Translate this page into:
Phytochemical investigation and antimicrobial activity of Derris scandens
⁎Corresponding author at: UoN Chair of Oman’s Medicinal Plants and Marine Natural Products, University of Nizwa, P.O Box 33, Postal Code 616, Birkat Al Mauz, Nizwa, Oman. Tel.: +968 25446770. Hidayat110@gmail.com (Hidayat Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Different fractions of root and stem of Derris scandens demonstrated good antibacterial (Escherichia coli, and Bacillus megaterium), antialgal (Chlorella fusca), and antifungal (Microbotryum violaceum) activities. Phytochemical investigation resulted in isolation of scandenin, scandenin A, betulinic acid, lupeol, β-amyran-3-one, β-amyrin, β-sitosterol and ß-sitosterol glucopyranoside. Study showed that scandenin has strong antibacterial activity against B. megaterium and good antifungal and antialgal properties. Scandenin A showed good antibacterial, antifungal and antialgal properties.
Keywords
Coumarin
Derris scandens
Antialgal
Antimicrobial
1 Introduction
Derris scandens Benth. (family Leguminosae) is distributed in South East Asia and North Australia (Mahabusarakam et al., 2004). Its stem is widely used in traditional medicine as an anti-tussive, diuretic, expectorant and anti-dysentery agent and for treatment of muscle pains (Mahabusarakam et al., 2004), cough and diarrhea (Sreelatha et al., 2010). Insecticidal constituents, viz., rotenone and lonchocarpic acid, were reported also from the roots of D. scandens, and these compounds demonstrated insect anti-feedant activity (Rani et al., 2013).
The use of D. scandens as a folk medicine and the antimicrobial activity of the crude extract prompt us to investigate phytochemical investigation. Previous studies indicated the presence of coumarins, isoflavones, flavones, isoflavone glycosides and phenyl coumarins as chemical constituents from D. scandens (Sreelatha et al., 2010; Mahabusarakam et al., 2004). Now we are reporting two coumarins (1 and 2), four triterpenes (3–6), and two steroids (7 and 8) from the roots and stems extracts of D. scandens. Working as anti-dysentery agent and in curing diarrhea lead us to propose the hypothesis that plant parts may have bacteriocidal, antifungal, anti-protozoa/antialgal properties. For the purpose we exploited phytochemical separation and analysis of different fractions and bioassay for testing of bacteriocidal/antifungal/antialgal activities.
2 Materials and methods
2.1 General experimental procedure
UV (MeOH) and IR (KBr) spectra were measured on Hitachi U-3200 and Shimadzu-8900 spectrophotometers, respectively. EI-MS and HR-EI-MS were carried out using MAT 8200 and Micromass LCT mass spectrometers, in m/z. The 1H NMR spectra were recorded on a Bruker AMX-500 spectrometer using TMS as an internal reference. The chemical shifts are reported in ppm (δ) while the coupling constants (J) are in Hertz. The 13C NMR spectra were recorded at 125 MHz on the same instrument. Column chromatography (CC) was carried out using silica gel (70–230 and 230–400 mesh; E-Merck, Darmstadt, Germany) and Aluminum sheets precoated with silica gel 60 F 254 (0.2 mm thick; E-Merck) were used for TLC to check the purity of the compounds and were visualized under UV light (254 and 366 nm) followed by ceric sulfate as the spray reagent.
2.2 Plant material
D. scandens plants were collected from Karachi, Pakistan, and identified through comparison with earlier identified herbarium specimen (voucher specimen, No. ICP-201) present in Herbarium, Department of Botany, University of Peshawar, Pakistan.
