Translate this page into:
Phytochemical analysis of Cymbopogon flexuosus (lemongrass) oil, its antifungal activity, and role in inhibiting biofilm formation in Candida albicans MTCC854
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background and objectives
Candida albicans is a highly adaptable dimorphic fungal pathogen capable of developing tolerance and resistance to antifungal agents. The treatment of candidiasis becomes more difficult due to the development of biofilms and their morphological variations. Lemongrass oil has been reported for various biological properties and is normally used in various medical applications. In the present study, Cymbopogon flexuosus (lemongrass) oil was analyzed for phytochemicals, studied for its antifungal, anti-biofilm on C. albicans, and cytotoxic effect on HaCaT keratinocytes.
Methods
Using gas chromatography-mass spectrophotometry, the quantitative phytochemical analysis of lemongrass oil was investigated. Antifungal activity and inhibitory activity on biofilm formation of lemongrass oil in C. albicans were evaluated. Cytotoxicity of lemongrass oil on HaCaT keratinocytes was determined by MTT assay.
Results
The GC–MS analysis revealed that lemongrass oil contains 16 different components. The major components were 4-tert-butylcalix, panaquinquecol 7, and diethyl-, 3, 4-dihydro-1-naphthalenyl ester. The minimum inhibitory concentration was 1.25 µl/ml and the minimum fungicidal concentration was 2.5 µl/ml. Complete inhibition of biofilm formation was observed at 0.5 µl/ml concentration of lemongrass oil and 90.9% inhibition was observed at 0.25 µl/ml concentration. The cell viability of HaCaT keratinocytes was maintained above 51.5% at 1.25 µl/ml concentration, and only a moderate cytotoxic effect was observed at a higher concentration.
Conclusion
Lemongrass oil showed antifungal activity against C. albicans and the lower concentrations inhibited biofilm formation. Cytotoxicity was not observed on HaCaT keratinocytes at lower concentrations. Further studies on the active components of lemongrass oil can be effectively useful in controlling biofilm formation in both medical devices and Candida related infections.
Keywords
Antifungal
Biofilm
Candida albicans
Lemongrass oil
Cytotoxicity
1 Introduction
Candidiasis, caused by Candida albicans is increasing extremely in high risk immunocompromised individuals such as patients suffering from cancer, HIV, SARS CoV-19 and in organ transplant recipients (Shekatkar et al., 2021). Oropharyngeal candidiasis is a common opportunistic infection where C. albicans is associated with biofilm formation to acquire antifungal resistance. Additionally, C. albicans develops different mechanisms to resist the effect of various antifungal agents (Vitale, 2021). C. albicans is considered as fourth common commensal causing nosocomial and opportunistic infections. Candidemia caused by azole-resistant strains of C. albicans is death-defying with few or has no treatment options (Baddley et al., 2008). Antifungal agents such as nystatin and miconazole are becoming unfeasible for treatment because of antifungal resistance and toxicity (Ferreira et al., 2021). Candidiasis has increased over the last decades due to an increase in the use of medical implants, immunocompromised patients, and patients getting immunosuppressive therapy (Pappas et al., 2018). Candida species becomes a real threat for hospitalized patients with invasive medical care and immune disorders, leading to catheter-associated infections and biofilm formation in medical implants (Yamin et al., 2021). Phytochemicals present in natural plant resources may help in the discovery of novel drugs to control fungal infections (Khameneh et al., 2021).
Lemongrass oil (LGO) extracted from the leaves of Cymbopogon sp. is considered a potential antifungal agent in traditional medicine (Moumni et al., 2021). LGO has been reported for anti-inflammatory, anti-microbial, and analgesic properties (Boukhatem et al., 2014). The chemical properties such as lipophilicity of LGO may inhibit the growth and multiplication of fungi by interacting with cellular lipid components, cell membranes and thereby inhibiting fungal enzymes required for its growth and metabolism (Singh et al., 2016). Antimicrobial and antibiofilm activities of Cymbopogon citratus against C. tropicalis have been reported (Sahal et al., 2020). However, there are only a few reports are available on C. flexuosus against C. albicans (Gao et al., 2020). In this study, antifungal and anti-biofilm properties of LGO on C. albicans have been evaluated along with their cytotoxic effect on HaCaT cells. The chemical components present in LGO were determined using gas chromatography-mass spectrophotometry.
