Translate this page into:
Physio-biochemical, anatomical and functional responses of Helianthus annuus L. and Brassica juncea (Linn) to cypermethrin pesticide exposure
⁎Corresponding author at: Department of Geology and Pedology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska1, 61300 Brno, Czech Republic. rahul.datta@mendelu.cz (Rahul Datta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pesticides are applied all over the world to protect plants from pests. However, their application also causes toxicity to plants, which negatively affects the growth and development of plants.This study compared the physiological, biochemical and anatomical modifications of two oil seed crops viz., Helianthus annuus L. and Brassica juncea under different levels of pesticides. The present research was aimed to assess responses at the biochemical, physiological and anatomical levels to Cypermethrin (CYP) pesticide to discriminate the effects of osmotic stress and micronutrients availability. The two different concentrations of CYP-pesticide (20 mg /kg and 40 mg /kg) were examined. The obtained results showed that as the concentration of cypermethrin increased in soil, the fresh and dry weight of growing plants gradually decreased. The application of 40 mg /kg −1 soil of cypermethrin reduced the fresh weight of root in both H. annuus and B. juncea (up to 33 % and 29.4 % %, respectively) decreased root length (up to 2.3 % and 11 %, respectively). While shoot length was increased in both plant species at both levels of CYP relative to control. Shoot fresh weight decreased at 20 mg /kg CYP-treatment in H. annuus whereas in B. juncea at same level of cypermethrin increased fresh weight of shoot was found relative to un-treated seedlings. Reactive oxygen species viz., H2O2 and MDA contents in roots increased by 40 mg/kg-1 CYP in B. juncea followed by H. annuus (upto 50.27 % and 19.79 %; 36.33 % and 35.21 %, respectively). Furthermore, pesticide increased the activities of antioxidant enzymes in H. annuus followed by B. juncea. SOD, POD and CAT enzyme activities significantly decreased in B. juncea under 40 mg/kg-1 CYP by 38.8 %, 44.78 % and 36.83 %, respectively. In 20 mg /kg cypermethrin treatment declined antioxidant enzyme activities were found in H. annuus shoot compared to B. juncea shoot. In case of B. juncea increased APX enzyme activity was observed at 20 mg /kg-1 cypermethrin treatment. While significantly decreased activities of an antioxidant enzymes were found to be at 40 mg /kg CYP-treatment. The main aimed of this study to determine the anatomical responses of H. annuus and B. juncea roots to the cypermethrin exposure. Cypermethrin stress (40 mg /kg-1) change the root diameter (smaller), damaged epidermis layer, irregular form of epidermal cells of both species. The area of vascular bundle is decreasing when increasing the cypermethrin pesticide. Moreover, in stem, epidermis thickness was observed only in B. juncea xylem thickness increased in H. annuus at 20 mg /kg treatment, while in B. juncea non-significant difference was observed at this trait relative to un-treated plants. Phloem thickness raises and pith area in both species at lower concentration of cypermethrin. Leaf collenchymatous cell area was increased at both plant species at 20 mg /kg. Epidermis, spongy mesophyll and palisade mesophyll thickness increased when increased cypermethrin. Mineral uptake by H. annuus and B. juncea from cypermethrin treated soil was measured and also Pearson correlation was measured between all analyzed traits of plants. PCA clearly differentiated all treatments and growth parameters of both plant species. Thus, findings of present study confirmed that overdose of pesticides exhibited toxic effects on different growth, physiological and biochemical attributes of tested plants and pesticide also alter structural changes in plant tissues.
Keywords
Anatomy
Antioxidant enzymes
Micronutrients
Oil crops and pesticides
1 Introduction
Pesticide residues on cultivated crops can have negative consequences for human health and the environment (Zikankuba et al., 2019; Hailu, 2016; Miraglia et al., 2009; Hajslova and Zrostlikova, 2003). Extensive pesticide use, various ecological activities have degraded, flora and fauna have suffered destruction, and toxic residue levels have increased (Shakir et al., 2018, Shakir et al., 2016; Molaei et al., 2017; Sarkar et al., 2021). Pesticides have been used to raise crops in agricultural fields all over the world, which may pose toxicity issues in developing countries in particular, as farmers are mostly under-educated about the risks of using such poisonous chemicals (Alengbawy et al., 2021; Ngowi et al., 2007; Obopile et al., 2008).
Pakistan is a developing country with an agricultural state where a wide area of about 22.2 million ha is used for the production of different crops (Food and Agriculture Organization, 2006). Agriculture is important to the nation's economy, with over 40 % of the population relying on it as a source of income and agriculture contributing over 21 % of the country's GDP (Food and Agriculture Organization, 2006). Like other pests, insects have a major impact on the production and growth of crops in Pakistan and Indian subcontinent, resulting huge economic losses (Maalik et al., 2013; Ganguly and Srivastava, 2020). Furthermore, plant diseases caused by pathogen attacks are often regarded as a major stumbling block in the production of crop yields (Muhammad et al., 2020; Hussain and Abid, 2011; Ramzan et al., 2021).
A pesticide is a substance that can be used to repel, destroy, or eliminate pests. Pesticides are categorized as herbicides, fungicides, or insecticides based on the target killed. Because of the growing demand for food as a result of population growth, farmer has been forced to use pesticides to improve crop production (Lamichhane, 2017; Tomer, 2013; Tomer, 2013; Sarkar et al., 2017). Cypermethrin is one of the most widely used synthetic insecticides for agricultural and domestic purposes, globally. Pesticides regulate or kill plants by inhibiting biological processes such as photosynthesis, mitosis, cell division, enzyme activity, root development, or leaf formation; interfering with pigment, protein, or DNA synthesis; destroying cell membranes; or promoting unregulated growth (Lydia and Nataliia, 2021). Nehru et al. (1999) observed the inhibitory effect of herbicide pendimethalin and trifluralin on seed germination and early growth in crop Vigna radiata L. and Zea mays L. chlorotoluron blocked the higher plant photosynthetic electron transport (Muyesaier et al., 2021; Fuerst and Norman, 1991) and disrupted PSII reaction center (Barry et al., 1990). Previous research has shown that pesticide deposition by plants has harmed plant development and induced metabolic disorders (Sharples et al., 1997). Plants can respond to environmental stresses in a variety of ways, including physiological responses and oxidative damage (Chatterjee et al., 2018; Shah et al., 2020; Sarkar and Rakshit, 2022). It is well recognized that the interaction of such radicals with macromolecules, especially lipoprotein, causes more severe peroxidative damage, as evidenced by the disruption of cell membranes (Muyesaier et al., 2021; Jan et al., 2012). As opposed to control, triazoles treatment inhibited electrolyte leakage and lipid peroxidation in carrot plants (Gopi et al., 2007). Numerous researchers reported that SOD and CAT enzyme activities were increased in different plant species by the presence of herbicides and fungicides (Zhang et al. (2001), Wu et al. (2002), Basantani et al. (2011). Several plant species have been shown to have CATs participating in herbicide resistance, or an uptick in CAT behavior during herbicide exposure (Radetski et al., 2000; Jung et al., 2006). Thus, it is important that the authorities of the country, track pesticides in goods and implement guidance centered on permissible limits (Syed et al., 2014). The interaction of pesticides with other agricultural amendments should also be studied (Brtnicky et al., 2021). Farmers are subjected to elevated pesticides levels on a daily basis, typically far higher than customers (Damalas, 2016; Fahad et al., 2021). So, current pesticide use in Pakistan is still on the rise (Shakir et al., 2016).
Among different pesticides, Cypermethrin (CYP) is a synthetic pyrethroid pesticide used to manage a wide variety of pests for fields in the world (Jin et al., 2010). It is a member of the fourth pyrethroid group (Casida, 1980). Cypermethrin has cancer causing and carcinogenic properties and can produce endocannabinoid-active compounds such as 3-phenoxybenzoic acid (Morgan et al., 2007) and estrogenic activity (Al-Hamdani and Narasinhachary, 2011). Cypermethrin is water insoluble, and it has a strong tendency to adsorb soil particles that cause surface soil and microbial degradation.
Only some researchers have assessed a move beyond that to examine the effectiveness of phytoremedation in contamination or the possibility of concurrent penetration of contaminants during phytoremedation (Sarkar and Rakshit, 2020; Altaf et al., 2021; Radziemska et al., 2021). Such researchers are using an outstanding pollutant with one group for contaminants or two plant species, but will report tolerance of plants in contaminated soil via analyzing the morophological, anatomical, and biochemical responses to spiked soil (Irfan et al., 2021; Saboor et al., 2021). The effectiveness for that method is focused on the targeted absorption capacity of plant root systems and on the ability of selected plants to bio accumulate, translocate and degrade (Sharma et al., 2015). The aim of this comparative research is to establish the shoot and root physiological and biochemical responses of B. juncea and H. annuus to Cyp excess as a function of concentration. This paper examines morphological, physiological and biochemical changes in the shoot and root system, which are important in terms of survival since these architectural changes can aid in Cyp adaptation.
2 Material and methodology
2.1 Experimental site and design
The experiments were performed at the Department of Botany; The Islamia University of Bahawalpur. Set complete randomized design with 3 treatments in spiked (20 mg/kg and 40 mg/kg) and unspiked soil (0 mg/kg), with 3 replicates each. Experimental soil used was bhal and cow dung, purchased from the nearby Bahawalpur nursery, was stained through a 2 mm stainless sieve. Soil spiked with different Cypermethrin (CYP) concentrations (20 and 40 mg/kg) were mixed to experimental unit of soil separately. All quantities were combined as a component of 250 g with soil, and combined with 750 g of soil to create 1000 g (total soil of each treatment is 6 kg). Contaminated soils were kept in shade for 1 month, pre-seed planting area in order to maintain a balance between solid - liquid phase.
