Translate this page into:
Phytochemical analysis, antiproliferative and antifungal activities of different Syzygium aromaticum solvent extracts
⁎Corresponding authors. asali@ksu.edu.sa (Ashraf Abdel-Fattah Mostafa), falotibi@ksu.edu.sa (Fatimah O. Al-Otibi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The outbreaks of fungal resistance to the different antifungal agents represent a public health problem resulting in a significant morbidity and mortality. Furthermore, chemotherapy represents a serious issue in the cancer treatment worldwide. Accordingly, the efficiency of clove extracts as anticandidal and antitumoral agents was evaluated. Disk diffusion and 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium bromide (MTT) techniques were achieved to evaluate the antimycotic and antiproliferative activities of different clove extracts. The active phytochemicals of clove extracts were analyzed using gas chromatography mass spectrometry. Resistance of Candida glabrata strain to clotrimazole was obvious while C. albicans and C. tropicalis expressed dose dependent susceptibility. In contrast, the clove acetonic extract expressed the highest antimycotic proficiency against C. glabrata, C. albicans and C. tropicalis strains with suppressive zones of 18.54 ± 0.24, 19.86 ± 0.34 and 22.6 ± 0.32 mm respectively. While, the ethanolic clove extract displayed the uppermost antiproliferative efficiency with relative IC50 of 6.80 µg/mL. Phytochemical analysis showed that eugenol compound was the most prominent active ingredient of clove acetonic and ethanolic extracts with corresponding proportions of 46.53 and 54.71 % respectively. Anticandidal activity of the main active ingredient, eugenol, was significantly higher than that of clotrimazole, used as control, against C. glabrata strain (P ≤ 0.05). The proficiency of antimicrobial activity of clove and its major constituent, eugenol, against candidal strains supports utilizing these extracts in fabrication of anticandidal drugs especially against the resistant C. glabrata strain. Also, these extracts could be a source of adjuvant anticancer therapies due to their potent antiproliferative activity.
Keywords
Syzygium aromaticum
Eugenol
Clotrimazole
Antimycotic
Anticancer
GC–MS
1 Introduction
Candida species, which are found in the gastrointestinal, skin, and genital tracts, are regarded as commensal human microbes (Talapko et al., 2021). On the other hand, Candida can cause life-threatening infections in people, notably those who are hospitalized, have impaired immune systems, and elderly patients (Lin et al., 2021a,b). In the United States, Candida sp. was considered one of the main etiological agents of nosocomial infections (Jahagirdar et al., 2018). Candida albicans is the main persistent candidal pathogen isolated from clinical species (37 %), succeeded by Candida glabrata (27 %) (Bhattacharya et al., 2020). Candidiasis is marked by extended spectrum of fungal infections varying from superficial cutaneous infections to severe invasive candidemia (Badiee and Hashemizadeh 2014). High mortality rate was recorded among candidemia cases recording about 43 %, owing to the candidal resistance to antifungal agents (José et al., 2019). Clotrimazole is an antifungal agent of the class of medications known as azoles and is frequently used to treat candidiasis (Mendling et al., 2020). Azoles disrupt ergosterol biosynthesis through binding to the 14α–demethylase (Erg11p) enzyme resulting in impairment of fungal cytoplasmic membrane and induction of cell death (Lv et al., 2016). The drug tolerance of C. glabrata to azole antifungals could be attributed to the increased efflux pump activity and mutations in ERG11 enzyme (Massa et al. 2018; Berman and Krysan 2020). Antifungal resistance of C. glabrata to clotrimazole, fluconazole, itraconazole, nystatin and terbinafine drugs has been reported in an earlier study (Yassin et al., 2020a). Another report demonstrated the clotrimazole resistance of C. glabrata strains isolated from patients suffering from vaginitis disease (Nejat et al., 2018). The clotrimazole resistance was detected in 21.5 % of total Candida species isolated from oral and diaper rash candidiasis in neonates (Mohamadi and Motaghi 2014). In addition, cancer is considered the second dominant cause of mortality worldwide after the cardiovascular disorders (Nagai and Kim 2017). Cancer mortality rate is predicted to increase from 7.1 million cases in 2002 to 11.5 million cases in 2030 (Lin et al., 2021a,b). The treatment of cancer is challenging as the chemotherapeutic agents used in cancer treatment cause severe side effects such as nausea, vomiting, and severe gastric ulcers (Zhang et al., 2018). In this regard, plant extracts were recorded as potential, safe and effective adjuvant therapies for cancer management because of their ingredients of phenolic compounds and flavonoids (Seca and Pinto 2018; Tungmunnithum et al., 2018). Syzygium aromaticum is a flower bud which is always known as clove and belongs to Myrtaceae family (Rajalekshmy and Manimekalai 2019). Clove has been stated as a precious source of antiviral, antibacterial, antiproliferative, and antimycotic activities (Batiha et al., 2020). High incidence of candidal resistance to different antifungal drugs results in high mortalities and morbidities worldwide (Yassin et al., 2020b). Moreover, cancer therapy is challenging due to the adverse side effects of chemotherapy. Accordingly, formulation of effective antifungal and anticancer agents is of great importance. Hence, the antifungal efficiency of clove extracts against the most frequent fungal strains causing candidiasis was evaluated, and their anticancer efficiency against HUH7 human hepatoma cell line was also assessed.