2 Materials and methods
2.1 Materials
Candida albicans MTCC854 (donated by Aakaar biotechnologies Pvt. Ltd., Lucknow, India) was used in the present study. The fungal culture was sub-cultured in brain heart infusion broth with glycerol and stored at −80 °C. For experiments, these cultures were inoculated in two sets of Sabouraud dextrose agar medium and incubated for 18 h at 36 ± 1 °C. Lemongrass oil (Cymbopogon flexuosus) (Essential oils) was purchased from the local market. As per the manufacturer’s claim on the label, the essential oil was isolated by steam distillation process from leaves, and the product was tested for 100% purity and certified (Bloomingdale IL60108 USA).
2.2 GC–MS analysis of lemongrass oil
The LGO was analyzed by gas chromatograph-mass spectrometry by using GCMS-QP2010 ultra gas chromatograph, (Shimadzu) by following the methodology of Zhao et al. (2021), with slight modification. Briefly, LGO (100 µl) was taken in a separating funnel along with a mixture of water (250 µl), and ethyl acetate (750 µl). The sample was mixed well by thorough shaking and the upper layer was collected and concentrated. Further, a mixture of N,O-Bis(trimethylsilyl)trifluoroacetamide and trimethylchlorosilane (50 µl) (BSTFA-99 µl + TMCS-1 µl) were added initially and at the final step pyridine (10 µl) was added. The sample was heated at 60 °C for 30 min and transferred to GC vials. The contents were dried using liquid nitrogen and then dissolved in methanol for GCMS analysis. The analytical conditions were as follows: injection temperature 250 °C; column oven temperature 120 °C; Total flow rate at 16.3 ml/min and column flow at 1.21 ml/min with a linear velocity of 41.3 cm/sec and purge flow of 3.0 ml/min with ion source temperature of 220 °C and inlet pressure of 100 kPa. Mass spectral data were compared with Wiley8, and NIST libraries.
2.3 Antifungal activity
For determining the antifungal activity, Candida albicans culture (100 μl; 1.5 × 108 CFU/ml) was inoculated by swabbing into Sabouraud dextrose agar. Filter paper discs loaded with different concentrations (10–50 μl/ml) of LGO diluted in dimethyl sulfoxide (DMSO) (for easy diffusion of the essential oil). Amphotericin B (100 U) disc was placed as positive control along with the other discs. The plates were incubated at 36 ± 1 °C for 24 h and the zone of inhibition was measured.
2.4 Minimum inhibitory concentration
The minimum inhibitory concentration of LGO was determined by using 100 μl Muller Hinton broth inoculated with 5 μl of liquid culture with 0.5 McFarland standard turbidity, (1.5 × 108 CFU/ml). Different concentrations of LGO diluted in DMSO (to attain a final concentration of 0.3. 0.6, 1.25, 2.5, 5.0, 10, 20 µl/ml) were added in each well and incubated at 36 ± 1 °C for 24 h. The concentration at which no visual growth was observed is taken as MIC. For determination of minimum fungicidal concentration 10 μl of the culture was inoculated into Sabouraud dextrose agar and incubated at 36 ± 1 °C for 24 h. The concentration at which there was no growth was considered as minimum fungicidal concentration.
2.5 Biofilm formation assay (spectrophotometric)
Log phase cultures were diluted to 0.5 McFarland standard and 10 μl was added to 100 μl of medium in 96 well microtiter plate with different concentrations of LGO. Plate was incubated at 36 ± 1 °C for 48 h. After incubation, 20 μl of 0.1% crystal violet filtered (0.44 µm filter) was added to each well and allowed to stain for 10 min. Planktonic cells were pipetted out using a micropipette from the corner of each well. The wells were washed three times successively and air dried for 15 min. Dimethyl sulfoxide (100 µl) was added to each well and kept for 15 min for solubilization of the dye. The contents of each well were mixed thoroughly and the absorbance was measured at 595 nm wavelength.
