Translate this page into:
Phytochemical analysis and antimicrobial activity of Enicostemma littorale
⁎Corresponding author. adilfarooq25@gmail.com (Adil Farooq Wali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Antimicrobial resistance has risen in morbidity and mortality due to delay in medical care and rising health care costs. The aims and objectives of this study were to explore plant molecular entities from Enicostemma littorale by isolation, fractionation and antimicrobial activity using four different bacterial strains, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis followed by the establishment of their chemical identity in order to pave the way for potential advancement of plant-based pure therapies for application in the production of new drugs. The ethyl acetate extract was found to possess significant antimicrobial activity when compared to other extracts an attempt was made to fractionate these extracts by column chromatography. On the bases of spectroscopic data IR, NMR and MS and comparing the data in the scientific literature, the chemical structure of the isolated are luteolin (1) and α-amyrin acetate (2). Both isolated compounds showed the highest antibacterial effect, with minimum inhibitory concentration values ranging from 16 to 500 μg/mL, against Gram-positive and Gram negative bacteria, respectively.
Keywords
Enicostemma littorale
Phytochemical screening
Secondary metabolites
Isolation
Lupeol
α-Amyrin acetate
Antimicrobial screening
- mL
Milliliter
- g
Gram
- TLC
Thin layer chromatography
- IR
Infrared spectroscopy
- H1NMR
Proton nuclear magnetic resonance
- MIC
Minimum Inhibitory concentration
- DMF
Dimethylformamide
- °C
degree celsius
- Lb
Pounds
- Psig
Pounds per square in gauge
- Rf
Retention factor
- CDCl3
Deuterated chloroform
Abbreviations
1 Introduction
The traditional medicinal system involves various drugs that originated from plants and trees and played an important role in medical history. Most of the civilized countries have their indigenous systems as their traditional medicines. The system in which the treatments are different than those used in mainstream medicine is defined as complementary medicine. The largest number of users of medicinal plants and also those who use the maximum diversity of species are rural households. The trees and plants contain various secondary metabolites called phytochemicals. These phytochemicals help the plants to protect against microbial contagions or pest infestations. These secondary metabolites possess various therapeutic properties and due to this, they are considered as a medicine or drug (Arvind, 2016). The steps involved in the phytochemical investigation of a plant are extraction of the plant material, separation, and isolation of the secondary metabolites, identification by characterization of the isolated compounds, evaluation of the biological activities of these compounds, and quantitative estimations.
Infectious diseases are the world's main cause of mortality, in particular in developing nations. Currently, epidemics of infections caused by drug-resistant and unknown microorganisms have raised major health concerns (Gupta and Birdi, 2017). This situation has called for renewed strategies on treatment and preventive approaches, one of which is the discovery of new antimicrobials agents (Khameneh et al., 2019). Recently, it has been found that pathogenic bacteria are increasingly growing to become multidrug resistant. This troubling condition originates from the unnecessary and frequently use of antibiotics in human and animal health care to manage and prevent bacterial infections (Adamczak et al., 2019). Newer drugs armed with a competent mechanism should be formulated to regulate this condition, the plant-derived antimicrobial agents may be the solution to these problems. Recently, medicinal plants have become the focus of intense study regarding their conservation and potential pharmacological effects. Indeed, the search for new pharmacologically active agents, through the screening of natural sources such as microbial fermentations and plant extracts, has led to the discovery of many clinically useful drugs that now play major roles in the treatment of human diseases (Fialova et al., 2017; Chandra et al., 2017; Guimarães et al., 2019).
Enicostemma littorale is a perennial herb 10–15 cm high. It is branched from the base with erect stems. Leaves are sessile and can be linear or linear-oblong (Nidhi et al., 2017). Flowers are white in whorled axillary clusters and are sessile. The fruits are capsule, one-celled with many small, pale, ridged ovate seeds. Ayurveda describes the plant as stomachic and laxative. The plant is very bitter. It can be used as an anthelmintic and it cures fever (Vaijanathappa and Badami, 2008; Akhtar, 2011).
This plant is known to be used as a substitute for chiretta (Swertia chirata), a well-known anti-diabetic drug (Sankaranarayanan, 2010; Jhan et al., 2009). The plant comprises of a number of different chemical compounds with different roles. The presence of glycosides and ophelic acid in the plant gives bitterness to it. Gentianine is an alkaloid which is also reported from this plant. A number of other compounds such as phytosterol, glucoside, sugars, and tannins have been reported in the same (Selvam and Muruganantham, 2018; Doss and Kuberapandian, 2016). A yellow bitter crystalline glycoside called Gentiopicrin is present in this plant (Tanna et al., 2010). The presence of a monoterpene alkaloid-enicoflavin and gentiocrucin has been reported. The remarkable activity of cardiac depression is shown by the alkaloidal fraction of the plant (Leong et al., 2016). This plant is found to contain iridoid glycosides, flavonoids, and xanthone (Garad et al., 2012a, 2012b). Tribal use of the plant has been done for jaundice with the use of the paste of the entire plant of Enicostemma littorale in water along with copper coin tied in the forearm of patients. Anti-pyretic activity has been shown by the aerial part of the plant (Leelaprakash and Mohan, 2012).