2 Materials and methods
2.1 Preparation of clove extracts
Flower buds of clove were purchased from Riyadh markets, Saudi Arabia. The clove plant was identified and deposited with voucher number of (KSU-14682) by of the Botany Department herbarium, College of Science, King Saud University. The active phytochemicals of clove were extracted using four different organic solvents (petroleum ether, dichloromethane, acetone and ethanol) with corresponding polarity indexes of 0.1, 3.1, 5.1, and 5.2, respectively. Difference in the polarity of different used solvents allows the extraction of all active phytochemicals. Disinfection of the clove buds was performed using 0.5 % sodium oxychloride (NaOCl) followed by washing of these buds three successive times with demineralized water and finally the buds were left in shade for complete dryness. Maceration of buds was done using a mechanical mortar to attain a homogenized powder. Fifty grams of clove powder were submerged in 150 mL of the four different organic solvents. The flasks were incubated over a magnetic stirrer for 48 h at 25 ± 2 °C then centrifuged to remove the residues. Filtration of the supernatant was performed using Whatman filter paper no. 1 to obtain translucent filtrates. The solvents were evaporated using a rotatory evaporator to concentrate the S. aromaticum solvent extracts and then refrigerated at 4 °C till use. The extraction yields were estimated according to the following equation.
Extract yield = (A/B) × 100; Where A is the clove extract weight and B is the weight of raw clove sample (Mostafa et al., 2018).
2.2 Candidal strains
Three different candidal strains namely, Candida albicans (ATCC 18804), Candida glabrata (ATCC 15545), and Candida tropicalis (ATCC 13803) were assayed for their susceptibility to clove extracts. Fresh isolates of candidal strains were obtained by subculturing the concerned strains onto Malt extract agar medium then incubated at 35 ± 2 °C to obtain fresh inoculums.
2.3 Antifungal susceptibility testing
Antifungal efficiency of different organic solvent extracts of S. aromaticum against different candidal pathogens was evaluated using the standard method for yeast disk diffusion testing no M44-A (CLSI, 2004). 24 hr old colonies of each candidal strain was picked up using a sterile loop and dispersed in Mueller Hinton broth (MHA) medium. Adjustment of the turbidity of the candidal suspension was performed using 0.5 McFarland standards which are corresponding to 106 colony forming unit per ml (CFU/ml). Mueller Hinton agar (MHA) medium was poured in sterile petri dishes and the plates were seeded with 0.2 mL of the prepared candidal suspension. 8 mm filter paper disks were autoclaved for complete sterilization and then loaded with 10 mg of different clove extracts. Filter paper disks, impregnated with clotrimazole antifungal agent at a concentration of 10 µg/disk were used as positive controls. The susceptibility of different candidal pathogens to clotrimazole drug was interpreted as follows; the strain was considered resistant if the clear zone diameter is ≤ 11 mm while the strain will be considered as a sensitive strain if the zone diameter is ≥ 20 mm. Moreover, the strain was regarded as a dose dependent if the suppression zone ranged from 12 to 19 mm. Finally, the Petridishes were kept in the refrigerator for 2 hr at 4 °C to allow clove extracts diffusion then the Petridishes were incubated at 35 ± 2 °C for 48 hr. After incubation, the clear zones were measured using Vernier caliper.