2.6 Biofilm formation assay (Microscopic)
Coverslip assay was used for the determination of anti-biofilm activity of LGO. C. albicans grown in Sabouraud dextrose broth in test tubes. Sterile polyvinyl plastic coverslips (Fisher Scientific, UK) (UV sterilized) were placed in the culture tubes after inoculation, to attain 90° angle relative to the bottom of the tube. Different concentrations of LGO (0.3125, 0.625, 1.25, 2.5 and 5 µl/ml) were added in broth. A tube without LGO was used as a negative control for the study. It was made sure that half of the coverslip was immersed in the medium and these tubes were incubated for 48 h at 36 ± 1 °C. The coverslips were carefully removed and washed slowly in sterile cold water to remove the planktonic cells from the coverslip. The coverslips were immersed in Gram staining reagents, washed, and then air dried. The biofilm formation and its inhibition were observed by using a light microscope (Mathur et al., 2006).
2.7 MTT assay
Cytotoxicity of LGO on HaCatT keratinocytes cell line was determined by 3-(4, 5-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT assay) (Rusanov et al., 2017). Briefly, cells (approximately 10,000 cells/well) were cultured in RPMI medium supplemented with 10% FBS and 1% antibiotic solution in a 96 well plate at 37 °C for 24 h with 5% CO2. After incubation cells were treated with different concentrations of LGO (0.02–10 µl/ml) prepared in an incomplete medium. After 24 h, MTT 25 μg/ml (final concentration) was added to cell culture and incubated for 2 h. The culture supernatant was removed and cell layer with matrix was dissolved in 100 μl DMSO (dimethyl sulfoxide) and the results were recorded by using an Elisa plate reader (BioTek instruments Inc, Vermont, USA).
2.8 Statistical analysis
All experiments were conducted on quadruplicate on separate occasions. The data obtained in the present study were analyzed, summarized by percentage and presented in mean ± standard error.
3 Results and discussion
Gas chromatography-mass spectrophotometry analysis revealed the presence of several biochemical components in LGO (Fig. 1). The major constituents present in the oil were tabulated (Table 1). The major component present in the essential oil was 4-tert-butylcalix. Calixarenes were known for its biological and pharmaceutical properties. Amide derivatives of calixarenes have been reported for antimicrobial activity against Rhizopus stolonifer, Gram positive and Gram-negative bacteria (Memon et al., 2017). Inhibition of biofilm formation in several microorganisms has been well documented by calixarenes and their derivatives (Shurpik et al., 2020). Other phytochemical compounds such as panaquinquecol 7, diethyl 3, 4-dihydro-1-naphthalenyl ester were present in LGO. These components also possess the antifungal activity and the sub MIC of LGO inhibited the formation of biofilm. The functional group of panaquinquecol is reported