Several studies proved that Enicostemma littorale possesses various therapeutic activities like antidiabetic, anti-inflammatory and anti-microbial activities (Roy et al., 2010; Vishwakarma et al., 2010; Gite et al., 2010). Its shown to possess good hepatoprotective activity against paracetamol-induced hepatic-toxicity in albino rats (Vaidya et al., 2009). The leaves have varied usages such as antiulcer and anti-nociceptive (Gopal and Udayakumar, 2008; Gupta et al., 2010). The whole plant of Enicostemma littorale also possess cytoprotective effect (Marcus et al., 2019; Alolika et al., 2019).
The important factors that can influence the anti-microbial tests are the size of the test inoculum, pH, metabolic condition of organisms, temperature’, the period of interaction with the organism, the strength of the inhibitor, and presence of interfering substances (Xuan et al., 2004).
To know the susceptibility of the micro-organisms, sensitivity testing can be done under specified conditions. Common aerobes and anaerobes if they can grow well overnight, disk diffusion method is a suitable method. There are different procedures for conducting disk diffusion methods. Kirby-Bauer method is one of the official methods for disc diffusion methods (Amita and Shalini, 2014). The minimum inhibitory concentration of the extracts should be evaluated before the disc diffusion method by the agar-dilution method.
The present study includes preliminary phytochemical screening, extraction, isolation of the chemical constituents, and characterization of the chemical components isolated from the entire plant, of Enicostemma littorale. and the anti-microbial activity of the various extracts.
2 Materials and methods
2.1 Plant collection and preparation of the plant extract
The entire plant of Enicostemma littorale (4Kg) was collected from Tamil Nadu, India, and was authenticated by the Botanical Survey of India, Southern Regional Centre, Coimbatore (No. BSI/SRC/5/23/2010-11/Tech-2051). The whole plant was sun-dried and was powdered mechanically (2 kg) and then soaked in ethyl alcohol and preserved for one week. The alcoholic layer was decanted after seven days. This procedure was continued until the plant material was exhaustively extracted with ethyl alcohol. The ethyl alcohol extract thus obtained was distilled and concentrated to get a sticky mass (350 g).
2.2 Preliminary phytochemical evaluation
The preliminary phytochemical evaluation of the various extracts was evaluated using the standard protocol to find out the presence of various secondary metabolites present in it (Zhou et al., 2014).
2.3 Fractionation of ethanolic extract
The ethanolic extract (300 g) was fractionated successively with petroleum ether, butanol, and ethyl acetate after stirring with one-liter water. These extracts were concentrated under reduced pressure. The yield of petroleum ether extract was (25 g), butanol extract was (30 g) and ethyl acetate extract was (25 g).
2.3.1 Preparation of petroleum ether extract
Saponification with 20% ethanolic potassium hydroxide (300 ml) has done for the residue (20 g) of petroleum ether extract for 2 h. To remove any traces of ethanol this was then evaporated and water was added from time to time to replace the lost quantity. Petroleum ether was added to the unsaponifiable portion and extracted (5 × 300 ml). All the petroleum ether fractions were collected together which gave a yellow residue (150 mg).
2.3.2 Isolation of phytoconstituents
150 mg of petroleum ether extract was taken and adsorbed on an alumina column saturated with petroleum ether. The elution was carried out with a gradient elution technique. Petroleum ether alone used for the first elution. Graded mixtures of petroleum ether and benzene with the ratios 95:5, 90:10, 80:20, and 50:50 were used next. Benzene was used alone next to the elution. Graded mixtures of benzene and chloroform with the ratios 95:5, 90:10, 80:20, and 50:50 were used and then with chloroform alone. Petroleum ether with a boiling point of 60–80 °C was used for this study.
The eluates were collected but all these were brown resinous masses, which have not proceeded more.
2.3.3 Preparation of butanol extract
Saponification with 20% ethanolic potassium hydroxide (300 ml) has done for the residue (20 g) of butanol extract for 2 h. To remove any traces of ethanol this was then evaporated and water was added from time to time to replace the lost quantity. Petroleum ether was added to the unsaponifiable portion and extracted (5 × 300 ml). All the fractions were collected which gave a yellow residue (250 mg).
2.3.4 Isolation of phytoconstituents from butanol extract
The butanol extract (20 g) was taken and adsorbed on an alumina column saturated with petroleum ether. The elution was carried out with a gradient elution technique. Petroleum ether alone used for the first elution. Graded mixtures of petroleum ether and benzene with the ratios 95:5, 90:10, 80:20, and 50:50 were used next. Benzene was used alone next to the elution. Graded mixtures of benzene and chloroform with the ratios 95:5, 90:10, 80:20, and 50:50 were used and then with chloroform alone. Petroleum ether with a boiling point of 60–80 °C was used for this study.
All the fractions were monitored by TLC and visualization is done with the spraying reagent sulphuric acid and vanillin. 10 ml of the eluate was collected from time to time. Similar eluates after checking with the TLC were mixed and kept in a refrigerator.