2.3 Compound isolation and identification
The dried and powdered roots and stem of D. scandens were extracted with methanol at r.t. for two weeks. The crude extract of roots (6.5 g) and stem (5.3 g) of D. scandens was subjected to column chromatography (n-hexane, n-hexane/CH2Cl2 and CH2Cl2/MeOH, in the order of increasing polarity) to separate different fractions. Two coumarins viz., scandenin (1, 5.4 mg) (Pelter and Stainton, 1964) and scandenin A (2, 5.7 mg) (Rao et al., 2007) were purified from fraction DSR-3 by silica gel column chromatography (CC) and with n-hexane/ethyl acetate (10:1) as the eluent. Fraction DSR-1 was further separated by silica gel column chromatography and eluted with n-hexane–EtOAc (9:1) to give triterpene named betulinic acid (3, 2.2 mg) (Ikuta et al., 1995). Similarly, another triterpene viz., lupeol (4, 3.0 mg) (Carvalho et al., 2001) was also isolated from fraction DSR-1, after elution with a mixture of n-hexane–EtOAc (8.5:1.5). Repeated column chromatography of fraction DSS-1 using n-hexane–acetone (9.5:0.5) as the eluent afforded β-amyran-3-one (5, 2.5 mg) (Krishnaveni and Rao, 2000). Moreover fraction DSR-4 gave one triterpene named β-amyrin (6, 8.0 mg) (Heupel, 1985) upon elution with n-hexane–EtOAc (9:1) and one steroid viz., ß-sitosterol (7, 3.7 mg) (Rubinstein et al., 1976) was isolated from fraction DSS-4 with n-hexane–EtOAc (8:2) as eluent. Finally DSS-7 gave ß-sitosterol glucopyranoside (8, 7.8 mg) (Seo et al., 1978) using n-hexane–EtOAc (2.5:7.5) as the eluent. All the above compounds (Fig. 1) were identified by an intensive comparison of their NMR spectral data reported in the literature.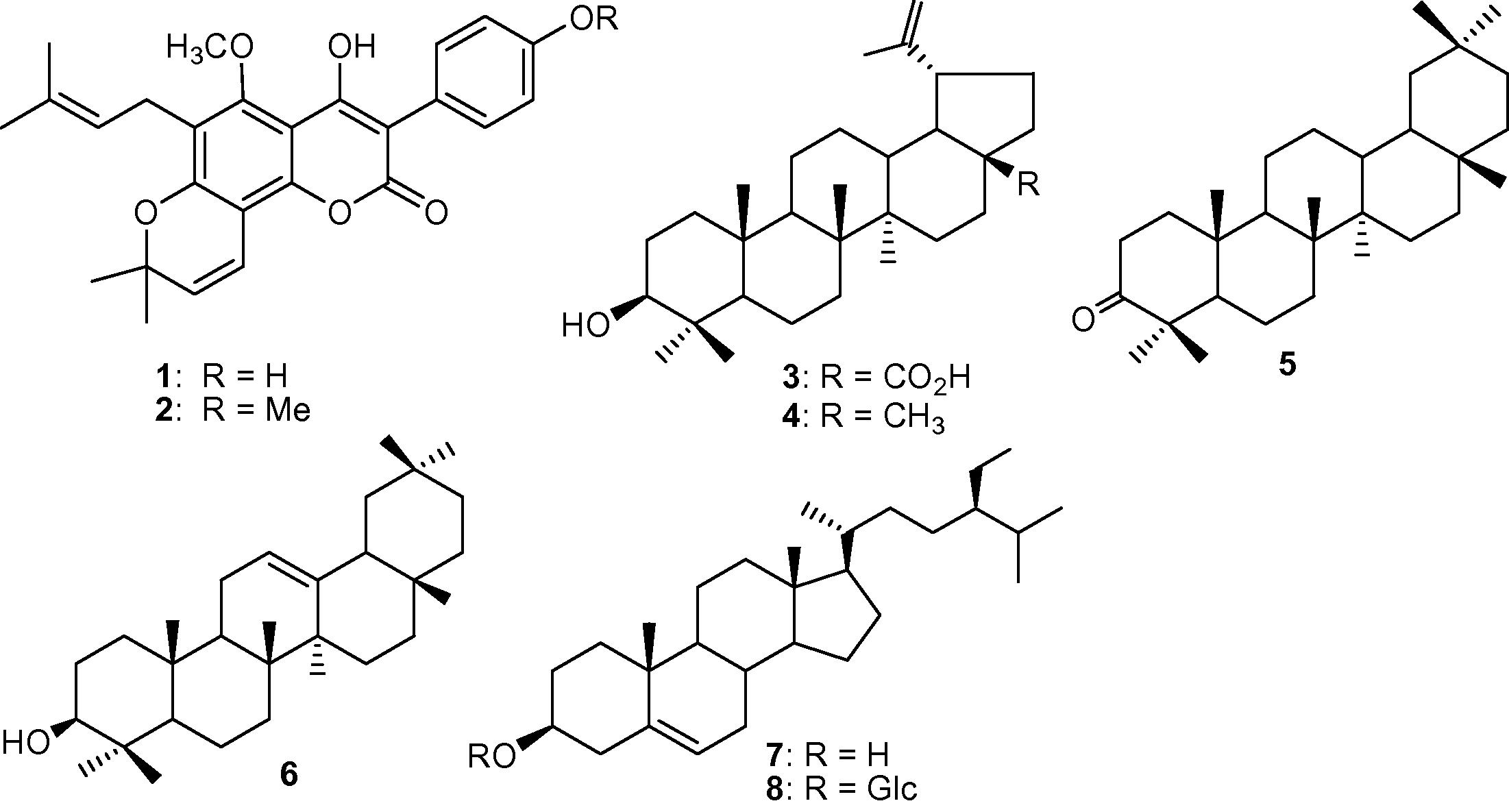
Structure of different compounds isolated from D. scandens.
2.4 Bioactivity test – agar diffusion test