for cytotoxic effects and studied for carcinostatic activity (Christensen, 2020) further contributing to antifungal activity. The presence of boronic acid, diethyl-, 3, 4-dihydro-1-naphthalenyl ester is also supported for antimicrobial and anti-biofilm activity. Branched peptides with boronic acid and its derivatives have been reported for antimicrobial activities against Candida, Staphylococcus and E.coli and the component inhibited biofilm formation in C. albicans (Wynn et al., 2017). Peak Retention Time Area Area % Molecular formula Name 1 6.301 87,636 1.23 2 6.515 92,069 1.29 3 6.954 86,188 1.21 4 7.807 39,568 0.55 5 11.382 631,503 8.84 6 12.071 345,262 4.83 7 12.772 324,814 4.55 8 13.153 118,648 1.66 9 13.201 33,123 0.46 10 13.253 580,956 8.13 11 14.190 85,026 1.19 12 16.896 46,006 0.64 13 18.939 87,326 1.22 14 22.075 145,285 2.03 15 23.826 259,402 3.63 16 26.441 417,929 58.52 714,204 100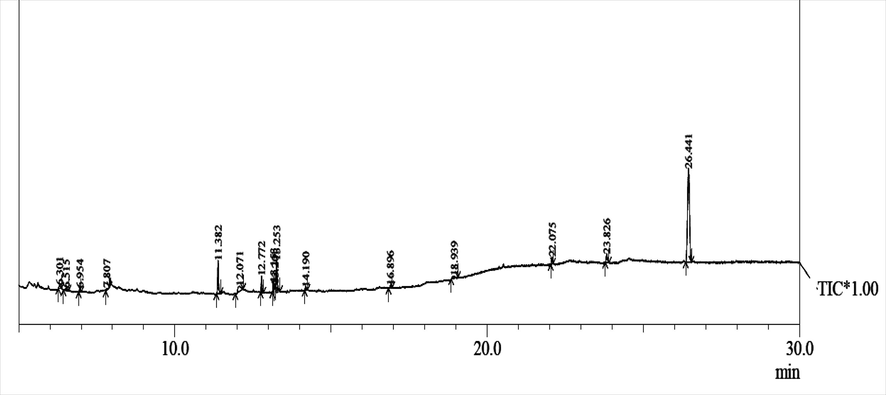
Gas chromatography mass spectrophotometer analysis of lemongrass oil.
C20H40O
Phytol
C14H28O
(E)-11 tertradecen-1-ol
C14H22O
3, 5-di-tert butylphenol
C8H16O
1,2-Dimethylpropylethyl ketone
C17H24O3
Panaquinquecol 7
C16H32O2
Hexadecanoic-d31 acid
C17H35NO2
(E,2R,3R)-2-aminoheptadec-4-ene-1,3-diol
C18H32O2
9,12-Octadecadienoic acid
C19H34O2
17-Octadecynoic acid, methyl ester
C14H19BO
diethyl-,3,4-dihydro-1-naphthalenyl ester
C16H22
1-ethynyl-4-octyl benzene
C34H58O4
Ditridecyl phthalate
C20H39NO
(Z)-N-ethyloctadec-9-enamide
C15H17I2NO2
Ioxynil octanoate
C42H63O3P
tris(2,4-ditert-butylphenyl)phosphite
C44H56O4
4-tert-butylcalix
Antifungal activity of LGO against C. albicans showed highest inhibition at 50 µl/ml concentration (11.33 mm). However, 20 µl/ml of the LGO showed an inhibition zone of 7.66 mm. The zone of inhibition increased with the concentration of the essential oil (Fig. 2). The present study on C. flexuosus consistent with the other antifungal activity reports C. citratus oil against C. albicans and other fungal pathogens that are showing resistance towards antifungal drugs. Lemongrass oil has been reported for antifungal activity against molds and yeast (Boukhatem et al., 2014; Sahal et al., 2020). Clinical isolates of C. albicans have been reported to form biofilm and thereby acquire higher resistance to antifungal agents (Sahal and Bilkay, 2018). To overcome these challenges natural antifungal agents from plant sources are explored and evaluated for antifungal activity to eradicate multidrug resistant pathogens (Lemos et al., 2020). Minimum inhibitory concentration of LGO for inhibiting C. albicans was 1.25 µl/ml and minimum fungicidal concentration of LGO was determined as 2.5 µl/ml (data not shown). The essential oil tested at a very low concentration was able to inhibit the multiplication or growth of C. albicans. A low concentration of pure LGO (0.65 µg/ml) was reported as fungistatic and 2.5 µg/ml was found to be fungicidal against