From the various fractions, the graded mixtures of petroleum ether and benzene with the ratio 80:20 gave two components. A residue of 80 mg was resulted after removing the solvent. Preparative TLC was done for this residue with a solvent system of petroleum ether and benzene with the ratio 80:20. Then the two compounds were separated as pure components. Compound I of 30 mg and compound II of 25 mg were collected. The eluates from all other solvents and graded mixture were brown sticky masses that were not processed further.
2.3.5 Preparation and isolation of phytoconstituents from ethyl acetate extract
The 20 g of the residue of ethyl acetate extract was taken and adsorbed on an alumina column saturated with petroleum ether. The elution was carried out first with ethyl acetate alone and with the mixtures of ethyl acetate, methanol, and water (75:15:10). The eluates were collected but it was brown sticky masses, which have not proceeded more.
2.4 Characterization
The characterization of the isolated compounds from butanol extract; compound I and compound II were done using IR (model: Thermo-Nicolet 6700, Range: 400–4000 cm−1), H1NMR (Bruker model-AV-400, 400 MHz NMR spectrometers-Rheinstetten, Germany) and mass spectroscopy.
2.5 Evaluation of anti-microbial activity of various extracts
The petroleum ether, butanol and ethyl acetate extracts of the whole plant of Enicostemma littorale were evaluated for its anti-microbial activity.
2.5.1 Minimum inhibitory concentration (MIC)
This was done by agar dilution method. The test compounds of different concentrations were prepared by serial dilution method and mixed into the prepared agar medium. The test organism was added on to the agar medium as tiny droplets. The presence or absence of the development of the microorganism was detected and MIC of all these extracts was calculated from the results.
2.5.2 Procedure
Six test tubes were numbered from 1 to 6. Dimethylformamide (DMF) was taken in each tube. 2 ml of DMF in tube-1 and all the remaining five tubes with 1 ml of DMF each. All the six tubes were kept in the autoclave at 120 °C for 20 min to sterilize them. Each of the tubes was closed with non-absorbent cotton wool. Dissolve aseptically transferred 16 mg of the test compound into the tube-1. 1 ml from test tube 1 was added to test tube-2 and mixed well. Again 1 ml of solution from Tube-2 is added to Tube-3. Similarly, serial dilution was done for all the remaining tubes until the fifth one. From the last one (tube-6) 1 ml of the solution was discarded. Transferred Molten Mueller Hinton agar (15 ml) aseptically into all the test tubes and mixed well. All the samples were allowed to solidify after pouring them aseptically into six sterile Petri dishes. Each plate had been divided into four and was inoculated with 10 ml of different test organisms 106 cells/ml) in all the four sections. Positive and negative controls were made in the same way without the test sample. All plates were incubated at appropriate conditions (37 °C for 24 h).
2.5.3 Evaluation of antimicrobial activity by disc diffusion method
By using the Modified Kirby-Bauer method (Vineet et al., 2019) the anti-bacterial evaluation of the extracts was done. The calculated amounts of the test sample were saturated to the circular paper disks (6 mm diameter) and were kept on the prepared Mueller Hinton agar medium containing the test organism. The zones of inhibition of all the test samples were calculated and compared with those obtained with the standard antibiotic (ampicillin).
The concentration of the extracts in all the Petri plates is shown in Table 2. The test extracts were taken and mixed with sterilized dimethylformamide (DMF) and 25 μL of these solutions were transferred to the disk using a micropipette. Ampicillin (10 μg) was used as a standard antibiotic. Aseptic conditions were maintained throughout the procedure. ‘+’ indicates presence of growth, ‘− ‘ indicates absence of growth.
S.No
Tests
Ethanolic extract
Pet. Ether extract
Ethyl Acetate
Butanolic extract
1
Alkaloids
+ve
+ve
+ve
+ve
2
Carbohydrates
+ve
+ve
+ve
+ve
3
Flavonoids
+ve
-ve
+ve
+ve
4
Triterpenoids
+ve
+ve
+ve
+ve
5
Proteins
−ve
−ve
−ve
−ve
6
Resins
−ve
−ve
−ve
−ve
7
Saponins
−ve
−ve
−ve
−ve
8
Steroids
+ve
−ve
−ve
+ve
9
Tannins
+ve
−ve
−ve
+ve
10
Starch
−ve
−ve
−ve
−ve
11
Glycosides
+ve
+ve
+ve
+ve
Plate number
1
2
3
4
5
6
Concentration, μg/ml
500
250
125
63
31
16
2.5.4 Preparation of the Mueller Hinton agar media
The definite amount of desiccated medium or Hi-Media in distilled water was prepared and 20 ml from this was transferred to the test tubes (Saleh et al., 2019). All the test tubes were previously autoclaved at 121 °C (15 lb psig) for 15 min for sterilization. Then the contents from these tubes were transferred into Petri dishes and kept for solidification.
Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis cultures were used for the evaluation of the antibacterial activity. The Petri dishes were inoculated with one of the test organisms using a cotton swab. For this, diluted suspension (106 cells/ml) of the organism was used. The test compound was added into the filter paper disk and kept at three altered places in the Petri dishes at an equivalent distance along with the standard ampicillin at two places. It is then kept for diffusion.
These diffused Petri dishes were incubated for 24 h (37 °C). All the results were compared with that of the standard drug ampicillin.