2.2 Selected pollutant
Pesticide, SPYKER (Cypermethrin 10 % EC) obtained from M/S chemical Estate patron, Multan. Registered by: M/S. Nuchem (PVT) ltd.
2.3 Green house experiment
Seeds were soaked in water for 6 hrs and sown in 1 KG plastic pots filled with thoroughly untreated soil (control) or CYP-spiked soil, three pots of untreated soil and three pots of contaminated soil have been prepared, and seeds were sown at the same distance in allocated pots. Plants were harvested after 75 days.
2.4 Germination index (GI)
GI was calculated as arthmethical sums of the total seeds germination every day up to a period of six days.
GI = (No. of germinated seeds) (days of first count)+…..+(No. of germinated seeds) (day of last count)
2.5 Morphological attributes
Morphological parameters were calculated using measuring scale. Shoot and root length by cm, shoot and root fresh weight and dry weight was calculated in grams. The dry mass of shoot and root was recorded after harvesting and keeping them in an oven at 80 °C for 72 h.
2.6 Electrolyte leakage
The fully develop leaves and roots were cut in 0.5 cm pieces. Leaf and root segments separately were placed in 10 ml glass tube contained sterilize water and leave for 24 hrs, next day primary leaf conductivity (EC-i) noted by EC meter and placed on rotatory shaker at 120 °C for 30 min and then cooling at room temperature and final conductivity (EC-f) noted. Electrolyte leakage calculated by given formula (Lutts et al., 1995). EL = (EC-i)/(EC-f) × 100
2.7 Chlorophyll contents determination
For chlorophyll contents fresh leaves were taken (0.5 g leaf segments) and dipped in acetone (10 ml) for overnight. Color intensity of extract was determined at 645 nm and 663 nm in a spectrophotometer () for Chl a and b respectively.
2.8 Chlorophyll fluorescence parameters
Chlorophyll fluorescence a parameters were determined with the help of photosyn Q meter V.2.0.
2.9 Soil phosphorus and micronutrients determination
Phosphorus and micronutrients were quantified by the soil di-acid wet digestion. 3 ml HNO3 was mixed to one gram air-dried soil and warmed for an hour on a hot plate at 145 °C. Thereafter, perchloric acid (HClO4) (4 ml) was added, and the temperature of the hot plate was increased to 240 °C for another hour. The flask was removed after clearing the solution and and kept at room temperature. When solution cooled, distilled water (50 ml) was added and filtered. Micronutrients were estimated by atomic absorption spectrophotometer. However, spectrophotometer was used to assess the phosphorus in through yellow color method (Jones et al., 2006).
2.10 Micronutrient determination in plant tissues
In a microwave oven, dry plant material (0.1 g) was digested in 3 ml of HNO3 using a temperature step gradient (maximum of 210 °C). Metal concentrations were measured by ICP-OES on an iCAP 6500 Series spectrometer (Thermo Fisher).
2.11 Determination of MDA and H2O2 contents
The spectrophotometric measurement of H2O2 was based on method given by Gay et al. (1999), and Gay and Gebicki (2000). The method for lipid peroxidation estimation was described by Heath and Packer (1968).
2.12 Determination of antioxidant analysis
Fresh leaves and roots (0.5 g) were soaked in phosphate buffer (0.1 M, pH7.5) individually and add EDTA (0.5 mM) in a chilled mortar and pestle. The homogenate was centrifuged for 10 min at 4 °C at 12,000 rpm after being rinsed through four layers of muslin cloth. Supernatant was used for the enzymes assay. The ability of superoxide dismutase (SOD; EC1.15.1.1) to suppress the photochemical reduction of NBT (Beauchamp and Fridovich, 1971) and absorbance was measured at 560 nm. Ascorbic per oxidase was assayed following a reduction in absorbance at 290 nm for 3 min, with the activity expressed as EU mg−1 protein. POD and APX enzyme activities measured by previously described methods by Chance and Maehly (1955) and Nakano and Asada (1981) respectively. Catalase activity was measured using the method of Aebi (1984), with the change in absorbance recorded at 240 nm for 3 min. CAT activity was expressed as unit mg−1 protein.
2.13 Preparation of slides
Cutting the stem transversally with a sliding microtome was used to produce the longitudinal sections. The sample was then held in 100 % alcohol for 24 h and stained with 1 % safranin in 70 % alcohol, followed by 3 min each in70%, 80 %, 95 %, 100 % I, and 100 % II alcohol solutions, and finally soaking the sample in a 3:1,1:1, and 1:3 combination of alcohol:xylol. The slicing stem was therefore smeared with Canadian balsam and covered with a cover glass before becoming placed in the microscope slide. The final samples were studied and measured using a microscope and capture photograph with an ocular camera attached.
For the production of root transverse sections, the root cutting was coated in paraffin (embedding). The primary root was cut 1 cm from the tip and immersed in FAA solution, a 5:5:10 mixture of formalin, glacial acetic acid, and alcohol, for 24 hrs. The root pieces were stained by soaking them in 1 % safranin in 70 % alcohol for 24hrs, subsequently removing the fixative solution and mixing it with70%, 80 %, 95 %, 100 % I, and 100 % II alcohol for 30 min respectively. The specimens were dealtcoholized by dipping them in a3:1,1:1, and 1:3 mixture of alcohol and xylol, with the last 30 mins in xylol. At 57 °C, the sample was soaked in a 1:9 xylol:paraffin mixture overnight. The xylol-paraffin solution was removed and replaced with pure paraffin at the same temperature and time interval. The old paraffin was replaced by new pure paraffin. After 1 h, the paraffin-containing specimens were sliced into blocks. The block containing the sample is sliced to a diameter of 4–6 u m using a rotary microtome. The slice was adhered to the microscope slide using a glycerin:albumin spiked with water mixture, after which heated to 45 °C to stretch the paraffin band. After cooling, the specimen fragments were smeared with Canadian balsam and covered with a glass cover. A microscope with an ocular camera was used to test the final specimen.
2.14 Statistical analysis
Data computation was made on Origin 2020b. Difference among treatment means was analyzed by using the Fisher’s least square difference (LSD) design at 0.5 % probability level (Sharples et al., 1997). Pearson correlation and PCA was performed by Origin 2020b software.
3 Results
3.1 Germination rate
Change in the germination rate of B. Juncea and H. annuus in cypermethrin contaminated soil was mentioned in Table-1. Germination was greater in B. Juncea when opposed to H. annuus. In both plant species i.e., H. annuus and B. Juncea has shown a substantial decrease in their germination rate at treatment rates (20 and 40 mg/kg). Over time, germination recovery was observed; however, germination at higher pesticide concentrations was still relatively poor compared to control. 20 mg/kg and 40 mg/kg levels reduced 8.9 % and 24.7 % germination, respectively in H. annuus relative to control. However, in B. juncea 9.7 % germination reduced at 20 mg/kg level and 33.25 % at 40 mg/kg level relative to control.
3.2 Shoot length, fresh and dry weight
Table.1 shows the growth response (shoot fresh weight, shoot dry weight and shoot length) of H. annuus and B. juncea seedlings to Cypermethrin stress (20 and 40 mg/Kg). Cyp stress 20 mg /kg reduced the 31.6 % fresh weight in H. annuus relative to the B. juncea (48.3 %) whereas, 40 mg/kg reduced fresh shoot weight by 22.9 % and 52.78 %, in H. annuus and B. juncea respectively, relative to the untreated control. Shoot dry weight increased by 17.33 % and 68.8 %, in H. annuus and B. juncea respectively, in the 20 mg/Kg treatment, relative to the untreated control. However, the 40 mg/kg treatment decreased shoot dry weight by 26.87 % and 11.7 %, respectively, relative to control. In 20 mg/kg Cyp-stressed seedlings, shoot length decreased by 12.4 % and increased 10 %, respectively, in H. annuus and B. juncea, with 40 mg/kg Cyp increased by 15.6 % and decreased 25 %, respectively, in H. annuus and B. juncea relative to untreated seedlings (Table.1). Notes: Similar letters in same column are statistically non-significant according to Duncan’s Multiple Range Test (p < 0.05), Data are means (n = 3 ± SD).
Plant species
Cypermethrin applied
Germination
Root length
Shoot length
Root fresh weight
Shoot fresh weight
Root dry weight
Shoot dry weight
mg Kg−1
%
cm
g plant−1
H.annus
0
60.5 ± 8.82a
16.16 ± 0.70a
123.3 ± 3.23c
14.26 ± 0.18a
52.0 ± 5.33a
8.92 ± 0.12a
17.31 ± 1.45a
20
55.1 ± 8.05b
16.33 ± 2.32a
138.6 ± 1.70ab
12.32 ± 1.04b
35.55 ± 6.16b
7.85 ± 0.66b
14.31 ± 1.53b
40
45.5 ± 3.25c
16.5 ± 0.46a
142.6 ± 1.45a
9.55 ± 0.18c
26.85 ± 1.17c
6.77 ± 0.24b
12.66 ± 1.20bc
B. juncea
0
100 ± 5.85a
15.16 ± 0.70b
120 ± 1.71c
12.27 ± 0.33a
21.12 ± 6.56bc
8.32 ± 0.12a
10.91 ± 1.42b
20
90.23 ± 5.00ab
14.5 ± 1.66b
132 ± 1.70b
10.24 ± 1.95b
41.32 ± 1.01a
7.31 ± 0.42b
18.34 ± 1.21a
40
66.75 ± 10.82b
16.83 ± 1.86a
150.22 ± 2.23a
8.66 ± 2.19c
25.88 ± 3.59b
6.24 ± 0.91b
9.66 ± 1.14b
3.3 Root length, fresh and dry weight
Root fresh weight decreased by 13.8 % and 16.5 %, respectively, in H. annuus and B. juncea in the 20 mg/kg, CYP- treatment, relative to the untreated seedlings. However, the 40 mg/kg treatment significantly decreased root fresh weight of H. annuus and B. juncea by 33 % and 29.4 %, respectively, relative to untreated seedlings, and was more destructive for H. annuus than the B. juncea (Table.1).
