Translate this page into:
Phytochemical investigation and antimicrobial activity of an endophytic fungus Phoma sp.
*Corresponding authors. Address: Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany. Tel.: +968 25446770 (H. Hussain) Hidayat110@gmail.com (Hidayat Hussain), k.krohn@upb.de (Karsten Krohn)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 18 August 2014
Peer review under responsibility of King Saud University.
Abstract
Phytochemical investigation of the endophytic fungi Phoma sp. resulted in the isolation of sclerodin (1), 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3), and sclerodione (4). Preliminary studies showed that sclerodin (1) displayed moderate antialgal activity while 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3), and sclerodione (4) displayed moderate antifungal activity. Furthermore 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2) and atrovenetinone (3) showed moderate antibacterial activity against Bacillus megaterium and additionally atrovenetinone (3) showed good antibacterial activity towards Eurotium repens. Furthermore atrovenetinone (3) and sclerodione (4) displayed very strong antifungal activity towards Ustilago violacea.
Keywords
Endophytic fungi
Phoma sp
Antimicrobial activity
1 Introduction
There is an alarming increase in health related problems which may be directly associated with current day cancers, drug-resistant bacteria, parasitic protozoans and fungi (Hussain et al., 2012b). It has been found that either unusual or rather specialized ecological environments produce some of the most valuable microorganisms for the production of secondary metabolites. Furthermore, intensive studies on these secondary metabolites have focused in on the endophytes present, since these have been recognized as having the best possibility for the development of new and unique medicinal agents for addressing the health hazards faced by society today (Hussain et al., 2012b). In continuation of our programme on phytochemical analysis of endophytic fungi (Hussain et al., 2009a,b; Hussain et al., 2011a,b; Hussain et al., 2012a,b), we investigated the endophytic fungus Phoma sp., (internal strain No. 7133), which was isolated from Senecio kleinii from Gomera and led to the isolation and structural determination of four compounds viz., sclerodin (1), 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3), and sclerodione (4) (Fig. 1). Compounds 1–4 were also isolated from another taxonomical unidentified fungal strain 3004 in our group. Antimicrobial studies showed that sclerodin (1) showed moderate