3 Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) and means were compared using Bonferroni’s comparison test, the mean diameters of inhibition zones of the test samples bearing different superscript letters (a, b, c, d, e, f) are significantly different (p < 0.05) compared to other mean values. Data are means of three replicates (n = 3) ± standard error.
4 Results
4.1 Preliminary phytochemical evaluation
The results ‘of the preliminary phytochemical evaluation of the various extracts of the entire plant of Enicostemma littorale is showed in Table 1. Various secondary metabolites are present in the plant. Tannins, triterpenoids, alkaloids, cardiac glycosides, flavonoids, and steroids were present in the ethanolic extract of the entire plant.
4.2 Spectral analysis of the eluted compounds
From the butanol extract, the combined petroleum ether and benzene (80:20) eluates gave two components. These compounds gave a positive result in Liebermann Burchard’s test for triterpenoids. From the melting point, IR, 1HNMR, and mass spectra the two components were identified as Lupeol (compound I) and α-Amyrin acetate (compound II). The spectral details are as follows
4.2.1 Spectral characteristics of compound I
Compound I obtained as white crystals with Rf value 0.71 and gave a pink spot on the TLC plate. The melting range of the compound was found to be between 212 and 215 °C.
4.2.1.1 IR analysis of the isolated compound I showed the following characteristics
The IR spectra of compound I. 2937.42 cm−1, 3326.49 cm−1, 1640.51 cm−1 (C = C str.), 2869.48 cm−1, (C—H str. in CH3 and CH2), 1041.47 cm−1, (C—O str. of secondary alcohol), 1449.07 cm−1 (C—H deformation in CH2/CH3), 890 cm−1 (CH3—C = CH2), 1374.96 cm−1 (C—H deformation in gem dimethyl) (Supplementary data).
The compound had a broad —OH signal at 3326.49 cm−1. The compound showed a C—H stretch of CH2 and CH3, at 2869.48 cm−1 and 2937.42 cm−1. The olefinic C = C stretch appeared at 1640.51 cm−1. The C—H deformation in CH2/CH3 was discernible at 1449.07 cm−1. The C—H deformation in the gem dimethyl group was evident from the signal at 1374.96 cm−1., The C—O stretch of secondary alcohol appeared at 1041.47 cm−1. The exocyclic CH2 group showed a prominent signal at 890 cm−1.
4.2.1.2 1HNMR: (CDCl3) of the isolated compound I showed the following characteristics
The 1HNMR spectra of compound I. δ 1.76 (s,1H, CH3-C = CH2), δ 0.7–1.026 (m, 18H, 6 × CH3), δ 4.700 (s, 1H, β-C-24H), δ 3.322 (s, 1H,OH), δ 4.581 (s, 1H, H-3), δ 0.8 to δ 1.74 (m, 25H for CH2 and CH protons), δ 4.88 (s, 1H, α-C-24H) (Supplementary data).
Methyl protons of the 6-methyl group appeared upfield between δ 0.7 and 1.026. The methylene protons and methine protons appeared between δ 0.8 and 1.74 accounting for 25 protons. The proton of the CH—OH appeared at δ 3.322. Two signals at δ 4.7 (β-C-24H) and δ 4.88 (α-C-24H) attributed to methylene groups at C-24. The H-3 proton appeared as a singlet at δ 1.76.
4.2.1.3 Mass spectra of compound I
Molecular formula of the compound was C30H50O and molecular weight was 426 m/z. The molecular ion peak of compound I was at m/z 426 (2.8%). The spectra showed further peaks at 409 (1.86%), 393 (2.8%), 234 (3.73%), 218 (8.41%), 203 (34.57%), 189 (19.62%), 175 (6.54%), 161 (7.47%), 149 (14.01%), 135 (12.14%), 119 (13.08%), 109 (16.82%), 85 (68%) and 83 (100%) (Supplementary data).
By interpreting all the data such as melting point, IR, mass, and 1HNMR, the compound I is ascertained as Lupeol.
4.2.2 Spectral characteristics of compound-II
Compound I obtained as white crystals with Rf value 0.48 and developed a pink spot on the TLC plate. The melting of the compound II was found to be 227 °C
4.2.2.1 IR analysis of the isolated compound II showed the following characteristics
The IR spectra of compound II. 1735.8 cm−1 (ester C = O str.), 1641.3 cm−1 (C = C str.), 1452.3 cm−1 (C—H, deformation in CH3), 2854.5 cm−1 (C—H str. in CH3 and CH2),1244 cm−1 (C—O str. of acetate), 1365.5 cm−1–, 1340.4 cm−1 (gem dimethyl str.), 2918.1 cm−1 (Supplementary data).
4.2.2.2 1HNMR: (CDCl3) of the isolated compound II showed the following characteristics
The 1HNMR spectra of compound II. δ 0.80 (s, 3H, H-28), δ0.93 (s, 3H, H-25), δ 0.81–0.87 (m, 12H, H-23, 24,29,30), δ 0.95 (s, 3H, H-26), δ 5.15 (s, 1H, H-12), δ 2.06 (s, 3H, OAC), δ 4.55 (t, 1H, H-3), δ 1.08 (s, 3H,H-27). The signals’ due to methylene’ and methine protons overlapped with one another and obtained as multiplets in the region of δ 1.0–1.92 integrating for 23 protons (Supplementary data).