2.4 Bioassay of clove acetonic extract
2.4.1 Determination of the minimum inhibitory concentration (MIC)
The microdilution technique was achieved using the 96-well microtiter plate to detect MIC of clove extracts. Different concentrations of crude clove solvent extracts were prepared (32, 62.5, 125, 250, 500, 1000, 2000 μg/ml) 10 % DMSO. Equal volumes (100 µL) of candidal suspension, and clove extracts of different concentrations were pipetted into the wells. Negative control wells with DMSO were prepared while wells with amphotericin B were used as positive control. The plates were incubated for 24 h at 37 °C and the candidal growth was measured using microplate reader. MIC values were detected as the least concentration of clove extracts inhibiting the candidal growth (Yassin et al., 2021).
2.4.2 Determination of the minimum fungicidal concentration (MFC)
For the evaluation of MFC values, 10 µL of the MIC wells showing no visible candidal growth were streaked over MHA plates then the plates were incubated 35 ± 2 °C for 48 hr and finally investigated for the candidal growth. The MFC concentration was detected as the least concentration of clove extracts showing no candidal growth (Salleh et al., 2016).
2.5 In vitro antiproliferative assay
HUH7 human hepatoma cell line was used in the current study and purchased from ThermoFisher Scientific, USA. MTT assay as described by Famuyide et al., 2019 was used to evaluate the antiproliferative efficiency of different clove extracts.
2.6 Gas chromatography–mass spectrometry of clove solvent extracts
Phytochemical characterizations of the two efficient clove (ethanolic and acetonic extracts) showing the highest antifungal and antiproliferative activities were done using (an Agilent 7890 gas chromatograph and an Agilent 5975C Mass Spectrometer, USA). The conditions of GC–MS were standardized as described in an earlier study (Yassin et al., 2020c). The active chemical compounds were identified by comparing GC–MS results with the reference data of NIST database.
2.7 Antifungal activity of eugenol as a standard active ingredient of clove
The antifungal efficiency of eugenol (Sigma-Aldrich, USA), the main phytoactive component of clove extract, against different candidal pathogens was evaluated using disk diffusion assay. Sterile filter paper disks (8 mm) were impregnated with 50 µg/disk of eugenol and placed over MHA agar plates seeded with the candidal suspension (Pavesi et al., 2018). Filter paper disks, impregnated with clotrimazole antifungal agent at a concentration of 10 µg/disk were used as positive controls. The plates were incubated at 35 ± 2° for 48 hr and then the clear zones were detected using Vernier caliper.
2.8 Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to investigate Candida susceptibility to various clove extracts using one-way analysis of variance and Tukey's test. For triplicates, the data was reported as mean ± standard error. When the P-value was less than 0.05, the data was considered statistically significant.
3 Results
3.1 Antifungal activity of organic solvent extracts of clove against Candida strains
The dichloromethane solvent exhibited the highest efficiency in extraction followed by ethanol, petroleum ether, and acetone solvents with relative yield percentage of 6.32, 4.41, 3.61 and 1.96 respectively. The different solvent extracts of clove exhibited anticandidal activity against the concerned strains as seen in Fig. 1.The acetonic extract of S. aromaticum revealed the highest antimycotic potency against C. albicans, C. glabrata and C. tropicalis with suppressive zone diameters of 19.86, 18.54 and 22.65 mm respectively. On the contrary, the clove ethanolic extract suppressed the candidal growth of C. albicans and C. tropicalis strains with inhibition zones of 16.76 and 19.68 mm respectively. Furthermore, dichloromethane extract of clove inhibited the microbial growth of the candidal pathogens with suppression clear zones ranged from 16.14 to 20.15 mm while the clove petroleum ether extract suppressed the candidal growth of the investigated strains exhibiting inhibitory zones ranged from 15.08 to 21.28 mm as seen in Table 1. The solvent extracts of S. aromaticum exhibited a potential activity against the clotrimazole resistant strain namely, C. glabrata.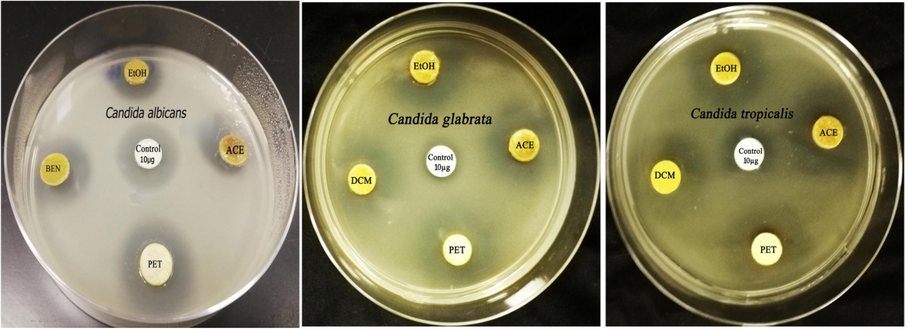
Antifungal activity of clove extracts (10 mg/disc) against different candidal strains.
