hydrolyze peptide bonds and degrading other proteins. They also can cause inflammation by controlling the expression and activity of pro-inflammatory cytokines, chemokines, and other immune components. The % inhibition of proteinase effect was estimated using a formula described by Sakat et al. (2010). where A1, absorption of the control sample, A2 = absorption of the EH extract.
2.8.3 Anti-lipoxygenase efficacy
Anti-lipoxygenase efficacy was established with minor modifications, using linoleic acid as substrate and lipoxidase as an enzyme (Eshwarappa et al., 2016). The said activity was performed because the lipoxygenase enzyme pathway plays an immense role in the formation of inflammatory disorders and the non-inflammatory drugs inhibit lipoxygenase activity and enhances tissue regeneration.
2.9 Statistical analysis
For all the parameters in the experiment, means and SEM (standard error of the mean) were determined. To assess the significant difference at p < 0.05, these means were statistically analyzed using a one-way ANOVA. Correlation between total flavonoids, phenolics and alkaloids was drawn with different anti-inflammatory efficacy.
3 Results
3.1 Yield with phytochemical screening
The yields of the extract from the conducted experiment were depicted in Fig. 1. Higher yield has been observed in ethanol extract of leaf (48.2 g w/w) followed by roots ethanol extract of EH (27.8 g w/w).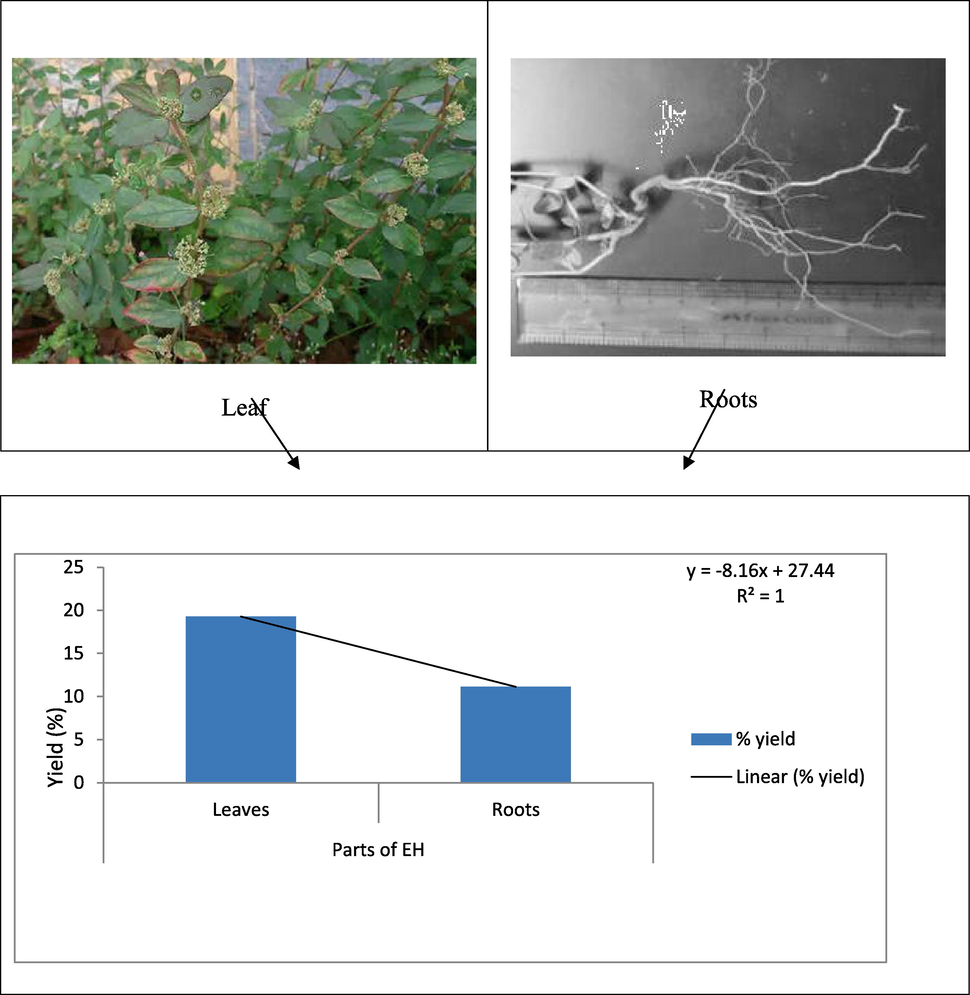
EH leaf and root parts and their yields (n = 1).