4.2.2.3 Mass spectra of compound II
The molecular formula was found to be C32H52O2, and molecular weight was 468 m/z. The mass spectrum of compound II showed molecular ion peak at m/z, 468 (M+), 453 (M+−CH3), 408 (M+CH3COOH), 358, 297, 270, 249, 218 (100%), 189, 175, 147, 121,107, 81, 69 (Supplementary data).
The mass fragmentation of compound II obtained was matching with that of an α-amyrin skeleton. The characteristic IR absorption at 1735.8 cm−1 indicates the presence of an ester linkage in the compound. The CH3 singlet at δ 2.069 is a characteristic of the acetyl group and this indicates the acetate nature of the ester. The peak obtained at m/z,’408 in the mass spectra shows the loss of acetic acid (60) from the molecular ion (M+) 468. All this indicates that the compound is in acetate form of amyrin. The melting point (184 °C) also supports with that reported for α-amyrin.
By interpreting all the data such as melting point, IR, mass, and 1HNMR, compound II was ascertained as α-amyrin acetate.
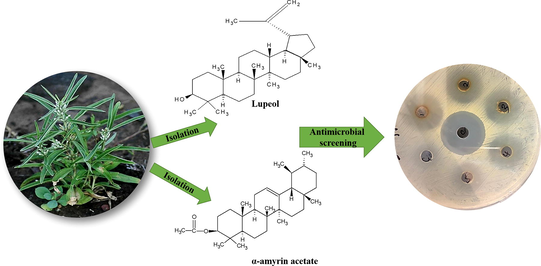
Figs. 1 and 2 represents the chemical structure of Lupeol and α-amyrin acetate respectively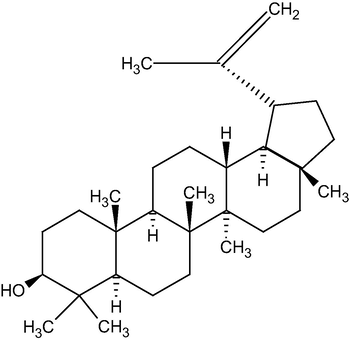
Represents the chemical structure of Lupeol.
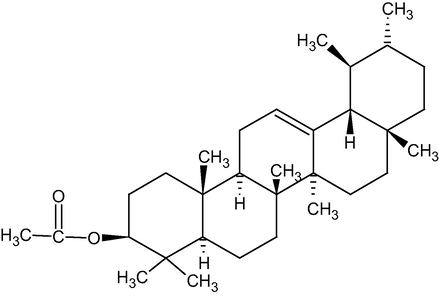
Represents the chemical structureα-amyrin acetate.
4.3 Antimicrobial activity of the various extracts and isolated compounds
The isolated compounds and extracts viz, petroleum ether, butanol and ethyl acetate of the whole plant of Enicostemma littorale were subjected to antimicrobial screening. The concentration that taken for the study is shown in Table 2. The results of antibacterial activity are given in Tables 3 and 4, which clearly show that all the extracts and isolated compounds at various concentrations have shown antibacterial activity equivalent to that of standard (Ampicillin) against the entire tested organisms. The minimum inhibitory concentration of the extracts was found to be 250 μg/ml. So at this concentration and above, all the extracts inhibited the growth of various test organisms used viz, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis Whereas, Lupeol was more effective against Bacillus subtilis and Staphylococcus aureus. α-amyrin was more effective against Pseudomonas aeruginosa and Escherichia coli. The MIC values ranging from 500 to 125 µg/mL were obtained for the two terpenoids against Bacillus subtilis, 500 to 16 µg/mL for Staphylococcus aureus, 500 to 31 µg/mL for Pseudomonas aeruginosa, and 500 to 25 µg/mL for Escherichia coli as shown in Table 3. ‘+’ indicates presence of growth, ‘−‘ indicates absence of growth. The data were analyzed by one-way analysis of variance (ANOVA) and means were compared using Bonferroni’s comparison test, the mean diameters of inhibition zones of the test samples bearing different superscript letters (a, b, c, d, e, f) are significantly different (p < 0.05) compared to other mean values. Data are means of three replicates (n = 3) ± standard error.
Extracts/Isolated compounds
Organism (Presence/absence of growth)