Cyp stress 20 mg/kg reduced the 11.9 % dry weight in H. annuus relative to the B. juncea (12.1 %). whereas, 40 mg/kg reduced dry weight by 24.1 % and 25.78 %, in H. annuus and B. juncea respectively, relative to the untreated control.
H. annuus showed Non-significant root lengths at both CYP levels and untreated seedlings. However 4.35 % root increase in B. juncea at 20 mg/kg level and 11 % root decrease in B. juncea at 40 mg/kg level in comparison with control.
3.4 Response of chlorophyll pigments
Total chl contents declined by 33.09 % and 51.51 %, respectively, in 20 mg/kg and 40 mg/kg CYP-treated H. annuus plants, relative to the control treatment. In B. juncea seedlings, total chl decreased by 19.35 % and 18.75 %, respectively, in 20 and 40 mg/kg treatments relative to untreated seedlings (Table.2). Notes: * represent values are statistically significant according to permissible limit, Data are means (n = 3 ± SD).
Plant species
Cypermethrin
Applied
(mg Kg−1)Phi2
PhiNPQ
NPQt
PhiNO
qL
Fv//Fm/
qP
LEF
H.annus
0
0.47 ± 0.03
0.16 ± 0.05
0.46 ± 0.18
0.36 ± 0.03
0.26 ± 0.02
0.76 ± 0.02
0.61 ± 0.04
0.67 ± 0.04
20
0.50 ± 0.01
0.16 ± 0.03
0.50 ± 0.12
0.33 ± 0.02
0.31 ± 0.01
0.76 ± 0.01
0.65 ± 0.00
0.76 ± 0.14
40
0.49 ± 0.02
0.15 ± 0.03
0.42 ± 0.09
0.35 ± 0.01
0.28 ± 0.01
0.77 ± 0.01
0.64 ± 0.33
0.75 ± 0.12
B. juncea
0
0.53 ± 0.01
0.1 ± 0.01
0.27 ± 0.03
0.36 ± 0.01
0.29 ± 0.01
0.79 ± 0.00
0.67 ± 0.01
0.78 ± 0.04
20
0.57 ± 0.00
0.13 ± 0.01
0.45 ± 0.08
0.29 ± 0.01
0.4 ± 0.01
0.77 ± 0.00
0.77 ± 0.00
0.9 ± 0.14
40
0.55 ± 0.00
0.10 ± 0.00
0.32 ± 0.03
0.33 ± 0.01
0.34 ± 0.01
0.78 ± 0.00
0.708 ± 0.01
0.83 ± 0.2
Fo
Fm
Fs
Total chlorophyll
PAR
F
1-qp
ETR
H.annus
0
3347 ± 285.63
14567.59 ± 723.47
7637.9 ± 321.87
37.48 ± 2.00
3.12 ± 0.00
1.24 ± 0.04
0.27 ± 0.03
0.63 ± 0.00
20
3118 ± 132.80
13265.21 ± 293.37
6584.4 ± 116.97
39.21 ± 3.48
3.40 ± 0.00
0.65 ± 0.08
0.26 ± 0.00
0.71 ± 0.00
40
2985.25 ± 130.9
13216.85 ± 511.43
6640.6 ± 379.21
34.57 ± 6.40
3.40 ± 0.00
0.64 ± 0.08
0.29
0.71 ± 0.00
B. juncea
0
2820.5 ± 204.86
13608.32 ± 774.31
6347.42 ± 506.71
44.41 ± 0.01
3.26 ± 0.02
0.67 ± 0.09
0.29 ± 0.02
0.73 ± 0.00
20
2986.8 ± 187.86
13004.56 ± 549.61
5550.22 ± 299.95
30.29 ± 0.02
3.5 ± 0.09
0.77 ± 0.02
0.25 ± 0.06
0.84 ± 0.00
40
3002.25 ± 19.64
14106.36 ± 257.81
6241.65 ± 181.90
43.98 ± 0.01
3.33 ± 0.04
0.708 ± 0.00
0.32 ± 0.03
0.78 ± 0.08
3.5 Photosynthetic efficiency
The effects of CYP, 20 and 40 mg/kg treatments on chlorophyll fluorescence parameters are shown in Table.2. The CYP-20 mg/kg treatment reduced Fv/Fm (by 6.60 %) in H. annuus and reduced 2.5 % in B. juncea, but increased ΦPSII (by 6.11 % and 7.30 %), and qP (by 22.58 % and 14.1 %) in H. annuus and B. juncea respectively, and also increased NPQ by 8.38 % and 66.0 % in H. annuus and B. Juncea respectively, relative to untreated control seedlings. Application of 40 mg/kg increased Fv/Fm, decreased ΦPSII, qP and NPQ in 40 mg/kg CYP-treated seedlings compared with un treated seedlings (Table.2).
3.6 Electrical leakage, MDA and H2O2 in leaves
Cypermethrin stresses (20 mg/kg and 40 mg/kg) not increased H2O2 production in shoot tissues of H. annuus relative to the control seedlings. The 20 mg/kg, and 40 mg/kg treatments increased H2O2 in B. juncea by 10.1 %, and 64.24 %, respectively, relative to control seedlings (Fig. 1). Cypermethrin stress (20 mg/kg) not significantly increased H2O2 content in roots of H. annuus and B. juncea by 1.39 %, and 2.84 %. The treatment 40 mg/kg increased 19.79 %, and 50.27 % H2O2 content in root tissues of H. annuus and B. juncea, respectively, relative to control seedlings (Fig. 1B).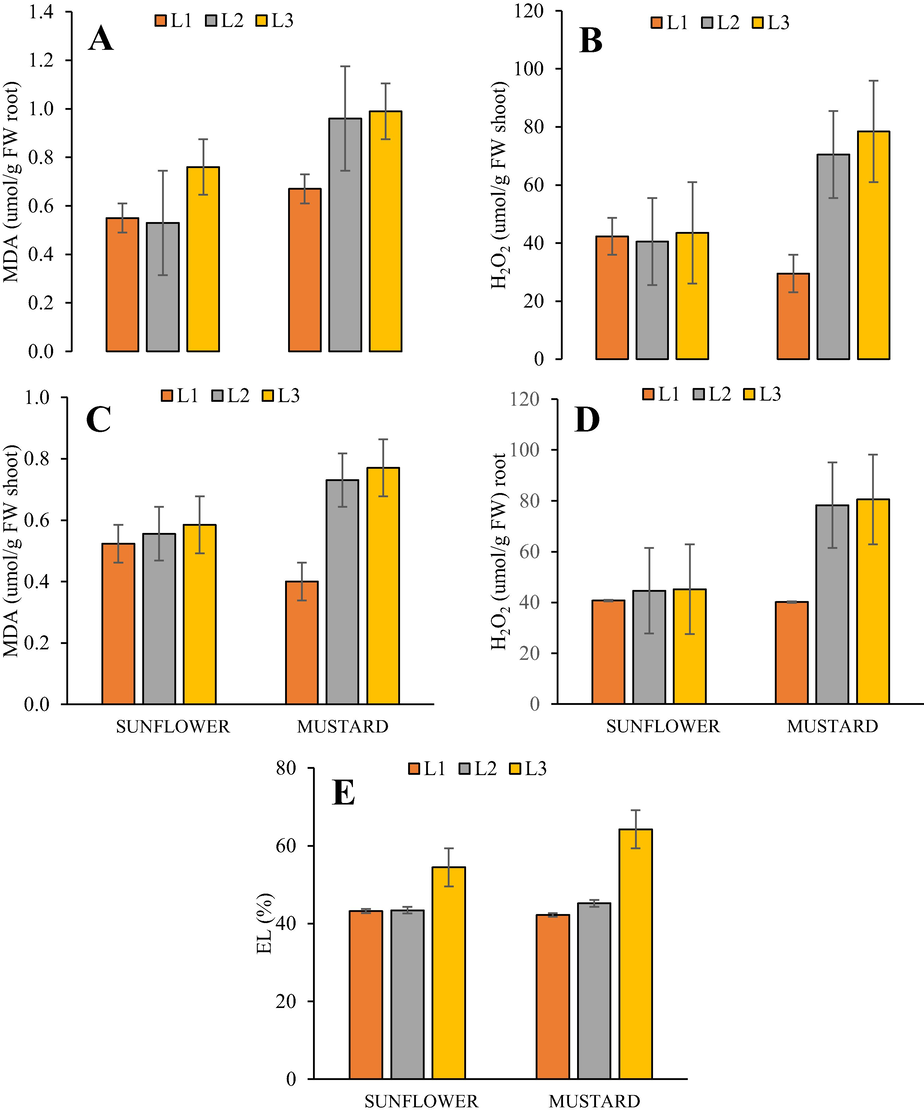
(A-E). Effect of cypermethrin on H. annuus and B. juncea under Cypermethrin stress (L1) control, (L2) 20 mg/kg and (L3) 40 mg/kg.