3.2 Phytochemical screening
Various phytochemical screening for both extracts were carried out separately and the presence of maximum phytochemical groups showed in the ethanol extract of the leaf rather than the root of the EH plant, according to the results. Both extracts contained alkaloids, flavonoids, sterols, tannins, and glycosides, but the ethanol leaf extract had a prominent impact on color change reactions (Table 1). (+++) = Prominent; (++) = Medium; (+) = very less present; (–) = Absent.
Phytochemicals
EH plant
Ethanol extract (Leaf)
Ethanol extract (Roots)
Glycoside
++
+
Alkaloids
++
+++
Tannins
++
+
Flavonoids
++
+
Sterols
++
+
Phenolics
++
+
Saponins
–
–
Terpenoids
–
+
3.3 Total phenolic contents
The TPC of various preparations of EH roots and leaf were determined using the Folin–Ciocalteu technique and gallic acid as the reference. The calibration curve was constructed using absorbance values obtained at various gallic acid concentrations (Fig. 2). The phenolic concentration of the ethanol EH leaf extract was the higher than the ethanolic root extract. The leaf extract had a phenolic concentration of 143.20 ± 0.21 mg GAE/g, whereas the root extract had a TPC of 124.39 ± 0.13 mg GAE/g (Table 2). Each value in the table is represented as mean ± SEM (n = 3).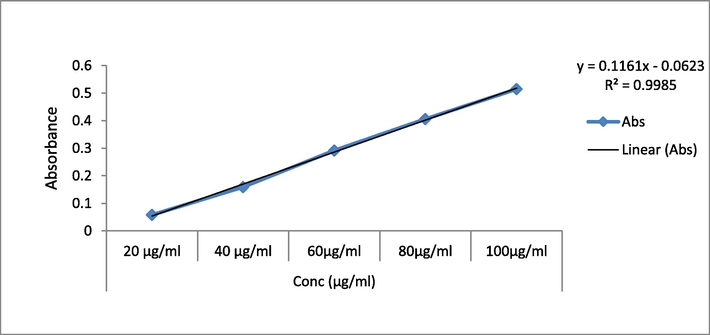
Calibration curve of Gallic acid at various concentrations.
Ethanol extract
Leaf of EH
Roots of EH
Total phenolics content
(mg gallic acid equivalent/g)143.20 ± 0.21
124.39 ± 0.13
Total flavonoid content
(mg quercetin equivalent/g)87.53 ± 0.30
63.41 ± 0.42
Total Alkaloid content
(mg Atropine equivalent/g)7.31 ± 0.24
13.01 ± 0.11
3.4 Total flavonoid contents
The TFC was estimated for extracts by the calibration curve's regression equation (Y = 0.114x; R2 = 0.994) and reported as milligrams of quercetin equivalents (QE) per gram of dry weight (mg/g) (Fig. 3). The TFC values followed the same pattern as the TPC values. The leaf extract of EH showed higher TFC value than the root extract. The TFC value was 87.53 ± 0.30 mg QE/g for ethanolic leaf extract, and it was 63.41 ± 0.42 mg QE/g for root extract (Table 2).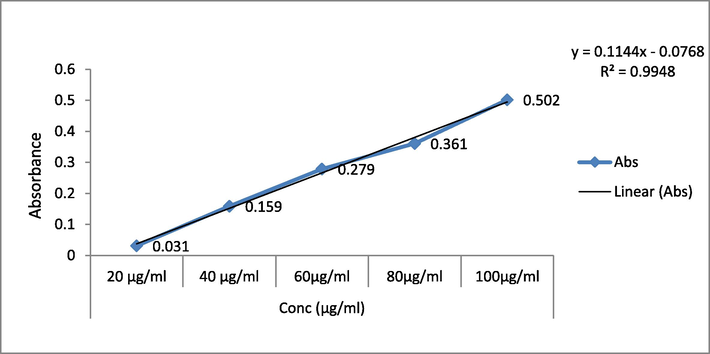
Calibration curve of Quercetin at various concentrations.