C. albicans (Madeira et al., 2016). This may be due to the varying biochemical constituents present in the LGO and its botanical origin. Previous reports revealed that 2 µl/ml of LGO inhibited ≥ 90% metabolic activities in C. albicans adherent cells (Taweechaisupapong et al., 2012). The phytochemicals such as citral, limonene, citronellal, myrcene, linalool and geraniol present in C. citratus oil were reported for antifungal activity against various fungal species (Boukhatem et al., 2014). The phytochemicals that inhibit the growth of microorganisms at 0.5 mg/ml are considered as strong inhibitors, 0.6 to 1.5 mg/ml are moderate inhibitors and above 1.6 mg/ml are weak inhibitors (Madeira et al., 2016). However, 2.5 µl/ml of LGO was required to inhibit C. albicans in the present study suggesting it as a weak inhibitor. Therefore, LGO was explored for anti-biofilm properties.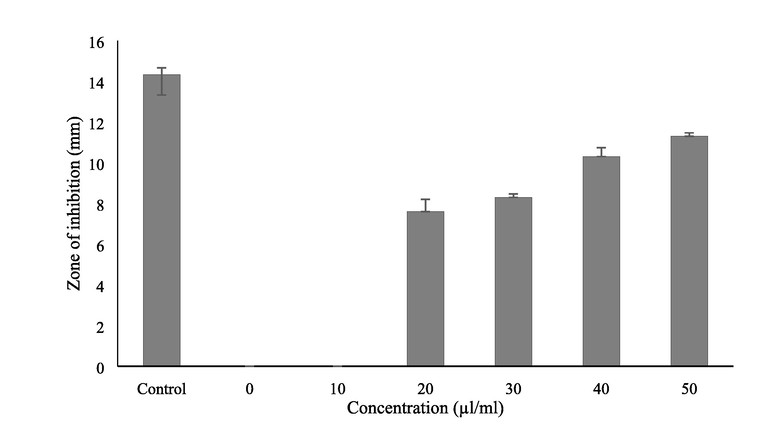
Antifungal activity of lemongrass oil on C. albicans at different concentration. Amphotericin B- (100 U) is taken as control.
In biofilm assay, the relative absorbance was reduced in presence of LGO due to less viability and adherence. Even at a lower concentration (0.03 µl/ml) of LGO only 30% of relative absorbance was observed. The biofilm activity was reduced at sub MIC level and increased concentration of LGO further reduced the cell adherence or biofilm formation. At an increased concentration of 0.5 µl/ml the biofilm formation was completely inhibited when compared with the control. Even at lower concentrations of LGO (0.25 and 0.12% concentration), relative reductions of 90.9 and 87.5% biofilm inhibition were observed respectively (Fig. 3). Microscopic assay of biofilm formation also revealed that low concentration below the MIC inhibited biofilm formation (Fig. 4). From the present study, it is evident that LGO exerted a toxic effect on C. albicans with antifungal and antibiofilm activity. This may be due to disruption of cellular structure and function, inhibiting several cellular activities such as membrane synthesis, respiration, and germination that are required for growth (Wilkinson and Cavanagh, 2005). The phytochemicals such as 4-tert-butylcalix, panaquinquecol 7 and boronic acid, diethyl-, 3, 4-dihydro-1-naphthalenyl ester interrupted cellular growth and function. Further, it decreased the adherence ability of C. albicans by inhibiting the quorum sensing molecules used for communication with other cells or inducing hyphal growth. Anti-quorum sensing activity of LGO has been reported in Vibrio campbellii (Viktorova et al., 2020). The present study suggests that the components present in LGO interrupt important steps in biofilm formation, thereby controlling the biofilm on materials as well as associated infections.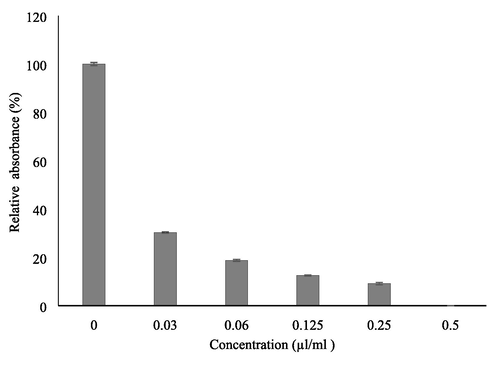
Relative absorbance with control in biofilm formation by C. albicans at different concentration of lemongrass oil.