Cypermethrin stress 20 mg/kg increased lipid peroxidation (estimated from MDA content) by 6.3 % and 5.47 % in H. annuus and B. juncea shoots respectively, relative to the control seedlings. The 40 mg/kg treatment increased MDA content by 11.8 %, and 45.2 % in H. annuus and B. juncea, respectively, relative to control shoots (Fig. 1C). Furthermore, 20 mg/kg CYP stress increased MDA content in roots of H. annuus and B. juncea by 30.2 %, and 30.4 %. The treatment 40 mg/kg increased 35.21 %, and 36.33 % MDA content in root tissues of H. annuus and B. juncea, respectively, relative to control seedlings (Fig. 1D). Cypermethrin stress (20 mg/kg) increased electrolyte leakage by 19.9 and 35.4 %, in H. annuus and B. juncea, relative to the control. The 40 mg/kg treatment increased electrolyte leakage by 19.61 %, and 45.4 %, respectively, relative to control shoot part (Fig. 1E).
3.7 Antioxidant enzyme activities
3.7.1 SOD enzyme activity in shoot and root tissues
The CYP-20 mg/kg treatment decreased SOD activity by 3.48 % and 7.47 % in H. annuus and B. juncea, respectively, relative to their control treatment. The CYP-40 mg/kg treatment slightly increased SOD activity in H. annuus (1.46 %), while in B. juncea SOD activity significantly decreased by 55.25 % relative to control seedlings (Fig. 2a). In case of roots the CYP-20 mg/ kg treatment not showed any effect on the activity of SOD in both H. annuus and B. juncea respectively, relative to their control treatment. The CYP-40 mg/kg treatment slightly decreased SOD activity in H. annuus (1.77 %), while in B. juncea SOD activity significantly decreased by 38.8 % relative to control seedlings (Fig. 2b).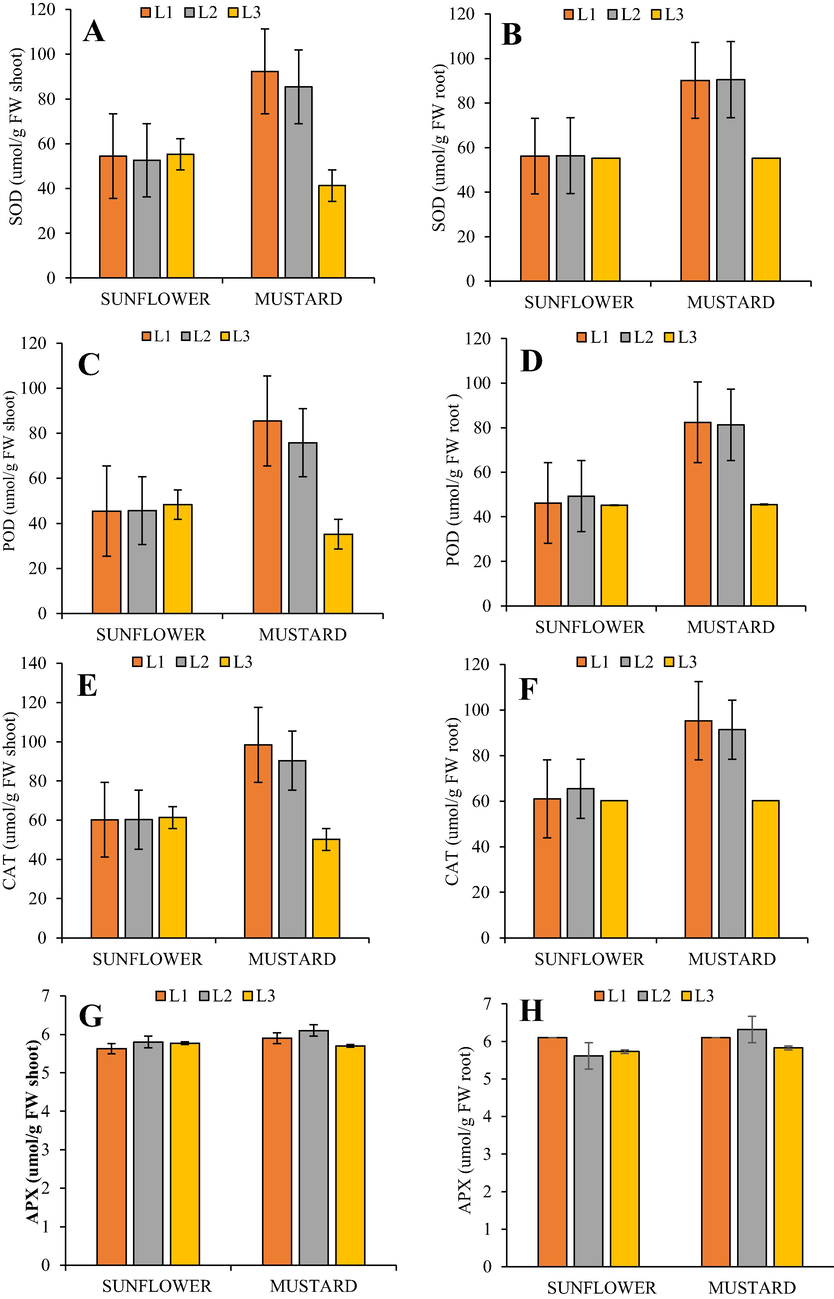
(A-H). Effect of cypermethrin on (Left) SOD shoot, Shoot POD, Shoot CAT, Shoot APX, and (Right) SOD root, root POD, root CAT, root APX, in H. annuus and B. juncea under Cypermethrin stress (L1) control, (L2) 20 mg/kg and (L3) 40 mg/kg. Different letters indicate significant difference at P ≤ 0.05.
3.7.2 POD enzyme activity in shoot and root tissues
The CYP-20 mg/kg treatment slightly increased POD activity by 0.44 % in H. annuus and decreased by 11.24 % in B. juncea relative to their control treatment. The CYP-40 mg/kg treatment slightly increased POD activity in H. annuus (6.15 %), while in B. juncea POD activity significantly decreased by 58.78 % relative to control seedlings (Fig. 2c). In case of roots the CYP-20 mg/kg treatment not showed any effect on the activity of POD in B. juncea while in H. annuus increased by 6.70 % relative to their control treatment. The CYP-40 mg/kg treatment slightly decreased POD activity in H. annuus (2.16 %), while in B. juncea POD activity significantly decreased by 44.78 % relative to control seedlings (Fig. 2d).
3.7.3 CAT enzyme activity in shoot and root tissues
The CYP-20 mg/kg treatment slightly increased CAT activity by 0.166 % in H. annuus and decreased by 8.13 % in B. juncea relative to their control treatment. The CYP-40 mg/kg treatment slightly decreased CAT activity in H. annuus (1.99 %), while in B. juncea CAT activity significantly decreased by 48.98 % relative to control seedlings (Fig. 2e). In case of roots the CYP-20 mg/kg treatment increased the activity of CAT by 7.21 % in H. annuus and decreased by 4.09 % in B. juncea relative to control treatment. The CYP-40 mg/kg treatment slightly decreased CAT activity in H. annuus (1.31 %), while in B. juncea CAT activity significantly decreased by 36.83 % relative to their control seedlings (Fig. 2f).
3.7.4 APX enzyme activity in shoot and root tissues
The CYP-20 mg/kg treatment increased APX activity by 3.01 % and 3.38 % in H. annuus and B. juncea, respectively, relative to their control treatment. The CYP-40 mg/kg treatment slightly decreased APX activity in H. annuus (2.46 %), and B. juncea (3.38 %) relative to control seedlings.
In case of roots the similar trend of Shoot APX activity was observed (Fig. 2 g & h).The CYP-20 mg/kg treatment increased APX activity by 3.01 % and 3.38 % in H. annuus and B. juncea, respectively, relative to their control treatment. The CYP-40 mg/kg treatment slightly decreased APX activity in H. annuus (2.46 %), and B. juncea (3.38 %) relative to control seedlings.
3.8 Mineral uptake
The CYP-20 mg/kg treatment impaired mineral uptake in the shoots and roots (Table.3). In the H. annuus shoots, reduced Zn, I, P, Mn, and Cu contents by 34.69 %, 40.33 %, 43.47 %, 48.62 %, 10.55 % and 32.45 %, respectively, relative to the control. However, in the shoot and root of B. juncea only Zn content 12.33 % increased and uptake of other micronutrients are reduced relative to the control. Notes: Similar letters in same column are statistically non-significant according to Duncan’s Multiple Range Test (p < 0.05), Data are means (n = 3 ± SD).