3.5 Total alkaloid content
The TAC was resulted as per the calibration curve's regression equation (Y = 0.111x; R2 = 0.991) and represented as mg atropine equivalents per gram of dry weight (mg/g) (Fig. 4). The root extract of EH has the greatest TAC value, followed by the leaf extract. The higher TAC value for ethanolic root extract was reported as 13.01 ± 0.11 mg atropine/g whereas the same for the leaf extract was 7.31 ± 0.24 mg atropine/g (Table 2).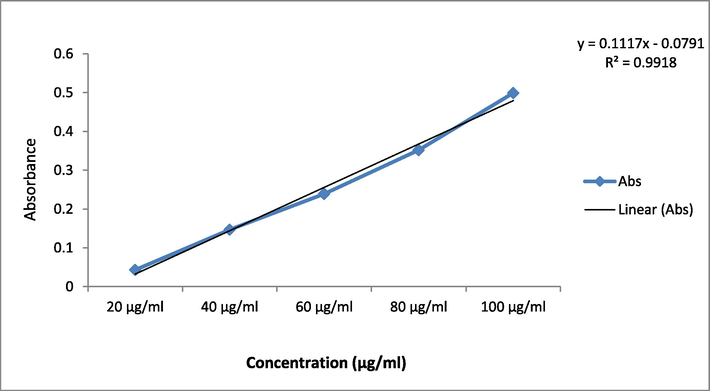
Calibration curve of Atropine at various concentrations.
3.6 In vitro anti-inflammatory effectiveness
3.6.1 Albumin denaturation study
The protein (albumin) denaturation inhibition was investigated in this work was studied and was found the maximum in ethanolic leaf of EH with 87.51 ± 1.10 % at 100 µg/ml, while root ethanol extract showed 64.20 ± 0.03 % at 100 µg/ml as compared to aspirin (102.32 ± 0.22 %) at 100 µg/ml concentration. Results are presented in Fig. 5.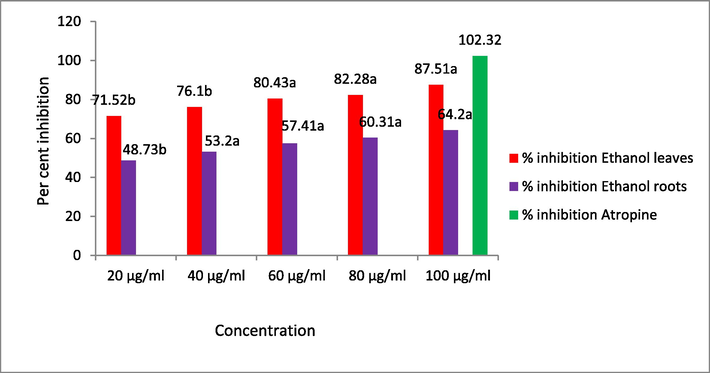
The inhibition of protein (albumin) denaturation. All values are expressed as mean ± SEM (n = 4), statistically significant at a = p < 0.05, b = p < 0.01; Values are compared with standard by using one way ANOVA followed by Dunnette’s multiple comparison test.