S. aromaticum extracts (10 mg/disc)
Inhibition zone diameter (mm) of Candida strains
C. albicans
C. glabrata
C. tropicalis
Acetonic extract
19.86 ± 0.34
18.54 ± 0.24
22.65 ± 0.32
Dichloromethane extract
18.42 ± 0.28
16.14 ± 0.43
20.15 ± 0.18
Ethanolic extract
16.76 ± 0.13
17.56 ± 0.32
19.68 ± 1.16
Petroleum ether extract
19.32 ± 0.53
15.08 ± 0.26
21.28 ± 0.16
Clotrimazole (10 μg/disk)
15.57 ± 0.21
9.12 ± 0.17
13.24 ± 0.28
3.2 Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
Minimal inhibitory concentrations (MICs) and MFC were evaluated for clove acetonic extract which recorded the highest efficiency against Candida spp. The suppressive effect of clove acetonic extract against C. albicans and C. glabrata showed MIC value of 125 µg/ml. On the other hand, C. tropicalis strain reported the highest sensitivity to clove acetonic extract recording MIC of 62.5 µg/ml as seen in Table 2. Moreover, the minimal fungicidal concentrations (MFCs) of clove acetonic extract against C. tropicalis was observed to be 125 µg/ml while the MFC of clove acetonic extract was 250 µg/ml against both of C. albicans and C. glabrata strains.
Clove extracts
Concentration of clove extracts (µg/ml)
C. albicans
C. glabrata
C. tropicalis
MIC
MFC
MIC
MFC
MIC
MFC
Acetonic extract
125
250
125
125
62.5
250
Dichloromethane extract
250
500
250
250
125
500
Ethanolic extract
250
500
250
250
125
500
Petroleum ether extract
125
250
500
250
125
1000
3.3 In vitro antiproliferative activity of different S. aromaticum extracts
The clove ethanolic extract showed the highest antiproliferative activity against HUH7 human hepatoma cell line exhibiting IC50 of 6.80 µg/mL while the lowest antiproliferative activity was employed by the dichloromethane extract demonstrating IC50 of 16.73 µg/mL. Furthermore, the clove petroleum ether and acetonic extracts showed antitumor activity against HUH7 human hepatoma cell line with corresponding IC50 of 11.69 and 8.53 µg/mL respectively as seen in Fig. 2.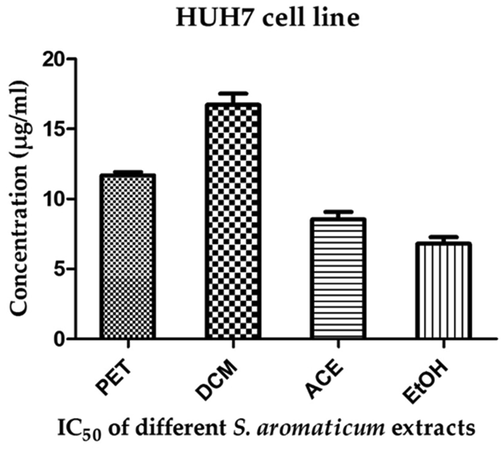
Antiproliferative activity of clove extracts against HUH7 human hepatoma cell line.
3.4 GC–MS analysis of different clove extracts
The phytochemicals of clove ethanolic and acetonic extracts were detected using GC–MS analysis as these extracts exhibited the highest antiproliferative and antimicrobial activities. The main active ingredient of the ethanolic and acetonic extracts was found to be eugenol with corresponding percentages of 54.71 and 46.53 % respectively. The other phytochemicals of the clove acetonic extracts were detected as eugenyl acetate (22.84 %), 5,6β-epoxystigmasterol (18.56 %) and isoeugenol acetate (11.99 %) as shown in Table 3. Furthermore, the other active components of clove ethanolic extract were found to be eugenyl acetate (17.40 %), caryophyllene (11.22 %), humulene (5.20 %), caryophyllene oxide (4.77 %), copaene (3.80 %) and β-cadinene (2.89). Mass spectrums of eugenol and eugenyl acetate compounds showing the highest abundance in clove ethanolic and acetonic extracts were shown in Fig. 3 and Fig. 4, respectively.