antialgal activity while 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3), and sclerodione (4) demonstrated moderate antifungal activity. Moreover 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2) and atrovenetinone (3) showed moderate antibacterial activity against B. megaterium and on the other hand atrovenetinone (3) demonstrated good antibacterial activity towards Eurotium repens. It is noteworthy that atrovenetinone (3) and sclerodione (4) displayed very strong antifungal activity towards Ustilago violacea.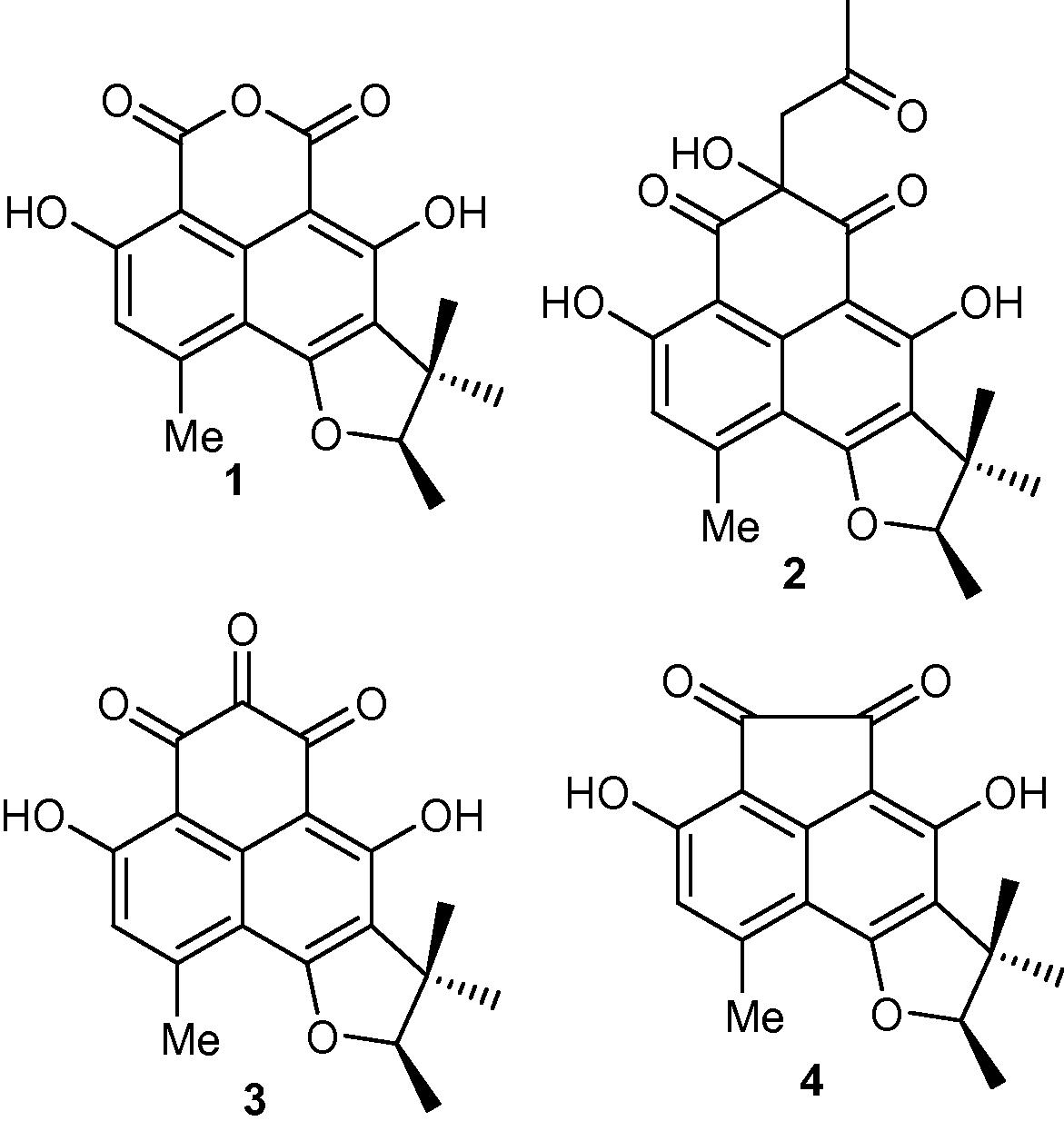
Structure of compounds 1–4 isolated from Phoma sp.
2 Materials and methods
2.1 General experimental procedure
Ultraviolet (UV) spectra were recorded in methanol on a Hitachi U-3200 spectrophotometer. Infra Red (IR) spectra were measured on Shimadzu-8900 spectrophotometer. EI-MS and HR-EI-MS were carried out using MAT 8200 and Micromass LCT mass spectrometers, in m/z. The 1H NMR spectra were recorded on a Bruker AMX-500 spectrometer using TMS as an internal reference. The chemical shifts are reported in ppm (δ) while the coupling constants (J) in Hertz. The 13C NMR spectra were recorded at 125 MHz on the same instrument. Column chromatography (CC) was carried out using silica gel (70–230 and 230–400 mesh; E-Merck, Darmstadt, Germany) and Aluminium sheets precoated with silica gel 60 F 254 (0.2 mm thick; E-Merck) were used for TLC to check the purity of the compounds and were visualized under UV light (254 and 366 nm) followed by ceric sulphate as the spray reagent. Microbiological methods and culture conditions are as described previously (Höller et al., 2000; Schulz et al., 1995).
2.2 Identification, culture, extraction, and isolation
The endophytic fungus Phoma sp., (internal strain No. 7133), was isolated from Senecio kleinii from Gomera, and was cultivated on biomalt solid agar medium (12 L, 5% w/v) at room temperature for 28 days. The endophytic fungus was identified by Dr. Siegfried Draeger and a voucher specimen (TUB-7133) was deposited in the culture collection of the Institute of Microbiology, Technical University of Braunschweig, Germany. The cultures were extracted with ethyl acetate to afford a residue (4.3 g). The extract was separated into three fractions by column chromatography on silica gel with a gradient of n-hexane/ethyl acetate (90:10, 50:50, 0:100) as the eluent. The sub-fraction F1 was further purified by silica gel column chromatography (CC) and preparative TLC with n-hexane/ethyl acetate (10:1 to 5:1) to give pure compounds 1 (4.5 mg), 2 (7.0 mg), 3 (5.3 mg), and 4 (4.3 mg).