3.6.2 Anti proteinase efficacy
Both extracts were tested for proteinase inhibitory efficacy and found to have a strong inhibitory effect. The higher concentration was reported in ethanol leaf extract, followed by EH root extract. Ethanolic EH leaf extract showed with 97.30 ± 0.58 %, and minimum in root extract 54.21 ± 0.11 % at 100 µg/ml concentrations (Table 3). All values are expressed as mean ± SEM (n = 4), statistically significant at (*) = p < 0.05 (**) = p < 0.01; Values are compared with standard by using one way ANOVA followed by Dunnette’s multiple comparison test.
Parameters
% Proteinase inhibitory action at various conc
20 µg/ml
40 µg/ml
60 µg/ml
80 µg/ml
100 µg/ml
Ethanol leaf of EH
75.10 ± 0.13*
83.10 ± 0.14*
88.31 ± 0.08 *
93.28 ± 0.32**
97.30 ± 0.58 **
Ethanol root of EH
34.67 ± 0.04*
38.41 ± 0.14*
44.36 ± 0.21*
49.24 ± 0.16**
54.21 ± 0.11**
Atropine standard
–
–
–
–
108.31 ± 0.04
Parameters
% Lipoxygenase inhibitory actionat various conc
20 µg/ml
40 µg/ml
60 µg/ml
80 µg/ml
100 µg/ml
Ethanol leaf of EH
73.63 ± 0.11*
78.10 ± 0.04*
84.14 ± 0.31 *
90.21 ± 0.02**
94.43 ± 0.23 **
Ethanol root of EH
29.77 ± 0.13*
36.21 ± 0.10*
40.66 ± 0.20*
44.14 ± 0.11**
48.21 ± 0.02**
Atropine standard
–
–
–
–
113.08 ± 0.22
3.6.3 Anti-lipoxygenase activity
Anti-lipoxygenase efficacy was revealed maximum in EH leaf (ethanol extract) with 94.43 ± 0.23 % whereas, minimum activity was observed in ethanol extract of root with 48.21 ± 0.02 % of inhibition at 100 µg/ml concentration (Table 3) but the activity was dose dependent manner.
3.7 Correlation study
Finally, the correlation between the TPC, TFC with anti-inflammatory efficacy was carried out. The significant positive correlation (p < 0.05) with the ethanolic extracts but higher with leaf extract which are depicted in Table 4. In the same manner the correlation study for ethanolic root extracts is also depicted in Table 5. Significant at (**p < 0.01); (*p < 0.05). Significant at (**p < 0.01); (*p < 0.05).
Leaf ethanol extract
Total phenolics
% Albumin inhibition
% Proteinase inhibition
% Lipoxygenase inhibition
Total phenolics
1
% Albumin inhibition
0.975*
1
% Proteinase inhibition
0.983*
0.998**
1
% Lipoxygenase inhibition
0.992**
0.993**
0.998**
1
Total Flavonoids
1
% Albumin inhibition
0.985*
1
% Proteinase inhibition
0.988*
0.998**
1
% Lipoxygenase inhibition
0.998**
0.996**
0.998**
1
Root ethanol extract
Total phenolics
% Albumin inhibition
% Proteinase inhibition
% Lipoxygenase inhibition
Total phenolics
1
% Albumin inhibition
0.971*
1
% Proteinase inhibition
0.980*
0.981*
1
% Lipoxygenase inhibition
0.996**
0.993**
0.991**
1
Total Flavonoids
1
% Albumin inhibition
0.981*
1
% Proteinase inhibition
0.983*
0.982*
1
% Lipoxygenase inhibition
0.988*
0.998**
0.989*
1
4 Discussion
The collected EH roots and leaf parts were subjected to ethanol extraction followed by yield estimation. The crude extract yield (w/w) was revealed to be higher with the ethanol leaf sample than with the root sample. This was due to the bioactive compounds of leaf being more dissolved in the ethanol solvent than the root part, which resulted in a higher yield. It was evident that when several solvents were tried for the extraction of the plant, ethanol among the solvents provided a higher yield than others and showed that the content of total alkaloids and flavonoids was higher than other solvents (Rastogi and Mehrotra, 2002). The same trend was followed in this research investigation.