Plant Species
Cypermethrin
Applied (mg Kg−1)Zinc (μg g−1 DW)
Iron (μg g−1 DW)
Phosphorus (μg g−1 DW)
Manganese (μg g−1 DW)
Copper (μg g−1 DW)
Shoot
Root
Shoot
Root
Shoot
Root
Shoot
Root
Shoot
Root
H. annus
0
39 ± 10.6a
33 ± 0.0b
190 ± 42.3a
200 ± 160.0b
17 ± 10.2a
19 ± 3.3a
17 ± 0.0a
19 ± 0.0a
16 ± 11.2b
10 ± 16.0b
20
36 ± 12.9b
28 ± 5.5c
186 ± 42.3b
205 ± 160.8b
16 ± 10.6a
16 ± 1.6b
16 ± 0.0a
16 ± 0.0a
19 ± 2.0a
12 ± 6.6ab
40
38 ± 12.1a
40 ± 5.5a
178 ± 10.2c
240 ± 18.4a
13 ± 2.0b
14 ± 1.6bc
13 ± ±0.1b
14 ± 0.0b
10 ± 9.2c
15 ± 13.8a
B. juncea
0
39 ± 3.3b
41 ± 32.0c
232 ± 3.6a
152 ± 2.4a
18 ± 10.6a
18 ± 3.2b
18 ± 0.0a
18 ± 0.1b
10 ± 8.0b
9 ± 2.4b
20
41 ± 0.9ab
43 ± 0.9b
215 ± 12.2b
145 ± 12.2b
16 ± 3.2b
21 ± 8.3a
14 ± 0.0ab
21 ± 0.0a
9 ± 2.4b
15 ± 4.6a
40
44 ± 20.3a
47 ± 28.1a
200 ± 32.0c
139 ± 10.2c
14 ± 3.2c
15 ± 12.0b
16 ± 0.0ab
15 ± 0.0b
17 ± 3.3a
14 ± 4.8a
In case of H. annuus shoots, the Cyp- 40 mg/kg alone treatment reduced Fe, P, Mn and Cu contents by 14.36 %, 22.85 %, 22.33 %, and 18.68 % respectively, relative to the control. Whereas enhanced the uptake of these micronutrients (Zn, Fe, and Cu) into roots by 25.33 %, 54.87 %, and 22.33 % while P and Mn uptake was decreased by 15.66 % and 20.74 %, respectively relative to the control (Table.3).
In B. juncea the Cyp- 40 mg/kg treatment reduced the uptake of micronutrients (Fe, P, and Mn) in the shoots (Table 3). In the shoots, these micronutrients are reduced uptake by 15.00 %, 18.48 %, and 7.05 %, respectively, relative to the control. Supplementation with Cyp-40 mg/kg treatment enhanced the uptake of Zn and Cu micronutrients into shoots by 32.65 % and 5.33 % respectively. In the roots, Cyp- 40 mg/kg treatment reduced Fe, P, Mn and Cu by 13.64 %, 11.39 %, 14.52 %, and 17.32 %, respectively, relative to the control. While with increase of 33.54 % in Zn, relative to the control (Table.3).
3.9 Correlation between micronutrient and plant growth parameters of H. annuus
The strongest positive correlation was between traits relating directly to the plant growth traits (i.e comprising fresh shoot weight, germination%, fresh and dry root weight, P < 0.001) and also between these traits and shoot phosphorus (P < 0.01, Fig. 3a). There were many strong positive correlations between the shoot copper concentration with root and shoot length (P < 0.001). Week negative relationships were between root Cu and soil P (r = −0.66), root Cu and root fresh weight (r = −0.57), root Mn and shoot dry weight and root length (r = −0.75), shoot Mn and shoot fresh weight (r = −0.71). there were few negative but strong relationships between the shoot Zn content and germination, fresh and dry shoot weight (r = −0.92, P < 0.01). There were negative relationships between root Zn and germination and with dry shoot weight (r = −0.95, P < 0.01) (Fig. 3a). The PCA resulted in three principal component groups having on eigenvalue of more than one thus contributing 94 % variability (Fig. 3b). The PC1 and PC2 groups contributed 71.7 %, and 22.9 % variability, respectively. Different parameters contributed both positively and negatively to different PC groups. In the PC1 group, soil Cu, EL, Shoot and root fresh and dry weight, while Root P, root and shoot Mn, Shoot Fe, germination, shoot P and chlorophyll recorded the positive correlation and highest variability. By contrast, root Zn, soil Fe, root Fe, root length, shoot length, Root Cu and soil P contributed negatively to the PC group. Maximum variability was observed for soil Mn, soil pH and shoot Zn and Cu in the PC2 group whereas the Ec of soil recorded higher variability then the other traits in PC2 group. Thus, the PC1 group showed the highest variability (73 %) for 20 mg/kg-Cyp tolerance contributing traits than the other groups. A smaller angle between different parameters in the same direction indicated a high association between corresponding parameters for classifying treatments (Fig. 3b).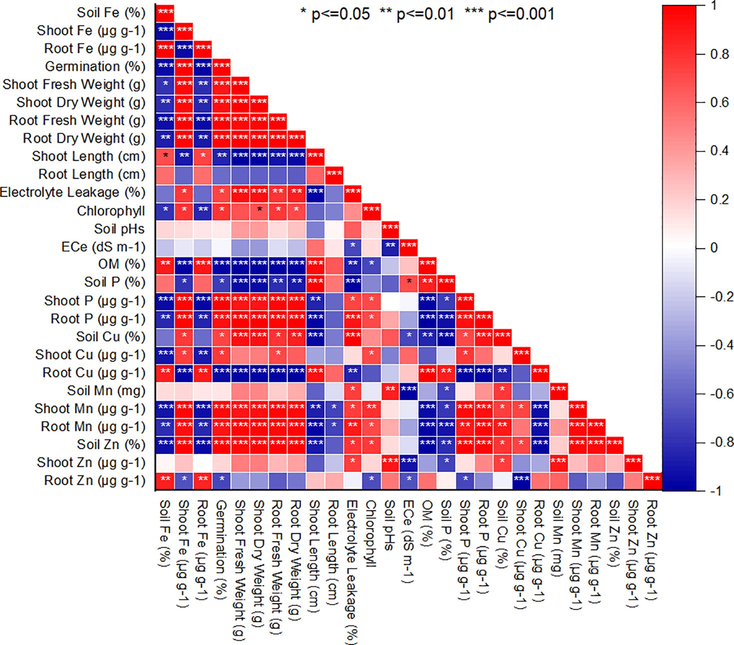
Pearson correlation for H. annuus growth, Chlorophyll and nutrients concentration affected by CYP levels.
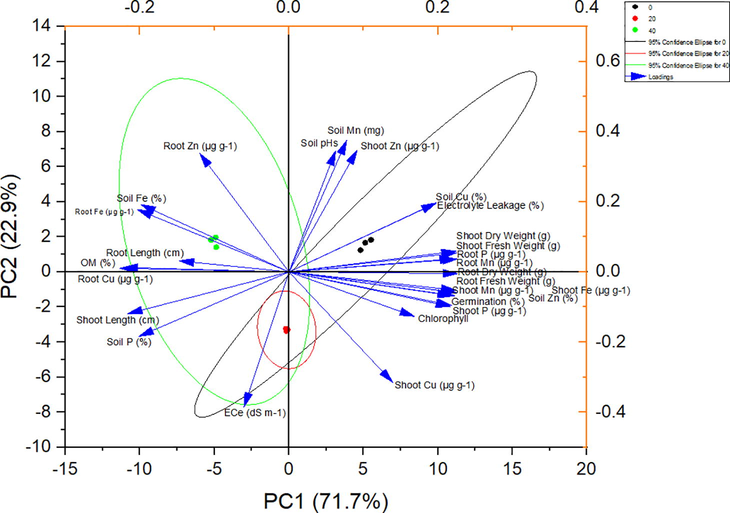
PCA for H. annuus growth, physiological attributes and nutrients concentration affected by CYP levels.
3.10 Correlation between micronutrient and plant growth parameters of B. juncea
Strongly positive correlations were presence between soil shoot and root Fe content and germination %, and with root fresh and dry wt (P < 0.001). There were positive correlations between shoot Fe and shoot fresh wt (r = 0.75, P < 0.001), and shoot dry wt (r = 0.70, P < 0.001). There was week but positive relationships presence between soil P with shoot and root fresh and dry weight, and with shoot length (P < 0.001). There were negative relationships between the root and shoot P with all plant growth parameters (P < 0.001). Shoot Mn strongly but negatively relationships with germination%, shoot and root fresh and dry wt, and also with shoot and root length (r = −0.65, P < 0.001). Root Mn and soil Zn were negative relationships with all plant growth traits by r = −0.70 and r = −0.80, respectively (Fig. 4a).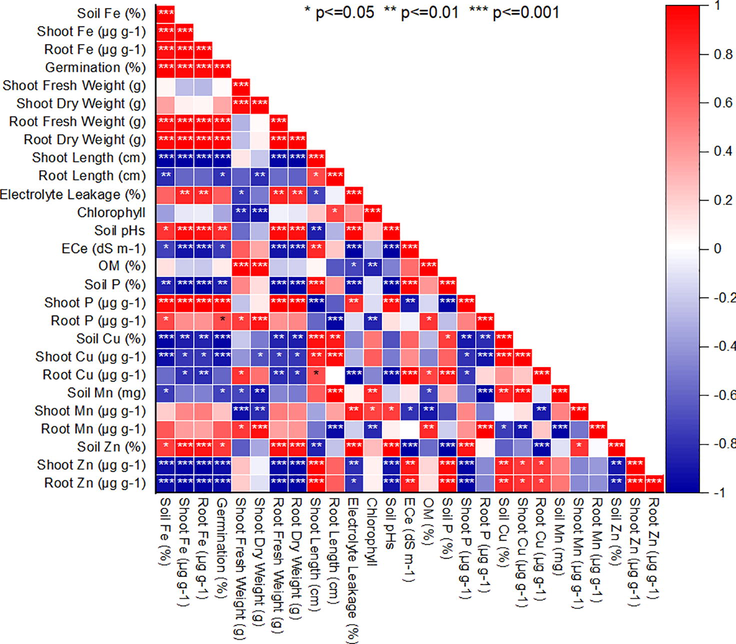
Pearson correlation for mustard growth, chl. flourescence and nutrients concentration affected by cypermethrin levels.