Tests for antifungal, antibacterial, and antialgal activities were performed as previously described (Schulz et al., 1995; Höller et al., 2000). The test organisms for the agar diffusion and screening tests were the bacteria Bacillus megaterium de Bary (Gram positive) and Escherichia coli (Migula) Castellani & Chambers (gram negative), the fungi Microbotryum violaceum [formerly known as Ustilago violacea (Pers.) Roussel (Ustomycetes), Mycotypha microspora Fenner (Zygomycetes)], and the alga Chlorella fusca Shih Krauss (Chlorophyceae). The crude extract of roots and stem of plant D. scandens was subjected to column chromatography and resulted in 12 fractions (DSR 1–12) for roots and 10 fractions (DSS1–10) for stems tested in an agar diffusion assay for their antifungal, antibacterial, and algicidal properties. Reference compounds were penicillin, nystatin, actidione, and tetracycline.
The extracted compounds were dissolved in acetone at a concentration of 1 mg/mL. Fifty microliters of the solutions (50 μg) was pipetted onto a sterile filter disk (Schleicher & Schuell, 9 mm), which was placed onto an appropriate agar growth medium for the respective test organism and subsequently sprayed with a suspension of the test organism (Schulz et al., 1995).
3 Results and discussion
All tested fractions of root and stem showed noticeable antibacterial (E. coli, and B. megaterium), antialgal (C. fusca), and antifungal (M. violaceum) activities (Table 1). Phytochemical investigation of roots resulted in the isolation two known coumarins, viz. scandenin and its methyl ether scandenin A. It is interesting to note that scandenin showed strong antibacterial activities against B. megaterium and E. coli. Recently it has been reported that an aqueous fraction of stems of D. scandens showed anti-bacterial activity against E. coli (Sittiwet and Puangpronpitag, 2009). The findings are of significance especially noting that E. coli causes serious nosocomial infection such as urinary tract infections, cardiovascular, and surgical-site infections (Sittiwet and Puangpronpitag, 2009). B. megaterium causes a plant disease viz., white to very light tan blotching and streaking of wheat leaves and considered soil saprophyte (Abdel-Monaim et al., 2012). Moreover compounds can be used as pesticide in agriculture which show activity toward C. fusca (Kotrikla et al., 1999).