Following that, the content of phytochemicals in both extracts was studied and revealed a more prominent presence of phytochemicals in leaf ethanol extract than in root extract. It indicated that the leaf ethanol extract contained more bioactive constituents in the dissolved form. It was observed that acidic soil pH (5–6) enhances the availability of most micronutrients to plants and accumulates in the leaf of the plant, which also favours plant growth (HoudaLezoul et al., 2020). An earlier report also revealed the same trend (Loncaric et al., 2008).
Further, TPC, TFC, and TAC for both the extracts were evaluated and revealed higher flavonoids and the presence of bioactive principles such as flavonoids, glycosides, tannins, steroids, and others in the leaf extract of the EH plant, which could be attributed to the presence of the above-mentioned constituents and others. The ethanol content of the leaf extract was higher than that of the root ethanol extract, which could be attributed to the presence of secondary metabolites (Basyal et al., 2021). Flavonoids and phenolics were discovered to have a key role in antioxidative activity due to their hydroxyl groups' ability to scavenge free radicals (Rana et al., 2017). Total alkaloids can be determined by spectrophotometer using bromocresol green, which is a simple and sensitive method. The proposed method has the benefit of confirmation and identification of alkaloid structure in the EH plant constituents and plays a significant role in inflammation reduction with its nitrogen atom in the cyclic structure (Falcão et al., 2005). Plant polyphenols (phenolic acids, anthocyanins, hydroxycinnamic acid derivatives, flavonoids, and non-flavonoids), which are synthesised from phenylalanine or its precursor, Shikimic acid, are important dietary antioxidants because of their structural chemistry. They are powerful free radical scavengers and show anti-inflammatory effects (Govindappa et al., 2011). Because of their redox properties, the presence of conjugated ring structures, and carboxylic groups, they inhibit lipid peroxidation (Saso et al., 2001). Therefore, the determination of total phenolics with flavonoid content in the present study was worthwhile to correlate with the anti-inflammatory activity. In our experiment, leaf showed the presence of both phenolics and flavonoids in high content, which provide a wider range of therapeutic activities than other parts of the EH plant, and the outcome was found to be consistent with previous scientific findings (Govindappa et al., 2011).
Because of the limited use of laboratory animals, ethical concerns, and a lack of logic for their use, in vitro models have been chosen. The anti-inflammatory property was assessed by estimating protein (albumin) denaturation, proteinase inhibition, and lipoxygenase inhibition bioassays. Tissue protein denaturation is a well-known example of inflammatory and arthritic disorders (Russo et al., 2000). Tissue injury could be caused by denaturation of cell protein components or intercellular material (Osman et al., 2016). As a result, a substance's capacity to prevent protein denaturation indicates that it has anti-inflammatory potential. Protein denaturation inhibition may be significant in the anti-rheumatic effect of NSAIDs. Inflammatory activity can thus be reduced by inhibiting protein denaturation. The denaturation of proteins in vivo may be the cause of auto-antigen production in certain arthritic conditions (Rackova et al., 2007). Proteinase inhibition activity was also related to the reduction of inflammation by indirect inhibition of the neutral proteinases or by suppression of the infiltration into inflammatory loci (Shigetomi et al., 2010). Thereafter, lipoxygenases are essential non heme enzymes in leukotriene and lipoxin production that regulate essential cellular responses in inflammation. These iron-containing enzymes use molecular oxygen for the diooxygenation of arachidonic acid to form hydroperoxides. As a result, anti-inflammatory medication research could benefit from medicines that prevent protein denaturation. Leukotrienes are involved in a variety of inflammatory illnesses, including arthritis, asthma, cancer, and allergies (Khasawneh et al., 2011). Arachidonic acid metabolism may play a major role in anti-inflammatory processes. As a result, lipoxygenase catalyses the deoxygenation of polyunsaturated fatty acids to produce cis- and trans-conjugated diene hydroperoxides (like leukotrienes), which are essential mediators in inflammatory diseases (Gunathilake et al., 2018). The flavonoids and phenolic compounds derived from EH leaf act as more potent anti-inflammatory mediators than the root. A similar activity was reported by earlier research (Ginwala et al., 2019).