2.2.1 Sclerodin (1)
Mp: 256 °C; IR (CH2Cl2): 3436, 1709, 1623, 1459, 1302, 1035 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.34 (s, 3H, 4′-H), 1.53 (d, 3H, J = 6.6 Hz, 1′-H), 1.59 (s, 3H, 5′-H), 2.83 (s, 3H, 7a-H), 4.75 (q, 1H, J = 6.6 Hz, 2′-H), 6.82 (s, 1H, 8-H), 11.38 (s, 1H, OH), 11.62 (s, 1H, OH); 13C-NMR (125 MHz, CDCl3): δ = 14.9 (C-1′), 21.1 (C-5′), 24.1 (C-7a), 25.8 (C-4′), 43.8 (C-3′), 92.4 (C-2′), 93.7 (s, C-3a), 97.4 (C-9a), 108.8 (C-6a), 117.6 (C-8), 119.5 (s, C-5), 135.6 (s, C-3b), 150.2 (C-7), 164.5 (s, C-4), 165.1 (C-3), 165.7 (C-1), 166.2 (s, C-9), 166.5 (s, C-6).; EIMS (200 °C) m/z (%): 328 [M]+ (37), 312 (100), 295 (29), 269 (58), 257 (30); HREIMS: 328.940 (calcd for C18H16O6, 328.947).
2.2.2 8,9-Dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2)
Mp: 231 °C; IR (CH2Cl2): 3407, 1711, 1645, 1632, 1382 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.30 (s, 3H, 4′-H), 1.33 (s, 3H, 4′-H), 1.49 (d, 3H, J = 6.6 Hz, 1′-H), 1.50 (d, 3H, J = 6.6 Hz, 1′-H), 1.54 (s, 3H, 5′-H), 1.55 (s, 3H, 5′-H), 2.22 (s, 6H, 2c-H), 2.75 (s, 6H, 7a-H), 3.31 (s, 2H, 2a-H), 3.36 (s, 2H, 2a-H), 3.68 (bs, 2H, OH), 4.66 (q, 2H, J = 6.6 Hz, 2′-H), 6.76 (s, 2H, 8-H), 12.84 (d, 2H, OH), 13.36 (d, 2H, OH); 13C-NMR (125 MHz, CDCl3): δ = 14.8 (C-1′), 15.0 (C-1′), 21.0 (C-4′), 24.6 (C-7a), 25.88 (q, C-5′), 26.1 (C-5′), 31.1 (C-2c), 31.2 (C-2c), 43.6 (C-3′), 43.7 (C-3′), 51.9 (C-2a), 52.2 (C-2a), 77.6 (C-2), 91.9 (C-2′), 103.0 (C-3a), 105.9 (C-9a), 110.0 (C-6a), 118.2 (C-8), 118.7 (C-5), 118.8 (C-5), 137.8 (C-3b), 149.4 (C-7), 149.5 (C-7), 165.7 (C-4), 165.8 (C-4), 166.3 (C-9), 166.4 (C-9), 166.5 (C-6), 166.5 (C-6), 197.5 (C-3), 197.5 (C-3), 199.7 (C-1), 206.4 (s, C-2b), 206.7 (C-2b); EIMS (200 °C) m/z (%): 398 [M]+ (13), 355 (25), 327 (55), 313 (100), 297 (75), 269 (35); HREIMS: 398.1360 (calcd for C22H22O7, 398.1366).