Phytochemical constituents
Chemical formula
Mol. weight
Retention time (min.)
% of Total
Clove acetonic extract
Eugenol
C10H12O2
164
9.814
46.53
Eugenyl acetate
C12H14O3
206
10.531
22.84
5,6β-epoxystigmasterol
C32H56O2Si
501
11.263
18.56
Isoeugenol acetate
C12H14O3
206
13.601
11.99
Clove ethanolic extract
Eugenol
C10H12O2
164.20
10.405
54.71
Copaene
C15H24
204.36
9.444
3.80
Humulene
C15H24
204.36
11.201
5.20
Caryophyllene
C15H24
204.36
10.933
11.22
β-cadinene
C15H24
204.36
12.597
2.89
Eugenyl acetate
C12H14O3
206.20
14.529
17.40
caryophyllene oxide
C15H24O
220.35
10.735
4.77
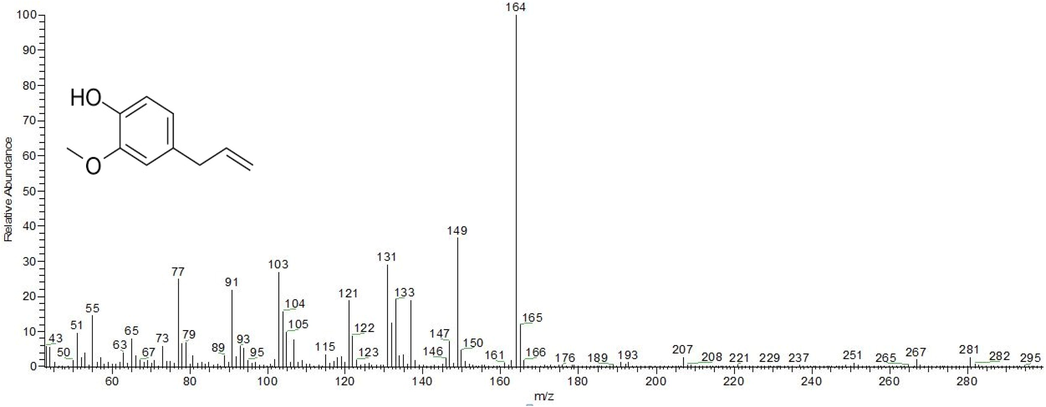
Mass spectrum and chemical structure of eugenol compound.
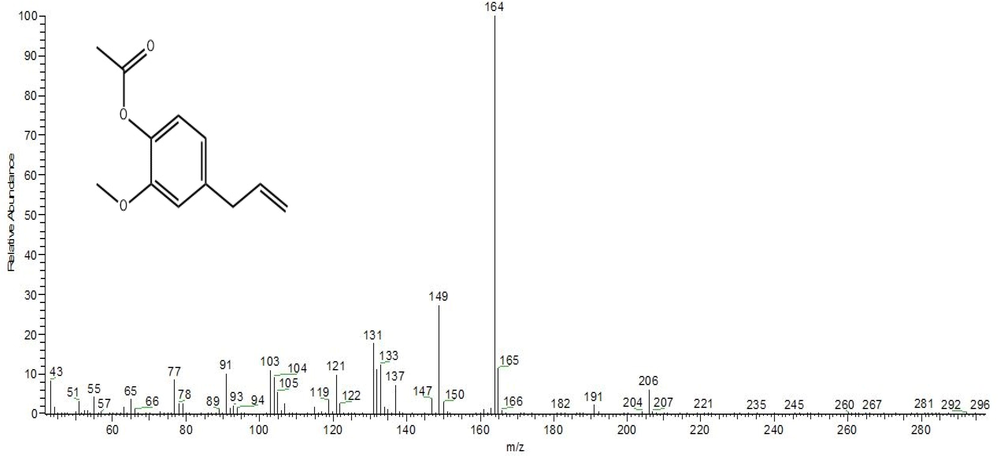
Mass spectrum and chemical structure of eugenyl acetate compound.