Finally, correlation study affirmed the strong and significant positive correlation among the flavonoids, phenolics with anti-inflammatory activity. In previous literature, it was shown that there was a link between anti-inflammatory activity, polyphenol content, and antioxidant capacity in various culinary herbs (Shahidi and Ambigaipalan, 2015; Zhang et al., 2018). In our present study followed the same trend where leaf showed higher anti-inflammatory activity than root extract of EH.
5 Conclusion
The results of this investigation demonstrated the ability of E. hirta extract to function as a potential natural source of anti-inflammatory action in various in vitro settings. Flavonoids and phenolic compounds were present in both the leaf and roots of EH. These compounds may be used to treat various illnesses and oxidative stress. Due to the high concentration of the flavonoids and phenolics in the leaf extract, it demonstrated greater action than the roots. Even the correlation study supported the leaf ethanol extract's favorable effects. The results may therefore be utilized to develop herbal medicines to treat diseases like inflammation brought on by oxidative stress. Additionally, the in vivo anti-inflammatory properties of these extracts must be assessed and linked to the mechanism of specific bioactive ingredients.
Funding
Majid Alhomrani would like to acknowledge Taif University for support No. TURSP (2020/257). One of the coauthors, Syed Mohammed Basheeruddin Asdaq, would like to thank AlMaarefa University, Riyadh, Saudi Arabia for extending support (TUMA-2021-1) to do this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti–inflammatory activity of Albizia lebbeck Benth, an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol.. 2009;125(2):356-360.
- [Google Scholar]
- Isolation and characterization of different constituents of Euphorbia hirta Linn. Curr. Sci.. 1980;49:311-312.
- [Google Scholar]
- Phytochemical screening and in vitro antioxidant and anti-inflammatory activities of aerial parts of Euphorbia hirta L. J. Nepal Chem. Soc.. 2021;42(1):115-124.
- [Google Scholar]
- Effect of cultural condition on evaluation of hepatoprotective activity of ethanolic bark extract of Anogeissus latifolia on ethanol induced hepatotoxicity. Asian J. Pharm. Clin. Res.. 2018;11(11):247-252.
- [Google Scholar]
- Evaluation of anti–inflammatory and mechanism of action of extract of Macrosiphonialongiflora (Desf.) Müll. Arg. J. Ethnopharmacol.. 2014;154(2):319-329.
- [Google Scholar]
- Phytochemical screening and metallic ion content and its impact on the antipsoriasis activity of aqueous leaf extracts of Calendula officinalis and Phlebodiumdecumanum in an animal experiment model. Turk. J. Pharm. Sci.. 2019;16(3):292-1202.
- [Google Scholar]
- Phytochemical screening and establishment of the antidiabetic potential of aqueous leaf extract of the endangered plant Decalepis nervosa in rats with alloxan-induced diabetes. Turk. J. Pharm. Sci.. 2020;17(3):319-328.
- [Google Scholar]
- Anti-lipoxygenase activity of leaf gall extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae). Pharmacognosy Res.. 2016;8(1):78-82.
- [Google Scholar]
- Review of the plants with anti-inflammatory activity studied in Brazil. Rev. Bras. Farmacogn.. 2005;15:381-391.
- [Google Scholar]
- Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel). 2019;8(2):35.
- [Google Scholar]
- Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of Crotalaria pallida Aiton. Afr. J. Pharm. Pharmacol.. 2011;5(21):2359-2371.
- [Google Scholar]
- Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7:22.
- [Google Scholar]
- Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules. 2020;25:4672.
- [CrossRef] [Google Scholar]
- Antioxidant, anti-lipoxygenase and cytotoxic activity of Leptadenia pyrotechnica (Forssk.) Decne polyphenolic constituents. Molecules. 2011;16:7510-7521.