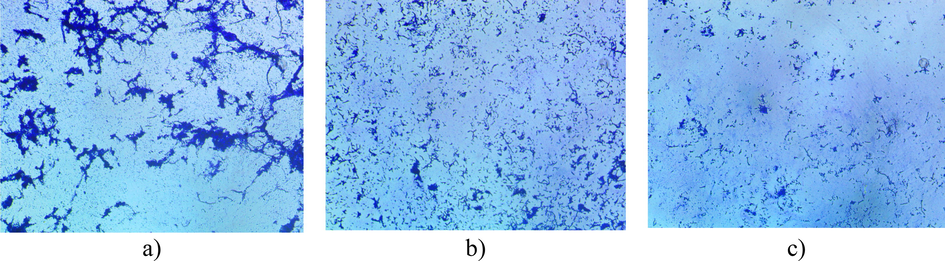
Representative light microscopic (100× magnification) images of biofilm inhibition properties of LGO on C. albicans on coverslip a) medium without lemongrass oil, b) lemongrass oil at 0.125% and c) 0.25% of lemongrass oil.
The cytotoxic studies on HaCatT keratinocytes cell line showed that a lower concentration of LGO maintained cell viability (Fig. 5). Cells showed 51.5 and 70.5% viability at 1.25 and 0.6 µl/ml concentrations of LGO respectively. Moderate toxicity was observed with LGO on HaCatT keratinocytes cell lines and higher concentration (5–10 µl/ml) of LGO and decreased the viability of cells below 10%. The IC50 value for LGO on HaCatT keratinocytes cells was approximately 1.26 µl/ml. Similarly, the citral component in LGO showed moderate cytotoxicity (IC50 = 39.48 µg/ml) and the viability of human dermal fibroblast cells was decreased up to 5% viability at 0.25% v/v (Adukwu et al., 2016). Toxicity studies of C. citratus on W138 cells showed moderate toxicity (IC50 = 39.77 µg/ml) (Kpoviessi et al., 2014). Therefore based on the present study, the lemongrass oil showed less cytotoxic effects at need further investigation on other types of cells before using as an anti-biofilm agent.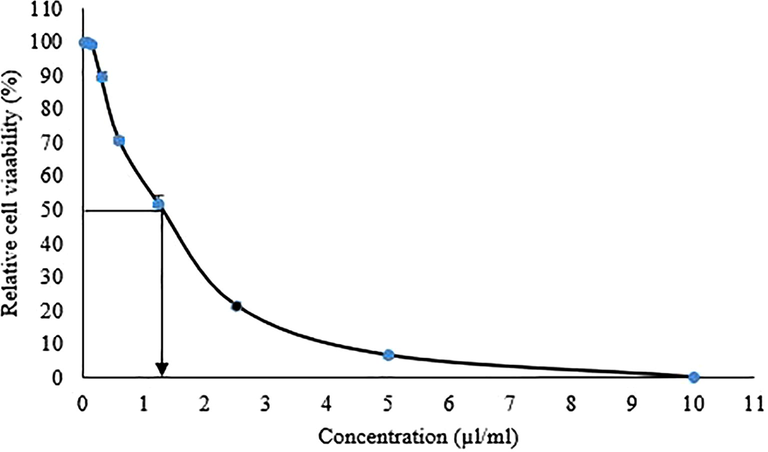
Dose dependent cytotoxic of lemongrass oil on HaCatT keratinocytes cell lines calculated as the relative cell viability using MTT assay (IC50 = 1.26 µl/ml).
4 Conclusion
Overall C. flexuosus oil showed antifungal activity and its ability to inhibit biofilm formation. The concentration below MIC and fungicidal activity of LGO could reduce the biofilm formation in C. albicans. Several chemical constituents such as 4-tert-butylcalix, panaquinquecol 7, boronic acid, present in C. flexuosus oil showed antifungal and antibiofilm activities. Therefore, LGO and its active components need further studies to be used in controlling Candida-associated infections and other invasive pathogens forming biofilms.