3.5 Antimycotic efficiency of eugenol active constituent against the candidal pathogens
Eugenol is the most predominant active ingredient detected in clove extracts hence, the antimycotic proficiency of the previous compound against investigated strains was evaluated. C. tropicalis was the most responsive strain to eugenol compound with suppression zones of 15.39 mm while C. glabrata showed the lowest susceptibility with suppressive zone diameter of 11.22 mm. Moreover, eugenol expressed anticandidal efficiency against C. albicans with suppressive zone of 14.74 mm as seen in Fig. 5. The inhibitory effect of eugenol against the clotrimazole resistant C. glabrata strain was significantly higher than that of control (P ≤ 0.05). In contrast, the antifungal efficiency of eugenol against C. albicans and C. tropicalis strains was non-significant compared to control (P > 0.05).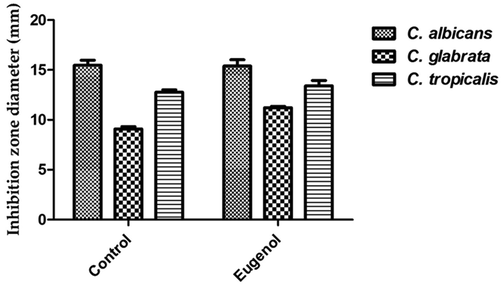
Antifungal activity of eugenol against different tested candidal pathogens.
4 Discussion
Different plant extracts were reported as efficient antimicrobial agents even against multidrug resistant strains (Mohanasundari et al., 2021; Narayanan et al., 2021a; Yassin et al., 2021), anticancer agents (Narayanan et al., 2022) while other phytoactive extracts were detected to possess larvicidal activity (Narayanan et al., 2021b). Hence, the bioactivity of different clove extracts was assessed to evaluate their antimycotic efficiency against drug resistant candidal strains.
The acetonic clove extract showed the maximum antimicrobial action against the different fungal pathogens, expressing clear zones ranged from 18.5 to 22.6 mm. Our findings matched those of a recent study, which stated that clove ethanolic extract (10 mg/ml) revealed antimycotic efficiency against C. albicans with suppressive zone of 24 mm (AbdelـFattah et al., 2019). Candida glabrata displayed clotrimazole resistance in the current investigation, which coincided with a previous study that assessed the antifungal sensitivity of 138 clinical isolates of C. glabrata strains to clotrimazole medication and found that 89 isolates were clotrimazole resistant (Costa et al., 2016). Another recent study reported that 62.5 % of the isolated C. glabrata strains expressed resistance to clotrimazole drug (Khan et al., 2018). The high antifungal efficiency of clove acetonic extract against the clotrimazole resistant strain, C. glabrata, highlights the implementation of clove extracts in the biofabrication of efficient antifungal agents and controlling the high incidence of candidal resistance.
The MIC value of clove acetonic extract against Candida albicans and Candida glabrata was 125 µg/ml. In contrast, the MIC value of clove extract against C. albicans was evaluated by a prior study which reported that clove extract showed antimicrobial activity against C. albicans with MIC value of 1 mg/ml (Hassan et al., 2020). Low MIC values of S. aromaticum extracts against the concerned candidal strains confirmed the capability of utilizing these extracts in biofabrication of natural antifungal drugs.
The microbicidal impact of clove extracts against candidal pathogens could be attributed to their ability to impair ergosterol biosynthesis resulting in formation of lesions in the candidal cytoplasmic membrane and induction of cell mortality (Chouhan et al. 2017). The hydrophobicity of clove extracts allows them to partition into the lipid bilayer of the fungal plasma membrane resulting in disablement of cell permeability, which causes the discharge of essential cellular components (Tariq et al. 2019).
The different solvent extracts of clove expressed potent antiproliferative effectiveness against the HUH7 human hepatoma cell line with IC50 ranged from 6.80 to 16.73 µg/ml. Our findings were in accordance with that of other researchers who verified the clove antiproliferative potency against MCF-7 human breast cancer cell line with corresponding IC50 of 17.6 µg/ml (Kumar et al., 2014). Cloves have been reported as carcinopreventive agents due to their content of betulinic acid and other triterpenes (Nirmala et al., 2019; El-Saber Batiha et al., 2020; Alexa et al., 2022). The anticarcinogenic effect of cloves against hepatoma cell line may be attributed to their ability to induce apoptosis and inhibition of cell proliferation (Bezerra et al., 2017). In this regard, eugenol (54.71 %) was detected as the main phytoactive constituent of the clove ethanolic extract, expressing the highest antiproliferative activity against HUH7 human hepatoma cell line. The eugenol compound was proved to possess high antioxidant activity contributing to the potent antiproliferative effect of clove (Yan et al., 2017).