The PCA resulted in PCA groups having on eginvalue of more than one, thus contributing 97.9 % variability (Fig. 4b). The PC1 and PC2 contributing 63.7 %, and 34.6 % variability, respectively. Different parameters contributed both positively and negatively to different PC groups. In the PC1 group near to 90° are shoot dry wt, root Mn and root P in control group of B. juncea plants. Whereas, <90° soil Fe, germination %, shoot Fe, and P, root fresh and dry wt, root Fe recorded the positive correlation and showed a smaller angle among them in the same direction indicated a high association between corresponding parameters for treatment 2 (20 mg/kg Cyp). by contrast, under the treatment three (40 mg/kg Cyp) root Cu, ECe, Soil P, shoot and root Zn, and shoot length contributing negatively to the PC1 group. Maximum variability and negative correlation was observed under same treatment in PC2, between soil Cu, shoot Cu, root length, soil Mn and chlorophyll. Thus the control treatment and 20 mg/kg Cyp showed positive correlation whereas, treatment 3 (40 mg/kg Cyp) clearly indicated negatively correlated parameters in PC2 group (Fig. 4a).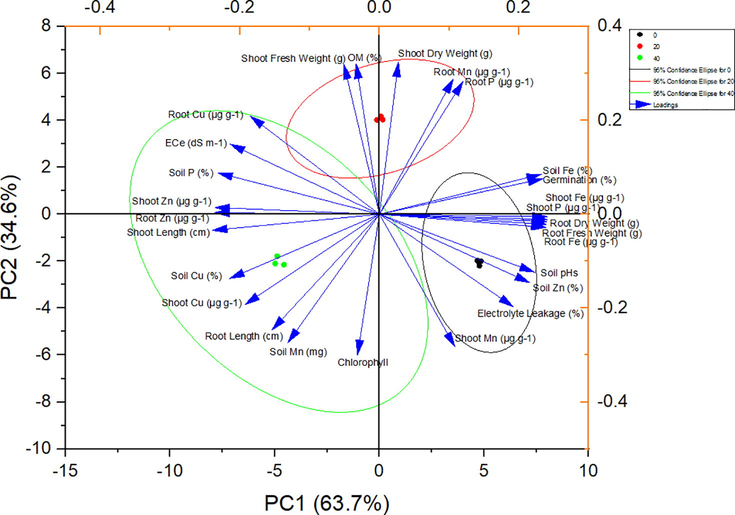
PCA Pearson correlation for mustard growth, chl. flourescence and nutrients concentration affected by cypermethrin levels.
3.11 Stem Anatomy
Results showed that 40 mg/kg CYP increased the epidermal thickness in B. juncea relative to H. annuus. Whereas reduced epidermal area of the stem was observed relative to in both tested plants. Cortex thickness was observed in B. juncea at higher concentration of cypermethrin compared to H. annuus. Xylem thickness increased in H. annuus at 20 mg/kg level while no change occurred in xylem thickness of B. juncea. Moreover, Phloem thickness raises in both plant species. Decreased metaxylem cell area was observed in both species (Fig. 5). While increased metaxylem area was observed at higher concentration. Increased pith area, and phloem thickness were recorded in H. annuus relative to B. juncea at lower concentration of cypermethrin compared to higher concentration which reduced the pith area and phloem thickness.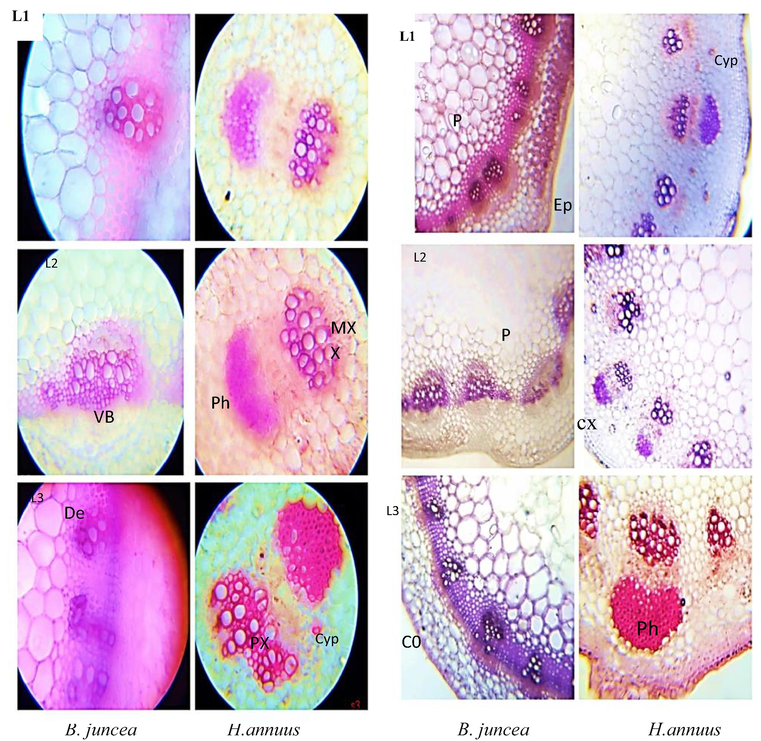
T.S 0f shoot of B. juncea and H. annuus. Different levels of cypermethrin are L1 = control, L2 = 20 mg/kg and L3 = 40 mg/kg. (Ep: epidermis; cx:vascular cortex and Co: collenchyma cells; Ph: phloem cell; MX: metaxylem; Vb: vascular bundle; Cyp: pesticide crystal; De: pestiside deposition; P: pith).
3.12 Leaf Anatomy
Cypermethrin (20 mg /kg) increased the collenchyma cell area in both H. annuus and B. juncea leaf tissues (Fig. 6). While higher dose of cypermethrin reduced this trait of leaf. Epidermis thickness increase with increased levels of cypermethrin. Spongy mesophyll thickness and palisade mesophyll thickness was observed at 40 mg/kg CYP-treated plant leaves. Metaxylem cell area decreased at higher concentration of cypermethrin while at lower concentration increased metaxylem area was observed only in B. juncea (Fig. 6). In B. juncea leaves increased midrib area was observed at higher concentration compared to H. annuus.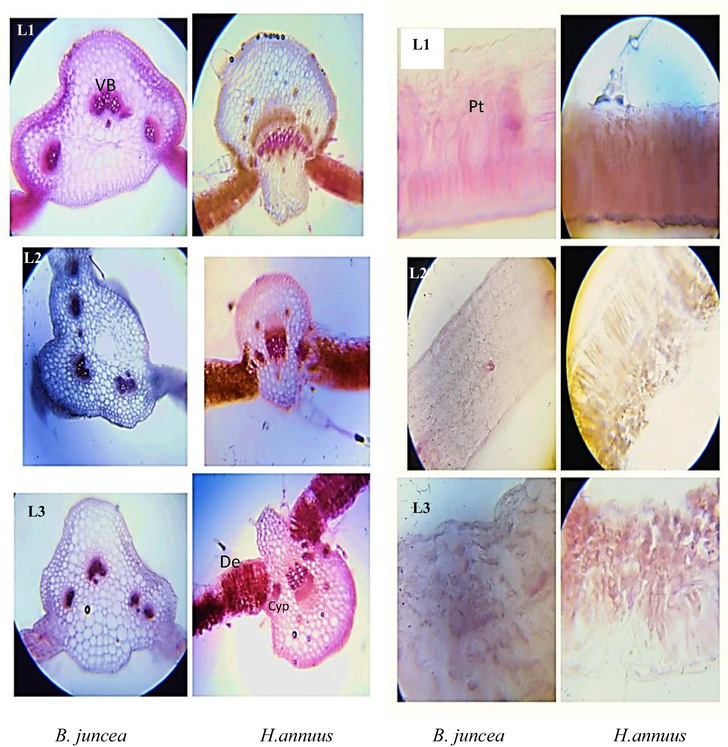
T.S 0f shoot of B. juncea and H. annuus. Different levels of cypermethrin are L1 = control, L2 = 20 mg/kg and L3 = 40 mg/kg. (Vb: vascular bundle; De: deposition of Cyp in palisade tissue; Pt: palisade tissue; Cyp: pesticide crystal).