Compound/Fractions
Antibacterial
Antibacterial
Antialgal
Antifungal
Ec
Bm
Bm
Mv
Scandenin (1)
8 + PI 10
14 + PI 16
8.5
PI 9
Scandenin A (2)
9 + PI 11
PI 8
7
PI 8
DSR-1
6
PI 6
6
6
DSR-2
5.5
7
6.5
6
DSR-3
6
6
6
6
DSR-4
6
7
6
6
DSR-5
6
8 + PI 9
6.5
6
DSR-6
6
8
6
PI 7.5
DSR-7
6
6
6
5 + PI 7
DSR-8
6
10 + PI 11
6
5 + PI 6
DSR-9
6
6
6
PI 4.5
DSR-10
7
PI 7
7
7
DSR-11
8
8
5.5
6
DSR-12
6
PI 6
7
0
DSS-1
7
PI 6
5.5
PI 6
DSS-2
6
PI 6
6
PI 6
DSS-3
6
PI 7
6
6
DSS-4
6
PI 6.6
6
PI 5.6
DSS-5
6
PI 6
6
PI 6
DSS-6
6
6
6
6
DSS-7
6
7
6.5
6 + PI 6.5
DSS-8
6
8
6.5
PI 6
DSS-9
8
7
7
5 + PI 6
DSS-10
7
PI 6
6.5
PI 6.5
‘
14
18
0
0
Tetracycline
18
18
PI 10
0
Nystatin
0
0
0
20
Actidione
0
0
35
50
Acetone
0
0
0
0
Similarly, all tested fractions and compounds viz., scandenin (1) and scandenin A (2) showed noticeable algicidal activity against C. fusca. Interestingly, fractions and compounds 1 and 2 showed appreciable antifungal behavior toward M. violaceum.
4 Conclusion
The overall result of the present study leads to the conclusion that different extracts of D. scandens exhibit appreciable antibacterial, antifungal and antialgal activities. Preliminary study showed that scandenin (1) showed strong antibacterial activity and convincing antifungal and antialgal properties. The results suggest further quantitative and elaborate investigation.
Acknowledgement
We thank Dr. Jahandar Shah for identification of plant material.
References
- Bacillus megaterium, a new pathogen on lupine plants in Egypt. J. Bacteriol. Res.. 2012;4:24-32.
- [Google Scholar]
- Acyl-lupeol esters from Parahancornia amapa (Apocynaceae) J. Barz. Chem. Soc.. 2001;12:556-559.
- [Google Scholar]
- Varietal similarities and differences in the polycyclic isopentenoid composition of sorghum. Phytochemistry. 1985;24:2929-2937.
- [Google Scholar]
- Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol. Res.. 2000;104:1354-1365.
- [Google Scholar]
- Triterpenoids from callus tissue cultures of Paeonia species. Phytochemistry. 1995;38:1203-1207.
- [Google Scholar]
- A new triterpene from callus of Pterocarpus santalinus. Fitoterapia. 2000;71:10-13.
- [Google Scholar]
- Toxic effects of atrazine, deethyl-atrazine, deisopropyl-atrazine and metolachlor on Chlorella fusca var-fusca. Global Nest Int. J.. 1999;1:39-45.
- [Google Scholar]
- A benzil and isoflavone derivatives from Derris scandens Benth. Phytochemistry. 2004;65:1185-1191.
- [Google Scholar]
- Isolation, characterization and chemobiological quantification of α-glucosidase enzyme inhibitory and free radical scavenging constituents from Derris scandens Benth. J. Chromatogr. B. 2007;855:166-172.
- [Google Scholar]
- Bioactivity evaluation of prenylated isoflavones derived from Derris scandens Benth against two stored pest larvae. J. Biopesticides. 2013;6:14-21.
- [Google Scholar]
- Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res.. 1995;99:1007-1015.
- [Google Scholar]
- Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc.. 1978;100:3331-3339.
- [Google Scholar]
- Antimicrobial activity of Derris scandens aqueous extract. J. Biol. Sci.. 2009;9:607-611.
- [Google Scholar]
- A new benzil derivative from Derris scandens: structure-insecticidal activity study. Bioorg. Med. Chem. Lett.. 2010;20:549-553.
- [Google Scholar]