The phytochemicals of clove ethanolic and acetonic extracts were detected using GC–MS analysis as these extracts exhibited the highest antiproliferative and antimicrobial activities. Chemical investigation of clove acetonic and ethanolic extracts using GC–MS analysis demonstrated that eugenol was the major component with corresponding percent of 46.53 and 54.71 % respectively. Other researchers confirmed these findings, reporting that clove oil contained eugenol as a main active component with a relative ratio of 52.53 % (Teles et al., 2021). Furthermore, a prior study found that S. aromaticum extract contained 83.785 % eugenol, 5.191 % caryophyllene, and 11.024 % caryophyllene, respectively (Das et al. 2018).
Candida tropicalis was the most susceptible strain to eugenol compound with clear zone of 15.39 mm while C. glabrata showed the lowest susceptibility with suppressive zone diameter of 11.22 mm. The current findings were supported by a previous study which evaluated the antifungal effect of eugenol (57 µg/disk) against C. albicans using agar disk diffusion method and stated its efficiency as antimicrobial agent, demonstrating inhibition zone diameter of 12.1 mm (Pavesi et al., 2018).
The fungicidal action of eugenol compound was elucidated by a previous report which stated that eugenol increases cell fluidity and permeability resulting in impairment of cell wall integrity (Latifah-Munirah et al., 2015). The antifungal activity of eugenol was further investigated by other researchers who reported that eugenol disrupts ergosterol biosynthesis leading to cytoplasmic membrane dysfunction (Zhao et al., 2021).
5 Conclusion
The potential anticandidal effectiveness of S. aromaticum solvent extracts against different fungal pathogens supports using of these extracts in fabrication of natural and effective antimicrobial agents especially against clotrimazole resistant C. glabrata strain. The main active ingredient of clove extracts, eugenol, suppressed the candidal growth of different strains. The potential antiproliferative effectiveness of clove solvent extracts highlight the capability of utilizing these extracts in fabrication of carcinopreventive agents.
Funding
This research project was supported by a grant from the Researchers Supporting Project number (RSP-2021/114), King Saud University, Riyadh, Saudi Arabia.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2021/114), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial activity of ethanolic extracts of clove and thyme. Arab. Uni. J. Agri. Sci.. 2019;27(1):491-499.
- [Google Scholar]
- Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J. Med. Sci.. 2014;139(2):195.
- [Google Scholar]
- Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2)
- [Google Scholar]
- The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients. 2017;9(12):1367.
- [Google Scholar]
- Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4(3):58.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts. CLSI m44-a, 23(6). Wayne, PA: Clinical and Laboratory Standards Institute; 2004.
- Clotrimazole drug resistance in Candida glabrata clinical isolates correlates with increased expression of the drug: H+ antiporters CgAqr1, CgTpo1_1, CgTpo3, and CgQdr2. Front. Microbiol.. 2016;7:526.
- [Google Scholar]
- Evaluation of therapeutic potential of eugenol-a natural derivative of Syzygium aromaticum on cervical cancer. Asian Pac. J. Cancer Prev: APJCP.. 2018;19(7):1977.
- [Google Scholar]
- Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement Altern. Med.. 2019;19(1):1-13.
- [Google Scholar]
- Synergistic antifungal activity of mixtures of clove, cumin and caraway essential oils and their major active components. J. Herb. Med.. 2020;24:100399
- [Google Scholar]
- Candida species as potential nosocomial pathogens–A review. Electron. J. Gen. Med. 2018;15(2)
- [Google Scholar]
- Invasive fungal infection in crtically ill patients: hurdles and next challenges. J. Chemother.. 2019;31(2):64-73.
- [Google Scholar]
- Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect Drug Resist. 2018;11:447.
- [Google Scholar]
- Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacogn. Res.. 2014;6(4):350.
- [Google Scholar]
- Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053) Front. Life Sci.. 2015;8(3):231-240.
- [Google Scholar]
- Advances in epidemiology and treatment of invasive Candida infection in the immunocompromised population. J. Shanghai Jiaotong Univ. (Med. Sci.). 2021;41(4):525.