2.2.3 Atrovenetinone (3)
Mp: 217 °C; IR (CH2Cl2): 3421, 1641, 1610, 1447, 1593 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.31 (s, 3H, 4′-H), 1.32 (s, 3H, 4′-H), 1.48 (d, 3H, J = 6.5 Hz, 1′-H), 1.50 (d, 3H, J = 5.5 Hz, 1′-H), 1.55 (s, 3H, 5′-H), 2.74 (s, 3H, 7a-H), 2.77 (s, 3H, 7a-H), 4.68 (pt, 1H, J = 6.5 Hz, 2′-H), 4.77 (pt, 1H, J = 6.6 Hz, 2′-H), 6.64 (s, 1H, 8-H), 6.71 (s, 1H, 8-H), 12.71 (s, 1H, OH), 12.93 (s, 1H, OH), 1.20 (s, 1H, OH), 13.82 (s, 1H, OH); 13C-NMR (125 MHz, CDCl3): δ = 14.9 (C-1′), 20.9 (C-4′), 24.6 (C-7a), 24.9 (C-7a), 25.6 (C-5′), 25.9 (C-5′), 43.6 (C-3′), 43.6 (C-3′), 86.9 (C-2), 92.2 (C-2′), 92.9 (d, C-2′), 102.2 (C-3a), 105.0 (C-3a), 108.2 (C-9a), 109.8 (C-9a), 110.3 (C-6a), 110.9 (C-6a), 118.4 (C-8), 119.0 (C-8), 119.1 (C-5), 119.5 (C-5), 138.3 (C-3b), 138.6 (C-3b), 150.4 (C-7), 152.1 (C-7), 166.7 (C-4), 167.2 (C-6), 168.2 (C-9), 168.7 (C-9), 177.0 (C-2), 179.2 (C-3), 179.5 (C-1), 193.7 (C-3), 196.0 (C-1); EIMS (200 °C) m/z (%):340 [M]+ (30), 327 (35), 312 (27), 297 (100), 269 (40); HREIMS: 340.0940 (calcd for C19H16O6, 340.0947).
2.2.4 Sclerodione (4)
Mp: 202 °C; IR (CH2Cl2): 3432, 1685, 1634, 1617, 1364 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.31 (s, 3H, 4′-H), 1.50 (d, 3H, J = 6.7 Hz, 1′-H), 1.55 (s, 3H, 5′-H), 2.76 (s, 3H, 7a-H), 4.70 (q, 1H, J = 6.3 Hz, 2′-H), 6.67 (s, 1H, 8-H), 7.63 (s, 1H, OH), 7.89 (s, 1H, OH); 13C-NMR (125 MHz, CDCl3): δ = 14.8 (C-1′), 21.4 (C-4′), 22.4 (C-7a), 26.0 (C-5′), 43.6 (C-3′), 92.3 (C-2′), 106.4 (C-3a), 107.4 (C-9a), 109.5 (C-6a), 117.8 (C-8), 119.9 (C-5), 146.8 (C-7), 151.5 (C-3b), 154.7 (C-9), 155.2 (C-4), 164.7 (C-6), 186.5 (C-3), 190.0 (C-1); EIMS (200 °C) m/z (%):312 [M]+ (28), 297 (75), 285 (20), 269 (100), 241 (20), 213 (32); HREIMS: 312.0990 (calcd for C18H16O5, 312.0998).
2.3 Bioactivity test-agar diffusion test
Tests for antifungal, antibacterial, and antialgal activities were performed as previously described (Schulz et al., 1995). The test organisms for the agar diffusion and screening tests were bacteria B. megaterium de Bary (gram positive) and Escherichia coli (Migula) Castellani & Chalmers (gram negative), the fungi U. violacea (Pers.) Roussel (Ustomycetes), Mycotypha microspora Fenner (Zygomycetes), E. repens Corda (Ascomycetes) and Fusarium oxysporum Schltdl. (Deuteromycetes) and the alga Chlorella fusca Shih Krauss (Chlorophyceae), where the inhibition of C. fusca is usually correlated with broader antialgal activity (Schulz et al., 1995). Compounds 1–4 were dissolved in acetone at a concentration of 1 mg/mL. Fifty microlitres of the solutions (50 μg) was pipetted onto a sterile filter disc (Schleicher & Schuell, 9 mm), which was placed onto an appropriate agar growth medium for the respective test organism and subsequently sprayed with a suspension of the test organism (Schulz et al., 1995).
3 Results and discussion
3.1 Structure elucidation
The ethyl acetate extract of endophytic fungus Phoma sp. was chromatographed on silica gel to give four compounds 1–4. These four compounds were identified as viz., sclerodin (1) (Ayer et al., 1986), 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2) (Ayer et al., 1986), atrovenetinone (3) (Ayer et al., 1986), and sclerodione (4) (Ayer et al., 1986) (Fig. 1) and their structures were confirmed by a comparison of their spectral data to the literature.