- [Google Scholar]
- Euphorbia hirta as a gold mine of high-value phytochemicals: A comprehensive review of its pharmacological activities and possible role against SARS-CoV-2. Biomed. Res. Ther.. 2022;9(2):4930-4949.
- [Google Scholar]
- Total and plant available micronutrients in acidic and calcareous soils in Croatia. Cereal Res. Commun.. 2008;36:331-334.
- [Google Scholar]
- Mangoale R.M., Afolayan A.J. Comaprative phytochemical constituents and antioxidant activity of wild and cultivated Alepideaamatymbica Eckl. & Zeyh. Biomed. Res. Int. 2020; 2020: Article ID: 5808624. 13 pages. http://doi.org/10.1155/2020/5808624.
- Evaluation of the anti– inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeckin rats. J. Tradit. Complement. Med.. 2015;6(2):172-215.
- [Google Scholar]
- Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn (Euphorbiaceae), a potential medication for asthma. Afr. J. Biotechnol.. 2009;8(24):7042-7750.
- [Google Scholar]
- In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult. Ethnopharmacol.. 2016;5(4):343-349.
- [Google Scholar]
- Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquafolium extract and isoquinoline alkaloids. J. Inflamm.. 2007;4:1-7.
- [Google Scholar]
- Anti-inflammatory activity of ethanol extract of Niebuhria apetala (roth) Dunn – In vitro models. Asian J. Pharm. Clin. Res.. 2019;12(5):278-281.
- [Google Scholar]
- In-vitro anti-inflammatory and antioxidant activity of ethanolic extract of Marchantia polymorpha in Kumaun region. World J. Pharm. Res.. 2017;7(9):864-875.
- [Google Scholar]
- Glossary of Indian Medicinal Plants. New Delhi, India: National Institute of Science Communication; 2002.
- Bioflavonoids as antiradical, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol.. 2000;16(2):91.
- [Google Scholar]
- In-vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci.. 2010;2:146-155. https://innovareacademics.in/journal/ijpps/Vol2Issue1/322.pdf. In this issue
- [Google Scholar]
- Inhibition of heat-induced denaturation of albumin by nonsteroidal anti-inflammatory drugs (NSAIDs): Pharmacological implications. Arch. Pharmacal Res.. 2001;24(2):150-158.
- [Google Scholar]
- Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects –A review. J. Funct. Foods. 2015;18:820-897.
- [Google Scholar]
- Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J. Pharm. Sci.. 2008;32:17-20.
- [Google Scholar]
- Anti-inflammatory actions of serine protease inhibitors containing the Kunitz domain. Inflamm. Res.. 2010;59(9):679-687.
- [Google Scholar]
- Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth) J. Taibah Univ. Med. Sci.. 2017;12(4):360-363.
- [Google Scholar]
- Euphorbia hirta Linn - an invasive plant: a review of its traditional uses, phytochemistry and pharmacological properties. Int. J. Pharm. Sci. Res.. 2021;12(12):6189-6201.
- [Google Scholar]
- A review - taxonomical study and medicinal uses of Euphorbia hirta (Linn.) in churu Rajasthan. World J. Pharm. Res.. 2017;6(10):1320-2134.
- [Google Scholar]
- Phenological and geographical effects on phenolic and triterpenoid content in Vaccinium vitis-idaea L. leaves. Plants. 2021;10:1986.
- [CrossRef] [Google Scholar]
- Ent-Kaurane Diterpenoids from Euphorbia hirta. Rec. Nat. Prod.. 2011;5(4):247-1225.
- [Google Scholar]
- Analgesic and anti-inflammatory activities of diethyl ether and n-hexane extract of Polyalthia suberosa leaves. Evid. Based Complement. Altern. Med.. 2018;2018:5617234.
- [Google Scholar]
- Antioxidant and anti-inflammatory effects of polyphenols extracted from Ilex latifolia Thunb. RSC Adv.. 2018;8:7134-7141.
- [Google Scholar]