B. subtilis
S. aureus
P. aeruginosa
E. coli
Dilution
1
2
3
4–6
1
2
3
4–6
1
2
3
4–6
1
2
3
4–6
Pet. EtherExtract
−
−
+
+
−
−
+
+
−
−
−
+
−
−
−
+
Butanol Extract
−
−
+
+
−
−
−
+
−
−
−
+
−
−
−
+
Ethyl acetate Extract
−
−
+
+
−
−
+
+
−
−
+
+
−
−
−
+
Ampicillin
−
−
−
−
−
−
−
−
−
−
−
−
−
−
−
−
Compound-I
+
+
+
−
+
+
+
+
+
+
+
+
+
−
−
−
Compound-II
+
+
−
−
+
+
+
+
+
+
+
+
−
−
+
+
+ve Control
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
−ve Control
−
−
−
−
−
−
−
−
−
−
−
−
−
−
−
−
S. No.
Extracts/Isolated compounds
Diameter of zone of Inhibition (mm)
B. subtilis
S. aureus
P. aeruginosa
E.coli
Pet. Ether Extract
11 ± 0.95a
11 ± 0.37b
12 ± 0.60c
14 ± 0.35f
Butanol Extract
10 ± 0.73b
11 ± 0.27c
12 ± 0.65b
13 ± 0.33d
Ethyl acetate Extract
10 ± 0.88d
10 ± 1.02d
11 ± 0.24a
12 ± 0.69e
Compound-I
13 ± 1.03a
12 ± 0.78f
12 ± 0.95d
10 ± 0.74d
Compound-II
13 ± 0.90a
11 ± 0.40c
13 ± 0.24b
09 ± 0.90d
Ampicillin (Standard)
15 ± 0.66b
13 ± 0.90f
14 ± 0.92d
17 ± 0.32d
5 Discussion
The current plant-related researches are based on the fact that natural medicine can be used for synthesizing many drugs for various ailments. To expand the therapeutic resources within nature, people try to understand the reason that rules the clinical or biological activity of many agents (Karina et al., 2019). Considerable efforts have been made for the technical development and extraction of biologically active phytoconstituents on a commercial scale by the industries and research organizations all around the world. With the help of these sophisticated isolation methods and pharmacological evaluation procedures, new plant drugs can be used as purified substances instead of previous galenical preparations.
Jahan et al reported the presence of catechins, saponins, steroids, sapogenin, triterpenoids, flavonoids and xanthones and a new flavone C-glucoside named as Verticilliside (Jahan et al., 2009). In previous report, Desai et al found six phenolic acids like vanillic acid, syringic acid, p-hydroxy benzoic acid, protocatechuic acid, p-coumaric acid and ferulic acid (Desai et al., 1966). Monoterpene alkaloids like enicoflavin, gentiocrucine and seven different flavonoids were isolated from the alcoholic extract and the structures were identified as apigenin, genkwanin, isovitexin, swertisin, saponarin, 5-o glucosylswertisin and 5-o glucosylisoswertisin were also isolated (Saranya et al., 2013).
From the result of preliminary phytochemical screening, it is observed that along with other secondary metabolites, triterpenoids are also present in the ethyl alcohol extract’ of the entire plant of Enicostemma littorale. From the Butanol extract, the combined Petroleum ether: Benzene (80:20) eluates gave two components. They showed a positive result in Liebermann Burchard’s test for triterpenoids. Among the various secondary metabolites present in the plants, triterpenes are of great importance. These are made up of isoprene subunits. From the melting point, IR, 1H NMR, and mass spectra, the two components were identified as Lupeol (compound I) and α-Amyrin acetate (compound II) (Thanyani et al., 2019). The hydroxyl group of Lupeol shows a peak at 3326.48 cm−1 and the olefinic moiety shows a peak at 1640.51 cm−1 (Imam et al., 2007). The pentacyclic triterpenoid nature of Lupeol is confirmed with the methyl singlets and the olefinic function in the 1H NMR spectrum (Macías et al., 2007). All these confirm the presence of Lupeol in Enicostemma littorale. Triterpenoids or phytosterols are known to possess various biological activities. Triterpenes mostly contain a chain of 28–29 carbons and one or two double bonds between the carbon atoms. Lupeol is known as dietary triterpene since this is present in most of the vegetables (Virgilio et al., 2015). It is reported to show many pharmacological activities such as anti-inflammatory, anticancer, and antidiabetic activities (Mohammad, 2009). Lupeol is found to possess antiurolithic and diuretic activity (Vidya and Varalakshmi, 2002) It is also reported that Lupeol has antifungal activity against various strains of Penicillium notatum (Manzano et al., 2013). It also reported showing cytotoxic effect against human breast cancer cell lines (Lambertini et al., 2005).
The spectral data of the compound II was compared with that of the earlier reports and thus identified as α-Amyrin acetate (Ali, 2013; Manguro et al., 2009; Medeiros et al., 2007). The methyl singlet at δ 2.069 shows the presence of acetyl group and this indicates the acetate nature of the ester. The peak obtained at m/z,'408 also supports the same (Nkeoma et al., 2014). α-Amyrin acetate is also a triterpene. It is also reported to possess antispasmodic and antibacterial activities. Lupeol and α-Amyrin acetate are the two triterpenoids isolated from the entire plant of E. littorale. Studies have been reported that these compounds possess very good wound healing activity (Walter et al., 2019). It is also reported that β-amyrin and α-Amyrin possess anti-inflammatory activity (Krishnendu et al., 2019). Many studies reported that the β-amyrin and α-Amyrin having anticancer activity on various cell lines. Since the plant is reported to have very good anti-diabetic activity it also might be due to the presence of these triterpenoids present in it (Fernado et al., 2019).