- [Google Scholar]
- Global Patterns and Trends in Gastric Cancer Incidence Rates (1988–2012) and Predictions to 2030. Gastroenterology. 2021;1:116-127.e8.
- [Google Scholar]
- The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn. Virulence. 2016;7(6):649-659.
- [Google Scholar]
- Antifungal activity of essential oils against azole-resistant and azole-susceptible vaginal Candida glabrata strains. Can. J. Microbiol.. 2018;64(10):647-663.
- [Google Scholar]
- Clotrimazole for vulvovaginal Candidosis: more than 45 years of clinical experience. Pharmaceuticals. 2020;13(10):274.
- [Google Scholar]
- Anti-fungal resistance in candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation. 2014;10(11):667.
- [Google Scholar]
- Evaluation of antibacterial efficacy of various solvent extracts of Evolvulus alsinoides and Mucuna pruriens against multidrug resistant (MDR) pathogenic bacteria. Appl. Nanosci. 2021:1-11.
- [Google Scholar]
- Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci.. 2018;25(2):361-366.
- [Google Scholar]
- Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis.. 2017;9(3):448.
- [Google Scholar]
- An in vitro investigation of the antidermatophytic, antioxidant, and nephroprotective activity of Solanum surattense. Process Biochem.. 2021;109:178-185.
- [Google Scholar]
- Phytochemical profile and larvicidal activity of aqueous extract of Ocimum americanum against mosquito vectors. Appl. Nanosci. 2021
- [Google Scholar]
- The pharmaceutical potential of crude ethanol leaf extract of Pedalium murex (L.) Process Biochem.. 2022;112:234-240.
- [Google Scholar]
- Molecular identification and antifungal susceptibility pattern of non-albicans Candida species isolated from vulvovaginal candidiasis, Iran. Biomed. J.. 2018;22(1):33.
- [Google Scholar]
- Antifungal and antibacterial activities of eugenol and non-polar extract of Syzygium aromaticum L. Int. J. Pharm. Sci. Res.. 2018;10(2):337-339.
- [Google Scholar]
- Comparative phytochemical analysis of the leaves of two Myrtaceae members-Pimenta dioica (L.) Merril and Syzygium aromaticum (L.) Merril and Perry. J. Pharmacogn. Phytochem.. 2019;8(3):3043-3045.
- [Google Scholar]
- Chemical composition and biological activities of essential oil of Beilschmiedia pulverulenta. Pharm. Biol.. 2016;54(2):322-330.
- [Google Scholar]
- Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int. J. Mol. Sci.. 2018;19(1):263.
- [Google Scholar]
- Candida albicans—the virulence factors and clinical manifestations of infection. J. Fungi.. 2021;7(2):79.
- [Google Scholar]
- A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog.. 2019;134:103580
- [Google Scholar]
- Teles, A.M., Silva-Silva, J.V., Fernandes, J.M.P., Abreu-Silva, A.L., Calabrese, K.d.S., Mendes Filho, N.E., Mouchrek, A.N., Almeida-Souza, F., 2021. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid Based Complement Alternat Med.
- Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93.
- [Google Scholar]
- Eugenol inhibits oxidative phosphorylation and fatty acid oxidation via downregulation of c-Myc/PGC-1β/ERRα signaling pathway in MCF10A-ras cells. Sci. Rep.. 2017;7(1):1-13.
- [Google Scholar]
- Yassin, M. T., Mostafa, A. A. F., Al Askar, A. A., 2021. In Vitro Evaluation of Biological Activities and Phytochemical Analysis of Different Solvent Extracts of Punica granatum L.(Pomegranate) Peels. Plants. 10(12), 2742.
- In vitro antifungal resistance profile of Candida strains isolated from Saudi women suffering from vulvovaginitis. Eur. J. Med. Res.. 2020;25(1):1-9.
- [Google Scholar]
- Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ. Sci.. 2020;32(3):2046-2052.
- [Google Scholar]
- Anticandidal efficiency of Cinnamomum zeylanicum extracts against vulvovaginal candidiasis. Curr. Sci.. 2020;118(5):796.
- [Google Scholar]
- Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol.. 2018;9:1253.
- [Google Scholar]
- Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest Manag. Sci.. 2021;77(7):3469-3483.
- [Google Scholar]