3.2 Antimicrobial activity
Antibacterial, antifungal and antialgal properties of the four pure isolated compounds viz., sclerodin (1), 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3) and sclerodione (4) are compiled in Table 1. The isolated compounds 1–4 were tested in an agar diffusion assay for their antifungal, antibacterial, and antialgal properties towards Chlorella fusca, U. violacea, E. repens, M. microspora, F. oxysporum, E. coli, and B. megaterium.
Compound
Antialgal
Antifungal
Antibacterial
Chl
Ust.
Eur.
Mm
F.o.
Ec
Bm
1
4
1
0
0
0
0
2
2
2
1
0
3
0
0
3
3
2
15
7
3
4
0
4
4
1
10
4
0
0
0
2
Sclerodin (1) which has a pyran-2,6-dione group showed moderate algicidal activity towards C. fusca and sclerodione (4) which has cyclopent-1,2-dione showed moderate antifungal activity towards E. repens. On the other hand 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2) and atrovenetinone (3) showed moderate antifungal activities towards M. microspora. Moreover compounds 1 and 4 were not active against M. microspora. Atrovenetinone (3) displayed moderate antifungal activity towards F. oxysporum while compounds 1, 2, and 4 were not active against F. oxysporum. It is noteworthy that compound 3 has a cyclohex-4-ene-1,2,3-trione group while compounds 1, 2, and 4 do have said group in their structures. On the other hand compound 2 has a propyl-2-one and hydroxyl group instead of oxygen which is present in sclerodin (1). Moreover atrovenetinone (3) has a third carbonyl group instead of oxygen which is present in sclerodin (1). In addition 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2) and atrovenetinone (3) showed moderate antibacterial activity against B. megaterium. Furthermore atrovenetinone (3) showed good antifungal activity towards E. repens. Interestingly atrovenetinone (3) and sclerodione (4) showed very strong antifungal activity towards U. violacea while compounds 1 and 2 were not active against U. violacea. It is noteworthy that this compound has a cyclohex-4-ene-1,2,3-trione group while compound 4 has cyclopentane-1,2-dione group in their structures. It is important to note that none of these compounds was active against E. coli. Compounds 1 and 2 were not active against E. repens and U. violacea.
4 Conclusion
The overall result of the present study concluded that a phytochemical investigation of the endophytic fungus Phoma sp. resulted in the identification of four compounds viz., sclerodin (1), 8,9-dihydro-3,5,7-trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-4H-phenaleno[1,2-b]furan-4,6(5H)-dione (2), atrovenetinone (3), and sclerodione (4). Preliminary studies showed that atrovenetinone (3) displayed good antibacterial activity towards E. repens. Furthermore atrovenetinone (3) and sclerodione (4) showed very strong antifungal activity towards U. violacea.
Acknowledgement
We thank BASF AG and the BMBF (Bundesministerium für Bildung und Forschung, project no. 03F0360A).
References
- Metabolites produced by the Scleroderris canker fungus Gremmeniella abietina. Part 1. Can. J. Chem.. 1986;64:1585-1589.
- [Google Scholar]
- Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol. Res.. 2000;104:1354-1365.
- [Google Scholar]
- New bioactive 2,3-epoxycyclohexenes and isocoumarins from the endophytic fungus Phomopsis sp. from Laurus azorica. Eur. J. Org. Chem. 2009:749-756.
- [Google Scholar]
- Bioactive chemical constituents of a sterile endophytic fungus from Meliotus dentatus. Rec. Nat. Prod.. 2009;3:114-117.
- [Google Scholar]
- Solid-state circular dichroism and hydrogen bonding absolute configuration of Massarigenin A from Microsphaeropsis sp. Chirality. 2011;23:617-623.
- [Google Scholar]
- Antimicrobial chemical constituents from the endophytic fungus from Phomopsis sp. from Notobasis syriaca. Nat. Prod. Commun.. 2011;6:1905-1906.
- [Google Scholar]
- Phomopsinones A–D: four new pyrenocines from an endophytic fungus, Phomopsis sp. Eur. J. Org. Chem. 2012:1783-1789.
- [Google Scholar]
- Pyrenocines J–M: four new pyrenocines from the endophytic fungus, Phomopsis sp. Fitoterapia. 2012;83:523-526.
- [Google Scholar]
- Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res.. 1995;99:1007-1015.
- [Google Scholar]