The findings of the present study showed that there were differences between the antimicrobial activities of plant extracts. These differences may be due to the different groups of secondary metabolites found in these extracts. Indeed, the antimicrobial activity of medicinal plants is correlated with the presence in their extracts of one or more classes of bioactive secondary metabolites (Nzogong et al., 2018). As mentioned previously, triterpenes are known to display significant antimicrobial properties (Catteau et al., 2018). Terpenoid compounds are present widely in the kingdom of flora; recent studies have shown that phenolic compositions have such a possible protective role against oxidative ailments. Results showed that α-amyrin acetate and lupeol compounds showed significant antimicrobial effects against different bacteria (Nzogong et al., 2018). Our results are similar to the earlier study, they noticed a rise in antimicrobial activity of Enicostemma littorale extracts with the increase in concentrations (Abirami et al., 2012). The results also revealed that there is a very week antimicrobial activity against low polar solvents. In other studies, the high polar solvents like alcohol showed significant antimicrobial effects against different bacteria (Saranya et al., 2013).
6 Conclusion
The findings of this study suggest that all extracts from the whole Enicostemma littorale plant may be a potential source of plant drugs for bacterial disease treatment. In addition, all the isolated compounds found to be active in this study might well be a useful step in the development of high therapeutic value newer compounds.
Acknowledgment
The authors thank Dr. D. Satyanarayana and Dr. Arun B Joshi for their support in conducting the study.
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/19), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- reliminary study on the antimicrobial activity of Enicostemma littorale using different solvents. Asian Pac. J. Trop. Med.. 2012;5(7):552-555.
- [Google Scholar]
- Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med.. 2019;9(1):109.
- [Google Scholar]
- Antioxidant potential of dried Enicostemma littorale. Pak. Biol. Sci.. 2011;14:956-995.
- [Google Scholar]
- Brine shrimp cytotoxicity of crude methanol extract and antispasmodic activity of α-amyrin acetate from Tylophora hirsuta Wall. BMC Complemenary Alt. Med.. 2013;13:135.
- [Google Scholar]
- Phytochemical screening, anti-oxidant and anti-microbial activity of leaf, stem and flower of Rangoon creeper: a comparative study. J. Med. Plants Stud.. 2019;7:123-130.
- [Google Scholar]
- Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharm. Phytochem.. 2014;2:115-119.
- [Google Scholar]
- Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: a review. Phytochem. 2018;17:11291163.
- [Google Scholar]
- Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials-a review. Plants (Basel, Switzerland). 2017;6(2):16.
- [Google Scholar]
- Chemical investigation of some Indian medicinal plants: Part II. Ind. J. Chem.. 1966;1966(4):457-459.
- [Google Scholar]
- Antidepressant activity of Enicostemma littorale Blume in Shp2 (Protein Tyrosine Phosphatase)-inhibited animal model of depression. Int. J. Prev. Med.. 2016;7:112.
- [Google Scholar]
- Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxid. Med. Cell. Longevity 2019:20.
- [Google Scholar]
- Plant natural agents: polyphenols, alkaloids and essential oils as perspective solution of microbial resistance. Curr. Org. Chem.. 2017;21:1875-1884.
- [Google Scholar]
- Aerial parts of Enicostemma littorale Blume serve as antipyretic and antacid: in vivo and in vitro evaluations. Pharm. Commun.. 2012;2(3):42-45.
- [Google Scholar]
- Aerial parts of Enicostemma littorale Blume serve as antipyretic and antacid: in vivo and in vitro evaluations. Pharm. Commun.. 2012;2:42-45.
- [Google Scholar]
- Hepato-protective activity of Enicostemma axillare in paracetamol induced hepato-toxicity in albino rats. J. Pharmocol.. 2010;1:50-53.
- [Google Scholar]
- Enzymatic and non-enzymatic antioxidant activity in p-DAB induced hepatocarcinoma in rats. Int. J. Pharmacol.. 2008;4:369-375.
- [Google Scholar]
- Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules (Basel, Switzerland). 2019;24(13):2471.
- [Google Scholar]
- Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med.. 2017;8(4):266-275.
- [Google Scholar]
- Enicostemma littorale: a new therapeutic target for islet of neogenesis. Int. J. Int. Biol.. 2010;9:1-50.
- [Google Scholar]
- Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica linn. Pak. J. Pharm. Sci.. 2007;20:125-127.
- [Google Scholar]
- Verticilliside, a new flavone C-glucoside from Enicostemma verticillatum. J. Asian Nat. Prod. Res.. 2009;11(3):257-266.
- [Google Scholar]
- Verticilliside, a new flavones C-glucoside form Enicostemma verticillatum. J. Asian Nat. Prod. Res.. 2009;11:257-260.
- [Google Scholar]
- α, β-Amyrin, a pentacyclic triterpenoid from Protium heptaphyllum suppresses adipocyte differentiation accompanied by down regulation of PPARγ and C/EBPαin 3T3-L1 cells. Biomed. Pharmacother.. 2019;109:1860-1866.
- [Google Scholar]
- Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob. Resist. Infection Control. 2019;8:118.
- [Google Scholar]
- Lupeol alters viability of SK-RC-45 (Renal cell carcinoma cell line) by modulating its mitochondrial dynamics. Heliyon. 2019;5
- [Google Scholar]
- Expression of estrogen receptor gene in breast cancer cells treated with transcription factor decoy is modulated by Bangladeshi natural plant extracts. Oncol. Res.. 2005;14:69-79.