3.13 Root Anatomy
The anatomical parameters measured at the roots of H. annuus and B. juncea are seen in Fig. 4. The root diameter of the 20 mg/kg treatment was slightly larger than that of the other treatments, although the root diameter of the 40 mg treatment was considerably smaller. Cypermethrin, which causes radial swelling in the roots. The 40 mg/kg treatment plant's limited root diameter could be unable to withstand the maximum concentration of Cyp, where cell growth has been stunted from the start due to Cypermethrin disruption to the cortex cells. The epidermis is the root's outermost layer, which is made up of one layer of closely packed cells. In comparison to other treatments, the root epidermis of H. annuus and B. juncea plants treated with 40 mg/kg Cyp was damaged. The epidermal layer of un-treated and 20 mg/kg Cyp treated plants is rectangular in form with intact and neat transparent cell walls, whereas the epidermis of 40 mg/kg Cyp B. juncea plants has thin layers around it that may be caused by cell wall tearing (Fig. 7). The outline of the epidermis cells became irregular and cell walls were broken in several places in H. annuus (40 mg/kg Cyp) plants, while the shape of the epidermis cells became irregular and cell walls were torn in several places in H. annuus (40 mg/kg Cyp) plants. H. annuus (20 mg/kg Cyp) epidermis cell area improved compared to un-treated and other treatments, while B. juncea epidermis cell area was non-significant. (Fig. 7).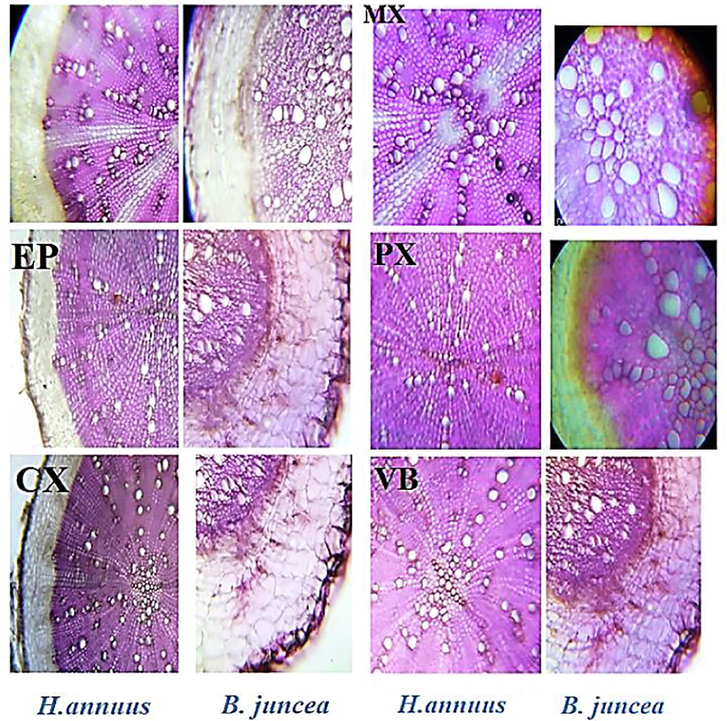
T.S of root of B. juncea and B = H. annuus. Different levels of cypermethrin are L1 = control, L2 = 20 mg/kg and L3 = 40 mg/kg. (Ep: epidermis; cx: cortex and Co: collenchyma cells; Ph: phloem cell; MX: metaxylem; Vb: vascular bundle).
The cortex, which is made up of parenchymal cells, lies under the epidermis membrane. The cortex is made up of several layers of cells. The cortex is made up of several layers of cells. Fig. 4 shows the anatomical findings. It is understood that in the H. annuus, both treatments have the densest cortical layer, whereas in the B. juncea, both treatments have the narrowest cortex. The cortex thickness difference between treatments is not significant, but the appearance of the cortex looked different. Cortex cell area is increased in both treated H. annuus plants relative to control. Moreover, in B. juncea decreased cortical cell area noted in all treatments. Insight the cortex vascular bundle area is present. The size of vascular bundle is varies among treatments in both tested plants (Fig.). The area of vascular bundle is decreasing when increasing the Cypermethrin dose. As comparison to H. annuus, the main vascular bundle in B. juncea is smaller in diameter and has less metaxylem components. (Fig. 7).
4 Discussion
Pesticides in this study induced germination of seeds in H. annuus and B. Juncea but it was blocked by higher doses. Numerous researchers have looked at the negative impact of pesticides on seed germination. Sammaiah et al. (2011) revealed that germination of solanum melogena seeds were inhabited, with elevated levels of pesticides i.e endosulfans and kitazin. Pendamethalin, reduced seed germination when increased concentration in Zea mays L. and also reported that seed germination was highest in the control set (95 %). Dubey and Fulekar (2011) studied the effect of three insecticides on Cenchrus setigerus, Vahl, pennisetumn and Pedicellatum Tan seedling germination based on spiked soil. In comparison to Cypermethrin and Fenvalerate, Chlorpyrifos showed a substantial decrease in seed germination and a delay in seed germination at higher doses. In the experiment of Wibawa et al. (2009), which applied Paraguat, glyphosate and glufosinate ammonium to Cucumis sativus L. and Zea mays L. at seed sowing stage and germination rate of the plants was determined to be more above 90 % normal (relative to untreated plots).
In the present research, the pesticide (cypermethrin) was tested, cypermethrin having the most toxic effect on sunflower and mustard growth parameters (root and stem length, fresh and dry weight). When subjected by elevated CYP, a decrease in significance had been reported in dry and fresh mass of root and shoot. Previously, in several species of plants, such as soya, maize, tomatoes and chickpea (Siddiqui and Ahmad, 1996'; Chahid et al., 2013and Tiyagi et al., 2004) growth has been affected by higher pesticide concentrations. Mishra et al. (2009) demonstrated that high concentrations of dimethoate insecticides decreased growth parameters in the term of stem and root length. Closely related findings have also been provided on Glycine max by Murthy et al. (2005). Nemat Alla et al., 2007, reported that rimsulfuron, imazethapyr, alachor, atrazine and fluometuron decrease the fresh and dry mass of maize seedlings.
Chlorotoluron blocked the higher plant photosynthetic electron transport (Fuerst and Norman, 1991) and disrupted PSII reaction centre (Barry et al., 1990). Propanil, a highly selective post-emergence herbicide, is utilized in a wide range of crops to suppress grass weeds. It is a photosynthetic blocker that disrupts photosystem II in chloroplasts and relates to the anilide family (Devine et al., 1993) The fungicide captan reduced chlorophyll a, b, total chlorophyll, and carotenoid content in pepper leaves, although the prescribed amount resulted in increased in chlorophyll a and carotenoid content when compared to higher concentrations and the control (Tort and Turkyilmaz, 2003). Electron transport is inhibited when atrazine binds to PSII's D1 protein (Zheleva et al., 1994). The effects of paraquat and norflurazon on Chl fluorescence parameters in Lemna minor were examined by Frankart et al. (2003), who discovered substantial decreases in Fv/Fm, qP, and PSII, but an increase in NPQ. Our findings revealed that as the exposure dosage rise, qP, NPQ, and PSII declined considerably in B. juncea followed by H. annuus, whereas at the lower dose of cypermethrin (20 mg/kg−1) the photosynthetic capability of both species remained strong, allowing crops to develop normally.
Iron, zinc, copper and manganese are considered as “borderline” ions and also compete to each other for the binding sites. These ions supposed to that displacement due to Cu (Casida, 1980). According to Bowen (1969), Cu, Zn, and Mn uptake is linked to oxidative phosphorylation, and Zn and Cu are transported through same channels. The findings of this investigation revealed that tissue concentrations of all these three ions in H. annuus were higher than in B. juncea, indicating that a rise in CYP concentration in the medium lowered tissue concentrations of these three ions in H. annuus. (Table.3).
Our data revealed a significant increase in the generation of H2O2 and MDA in 40 mg/kg CYP- stressed B. juncea followed by H. annuus (Fig. 1). This could be because pollutant-induced free radical generation alters membrane structure, increasing permeability (Ahmad et al, 2016). Oxidative bursts triggered by abiotic stress conditions due to disruption of the antioxidant protection mechanism may be one of the reasons for the development of ROS (Khooharo et al., 2008a; Khooharo et al., 2008b). Similar findings were alignment with Cui et al., 2010, and Li et al., 2012, they revealed that MDA content increased in root tissues of Brasica napus and Zea mays after the exposure of Napropamide and Atrazine respectively. However, increased H2O2 content was noted in Dichlorobenzene exposure Z. mays root tissues. Tan et al., 2012 and Radwan, 2012 reported increased contents of H2O2 and MDA in the leaf tissues of Brassica napus and Z. mays by Naropamide and Clethodium respectively. In the present study low dose of cypermethrin decreased the EL, H2O2 and MDA contents in the leaf tissues of tested plant species. These findings are align with the studies of Singh et al., 2012, those revealed that decreased oxidative stress was observed in the leaf tissues of Z. mays and Vigna mungo by Glyphosate and Pendimethalin.
Plants' primary reaction to abiotic and biotic stresses is oxidative stress (Rahman et al., 2017; Adrees et al., 2015, Ali et al., 2015; Jan and Parray, 2016). To protect the seedling from the deleterious effect of these stresses by some antioxidant enzymes which are stimulated to protect from free radicals and peroxides (Parween et al., 2012). Present results confirmed the findings of previous researches that increased the pesticide doses decrease the activities of antioxidant enzymes in shoot tissues of the plants. For instances. Tan et al., 2012, Radwan, 2012; observed that Acetochlor and Clethodium decreased levels of CAT, POX, SOD enzymes in leaf tissues of Vitis vinifera L. and Z. mays, respectively. In the present study we observed that no changes occurred by Cypermethrin in the activities of SOD, POD and CAT enzymes in the leaves of H. annuus. Similar findings were observed by Štajner et al., 2003, Wu et al., 2010 and Miguel et al., 2012.
In the present study root tissues expressed different enzymes i.e., SOD, POD, CAT and APX was high in 20 mg/kg CYP relative to double dose of CYP in B. juncea followed by H. annuus (Fig. 2). Shakir et al. (2018) revealed that activation as well as suppression of SOD describe the magnitude of phytotoxicity at high concentration of pesticide residues at the level of treatment. POD enzyme induced to higher level of H2O2 result is the scavenging of ROS (Breda et al., 1993; Shakir et al., 2018. Parween et al., 2012 reported that CAT enzyme activity increased in Vigna radiate, under Chlorpyriphous stress. Moreover, increase activity of APX in root tissues indicates that detoxify H2O2 in plants exposed to insecticide stress (Morimura et al., 1996). Same findings have been documented regarding triazole fungicide (Jaleel et al., 2006). Glyphosate and Atrazine increased activities of APOX, CAT and POD enzymes in root tissues of different plants has previously been observed (Moldes et al., 2008; Zhang et al., 2014; Jiang et al., 2016).