Acknowledgement
The author extends his appreciation to the Deanship of Research, Shaqra University, Kingdom of Saudi Arabia.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol.. 2016;100(22):9619-9627.
- [Google Scholar]
- Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother.. 2008;52(9):3022-3028.
- [Google Scholar]
- Lemongrass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med.. 2014;9(1):25431.
- [Google Scholar]
- Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules (Basel). 2020;25(11):2568.
- [Google Scholar]
- The use of essential oils and their isolated compounds for the treatment of oral candidiasis: A literature review. Evid. Based Complement. Alternat. Med.. 2021;2021:1-16.
- [Google Scholar]
- Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida Species. Front. Cell Infect. Microbiol.. 2020;10:603858
- [Google Scholar]
- Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics (Basel). 2021;10(9):1044.
- [Google Scholar]
- Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol.. 2014;151(1):652-659.
- [Google Scholar]
- Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front. Microbiol.. 2020;11:1525.
- [Google Scholar]
- In vitro effects of lemongrass extract on Candida albicans biofilms, human cells viability, and denture surface. Front. Cell. Infect. Microbiol.. 2016;6:71.
- [Google Scholar]
- Detection of biofilm formation among the clinical isolates of Staphylococci: an evaluation of three different screening methods. Indian J. Med. Microbiol.. 2006;24(1):25-29.
- [Google Scholar]
- Synthesis, characterization, and exploration of antimicrobial activity of copper complex of diamide derivative of p-tert-butylcalix[4]arene. Polycycl. Aromat. Compd.. 2017;37:362-374.
- [Google Scholar]
- Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics (Basel). 2021;10(2):104.
- [Google Scholar]
- Sodium dodecyl sulfate cytotoxicity towards HaCaT keratinocytes: comparative analysis of methods for evaluation of cell viability. Bull. Exp. Biol. Med.. 2017;163(2):284-288.
- [Google Scholar]
- Distribution of clinical isolates of Candida spp. and antifungal susceptibility of high biofilm-forming Candida isolates. Rev. Soc. Bras. Med.. 2018;51(5):644-650.
- [Google Scholar]
- Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol.. 2020;246
- [Google Scholar]
- Oral candidiasis in human immunodeficiency virus-infected patients under highly active antiretroviral therapy. Dis. Mon.. 2021;67(9)
- [Google Scholar]
- Antimicrobial activity of calixarenes and related macrocycles. Molecules (Basel). 2020;25(21):5145.
- [Google Scholar]
- Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Rev. Soc. Bras. Med. Trop.. 2016;49(4):465-472.
- [Google Scholar]
- Inhibitory effect of lemongrass oil and its major constituents on Candida biofilm and germ tube formation. S. Afr. J. Bot.. 2012;81:95-102.
- [Google Scholar]
- Lemongrass essential oil does not modulate cancer cells multidrug resistance by citral-Its dominant and strongly antimicrobial compound. Foods (Basel). 2020;9(5):585.
- [Google Scholar]
- Role of antifungal combinations in difficult to treat Candida infections. J. Fungi (Basel). 2021;7(9):731.
- [Google Scholar]
- Antibacterial activity of essential oils from Australian native plants. Phytother. Res.. 2005;19(7):643-646.
- [Google Scholar]
- Branched peptides: Acridine and boronic acid derivatives as antimicrobial agents. ACS Med. Chem. Lett.. 2017;8(8):820-823.
- [Google Scholar]
- Risk factors of Candida parapsilosis catheter-related bloodstream infection. Front. Public Health.. 2021;9:631865
- [Google Scholar]
- Chemical characteristics of three kinds of Japanese soy sauce based on electronic senses and GC-MS analyses. Front. Microbiol.. 2021;11:579808
- [Google Scholar]