- [Google Scholar]
- Antimicrobial activity and phytochemical screening of methanol extract of Enicostemma axillare. Int. J. Pharm. Pharm. Sci.. 2012;4:342-348.
- [Google Scholar]
- systematic review of the protective role of swertiamarin in cardiac and metabolic diseases. Biomed. Pharmacother.. 2016;84:1051-1060.
- [Google Scholar]
- Pentacyclic triterpenes with selective bioactivity from Sebastiania adenophora leaves, Euphorbiaceae. J. Chem. Ecol.. 2007;33:147-156.
- [Google Scholar]
- Triterpenes of Commiphora holtziana oleo-gum resin. Can. J. Chem.. 2009;87:1173-1179.
- [Google Scholar]
- Pentacyclic triterpenoids with antimicrobial activity from the leaves of Vernonanthura patens (Asteraceae) Emir. J. Food Agric.. 2013;25(7):539-543.
- [Google Scholar]
- Phytochemical and anti-microbial screening of Phyllantus fratenus and Taraxacuim officinale leaves. Biochem. Anal. Biochem.. 2019;8:1.
- [CrossRef] [Google Scholar]
- Mechanisms underlying the inhibitory actions of the pentacyclic triterpene alpha amyrin in the mouse skin inflammation induced by phorbol ester 12 O-tetradecanoylphorbol-13-acetate. Eur. J. Pharmacol.. 2007;559:227-235.
- [Google Scholar]
- Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett.. 2009;285(2):109-115.
- [Google Scholar]
- An Ayurvedic herb: Enicostemma littorale blume-a review article. J. Med. Plants Stud.. 2017;5(1):78-82.
- [Google Scholar]
- Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol.. 2014;52(11):1478-1486.
- [Google Scholar]
- Antimicrobial and antioxidant activities of triterpenoid and phenolic derivatives from two Cameroonian Melastomataceae plants: Dissotis senegambiensis and Amphiblemma monticola. BMC Complementary Alt. Med.. 2018;18(1):159.
- [Google Scholar]
- Antiulcer and anti-inflammatory activity of aerial parts of Enicostemma littorale Blume. J. Young Pharm.. 2010;2(4):369-373.
- [Google Scholar]
- phytochemical composition and biological activities of wild Scolymus maculatus L. Medicines. 2019;6:53.
- [CrossRef] [Google Scholar]
- Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu, India. J. Med. Plants Res.. 2010;4(12):1089-1101.
- [Google Scholar]
- Pharmacognosy of Enicostemma littorale: a review. Asian Pac. J. Trop. Biomed.. 2013;3(1):79-84.
- [Google Scholar]
- phytochemical screening and evaluation of in vitro antioxidant efficacy of Enicostemma littorale Blume leaves extract. Int. J. Pharm. Sci. Rev. Res.. 2018;49(1):113-120.
- [Google Scholar]
- Physico-phytochemical evaluation of aqueous extract of Mamajjaka Enicostemma littorale. Int. J. Pharm. Biol. Arch.. 2010;1(3):309-312.
- [Google Scholar]
- Antibacterial and antimycobacterial activity of crude extracts, fractions, and isolated compounds from leaves of sneeze wood, Ptaeroxylon obliquum (Rutaceae) Nat. Prod. Commun.. 2019;14(9):1-7.
- [Google Scholar]
- Swertiamarin: a lead from Enicostemma littorale Blume for antihyperlipidaemic effect. Eur. J. Pharmacol.. 2009;617(3):108-112.
- [Google Scholar]
- In vitro antioxidant activity of Enicostemma axillare. J. Health Sci. 2008:524-528.
- [Google Scholar]
- Evaluation of the effect of triterpenes on urinary risk factors of stone formation in pyridoxine hyperoxaluric rats. Phytother. Res.. 2002;16(6):514-518.
- [Google Scholar]
- Antibacterial activity of Indian medicinal plant extracts antibiotic resistant Pseudomonas aeruginosa isolated from clinical samples. J. Agric. Environ. Res.. 2019;1(1):29-40.
- [Google Scholar]
- Terpenoids and sterols from Hoya multiflora Blume. J. Appl. Pharm. Sci.. 2015;5(4):033-039.
- [Google Scholar]
- Evaluation of effect of aqueous extract of Enicostemma littorale Blume in streptozotocin-induced type 1 diabetic rats. Ind. J. Exp. Biol.. 2010;48:26-30.
- [Google Scholar]
- Inclusion complexes of β and HPβ-cyclodextrin with α, β amyrin and in vitro anti-inflammatory activity. Biomolecules. 2019;9:241.
- [Google Scholar]
- Kirby-Bauer disc approximation to detect inducible third-generation cephalosporin resistance in Enterobacteriaceae. Ann. Clin. Microbiol. Antimicrob.. 2004;3:13.
- [CrossRef] [Google Scholar]
- Ag nanoparticle–ZnO nanowire hybrid nanostructures as enhanced and robust antimicrobial textiles via a green chemical approach. Nano Technol.. 2014;25(145702):8.
- [Google Scholar]