After being exposed to a greater dosage of cypermethrin, the thickness of the stem epidermis was decreased. The epidermal layer of the stem on control plants is the thickest, whereas the epidermal layer on 40 mg/kg treated plants is the thinnest (Fig. 5). The decrease in epidermal cells is likely linked to CYP-induced reduced flexibility of cell walls, which causes the cells to become stiffer and unable to grow correctly. The smaller the epidermal cells of stem generated, as well as the thickness of epidermal cell outer walls, the greater the cypermethrin concentration supplied. The cuticle layer is most likely responsible for the thickening of epidermal cell outer walls.
The diameter of the stem cortex reduced when the pesticide concentration was raised (Fig. 5). The thickness of the stem cortex reduced when the pesticide dosage was raised. The control group and the 20 mg/kg treatment have polygonal stem cortical cells, but the 40 mg/kg treatment has irregular in shape and smaller cortical cells (Fig. 5). Similar to the cause of decreasing stem epidermal cells, the consequence of decreasing stem cortex cells is likely a decline in cell elasticity due to the presence of CYP in toxic concentrations, such that the cell wall cannot be stretched optimally and the cortex layer formed is thinner than in control plants due to the smaller size of its constituent cells.
As the concentration of CYP grows, the thickness of the stem vascular bundle decreases, and if the size of the tracheal cells created is measured from its constituent cells, the higher the pesticide concentration, the smaller the size of the tracheal cells made Gomes m. p. et al. (2011) demonstrated that the vascular bundle's thickness is likely reduced in response to Cr toxicity in plants, which inhibits Cr transport into photosynthetic tissue. In the present study reduction in the region of metaxylem cells was observed at lower concentration in both tested plant species. This character can increase water and nutrient conduction efficiency in the stem (Ali et al., 2009; Hameed et al., 2009).
In the current study, the root was the most damaged part of sunflower and mustard by pesticide. Reduction in the area of epidermal cells was reported in the resent study at treatment L3 (40 mg/kg), but increased epidermal area was reported at treatment L2 (20 mg/kg), both plant species showed increased thickness at both treatment levels (Fig. 7). In this study, sunflower and mustard showed an increase in the area of cortical cells under pesticide stress, but there was also a decrease in mustard in L3 (CYP 40 mg/kg) relative to control. In most cases, size and thickness of cortex cells increased in grass under saline stress, which is very reasonable. Such species are capable of storing extra water in parenchyma cells of the cortex, has become a major element in their sustainability, which, as stated by Grigore and Toma (2007). The tearing of the epidermis and cortex caused damage to some areas of the epidermis in the root, according to Jones et al. (2006). The activation of vacuole enlargement by Al is likely to be the cause of cortex cell enlargement (Wang et al., 2016). Reduction of the vascular area was also observed in both tested plant species (Fig. 7). Comparatively more resistant plants either equipped with narrower metaxylem and protoxylem area or decreased sizes with increased cypermethrin stress (40 mg/kg) have been observed in the present study. However, smaller vessel is less vulnerable to damage due to osmotic stress and this is important in the restricted supply of water (Hameed et al., 2009). In the current study, it was observed that, in the case of pesticide treatment, the area of the sunflower metaxylem vessel decreased, increased in mustard was recorded, which may enhance nutrient and water conductivity (Guo and Miao, 2010). The decreasing tendency of the xylem vessel area was contrary to the drift in the vascular bundle area by increasing stress, and is in agreement with Uga et al. (2008) whose work suggests that the vascular bundle area and the thickness or region of the xylem vessels are in distinct genetic control.
5 Conclusion
Pesticides applied in excess of the recommended amount had a negative effect on the growth of H. annuus and B. juncea, according to the findings of this study. Pesticide overdoses also caused phytotoxicity by producing oxidative stress, the generation of reactive oxygen species (ROS), and electrolyte leakage. Pesticides at greater concentrations resulted in lower protein and sugar levels. Different antioxidants' activities (SOD, POD, CAT, and APX) were shown to be reduced. Furthermore, pesticides in high quantities disrupted the interior structures of leaves, stems, and roots, which had a detrimental effect on overall plant morphological traits. The current findings are significant in terms of understanding plant responses to pesticide stress. More research will be stimulated in order to have a better understanding of the processes that affect gene expression.
Furthermore, H. annuus displayed better growth characteristics at low doses against Cypermethrin insecticide than B. juncea, according to the findings.
6 Compliance with ethical standard
N/A.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2022/315) King Saud University, Riyadh, Saudi Arabia.The publication of the present work is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1F1A1055482).
References
- Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf.. 2015;119:186-197.
- [Google Scholar]
- Heavy metals and pesticides toxicity in in agricultural soil and plants: Ecological risk and human health implications. Toxics. 2021;9:42.
- [Google Scholar]
- Endocrine disruptive action of cypermethrin in male mice. Toxicol. Environ. Health Sci.. 2011;3:69-79.
- [Google Scholar]
- Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res.. 2015;22:10601-10609.
- [Google Scholar]
- Heavy metal accumulation by roadside vegetation and implications for pollution control. PLoS ONE. 2021;16(5):e0249147.
- [Google Scholar]
- Photodestruction in higher plants by herbicide action. J. Exp. Bot.. 1990;41:123-126.
- [Google Scholar]
- Elevated antioxidant response and induction of tau-class glutathione S-transferase after glyphosate treatment in Vigna radiata (L.) Wilczek. Pest. Biochem. Physiol.. 2011;99:111-117.
- [Google Scholar]
- Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal. Biochem.. 1971;44:276-278.
- [Google Scholar]
- Differential expression of two peanut peroxidase cDNA clones in peanut plants and cells in suspension culture in response to stress. Plant Cell Rep.. 1993;12:268-272.
- [Google Scholar]
- A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ.. 2021;796:148756
- [Google Scholar]
- Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect.. 1980;34:189-202.
- [Google Scholar]
- Effect of Alpha cypremethrin on morphological parameters in tomato plants (Lycopersicon esculentum Mill.) Am. J. Environ. Protect.. 2013;2:149-153.
- [Google Scholar]
- Assay of catalase and peroxidase. Methods Enzymol 2: 764–775 Coskun, Y., Kilic, S. and Duran, R. E. (2015). The effects of the insecticide pyriproxyfen on germination, development and growth responses of maize seedlings. Fresenius Environ. Bull.. 1955;24:278-284.
- [Google Scholar]
- On-farm seed priming interventions in agronomic crops. Acta Agric. Slovenica.. 2018;111(3):715-735.
- [Google Scholar]
- Salicylic acid reduces napropamide toxicity by preventing its accumulationin rapeseed (Brassica napus L.) Arch. Environ. Contam. Toxicol.. 2010;59(1):100-108.
- [Google Scholar]
- Herbicidal inhibition of photosynthetic electron transport. In: Physiology of Herbicide Action. Englewood Cliffs, NJ: Prentice Hall; 1993. p. :113.
- [Google Scholar]
- Effect of pesticides on the Seed Germination of Cenchrus setigerus and Pennisetum pedicellatum as Monocropping and Co-cropping System: Implications for Rhizospheric Bioremediation. Rom. Biotechnol. Lett.. 2011;16(1):5909-5918.
- [Google Scholar]
- Bio-based integrated pest management in rice: An agro-ecosystems friendly approach for agricultural sustainability. J. Saudi Soc. Agric. Sci.. 2021;20(2):94-102.
- [Google Scholar]
- Food and Agriculture Organization, 2006. Global Forest Resources Assessment 2005: Progress towards sustainable forest management, FAO Forestry Paper 147.
- Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. –. Environ. Exp. Bot.. 2003;49:159-168.
- [Google Scholar]
- Interactions of herbicides with photosynthetic electron transport. Weed Sci.. 1991;39:458-464.
- [Google Scholar]
- Comparative biology of Spodoptera litura fabricius on different food sources under controlled conditions. Exp. Zool. India.. 2020;23(1):681-684.
- [Google Scholar]
- Hyderoperoxide assay with the ferric-xylenol orange complex. Anal. Biochem.. 1999;273:149-155.
- [Google Scholar]
- A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal. Biochem.. 2000;284:217-220.
- [Google Scholar]
- Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci. Agri.. 2011;68(5):566-573.
- [Google Scholar]
- Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucas carota L. Colloids Surf B: Biointerfaces. 2007;60:180-186.
- [Google Scholar]
- Histo-anatomical strategies of Chenopodiaceae halophytes: Adaptive, ecological and evolutionary implications. WSEAS Trans. Biol. Biomed.. 2007;12:204-218.
- [Google Scholar]
- Growth changes and tissues anatomical characteristics of giant reed (Arundo donax L.) in soil contaminated with arsenic, cadmium and lead. Central South Univ. Forestry Technol.. 2010;17:770-777.
- [Google Scholar]
- Hajslova J, Zrostlikova J (2003) Matrix effects in ultra-trace analysis of pesticide residues in food and biotic matrices. J Chrom A 1000: 181-197. H. Willekens, S. Chamnongpol, M. Davey, M. Schraudner, C. Langebartels, M.S. K. Shakir, S. Irfan, B. Akhtar, S. ur Rehman, M. K. Daud, N. Taimur, et al.,(2018), ''Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings'', Ecotoxicology, 1-17.
- Anatomical adaptations to salinity in cogon grass (Imperata cylindrica (L.) Raeuschel) from the Salt Range, Pakistan. Plant Soil. 2009;322:229-238.
- [Google Scholar]
- Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 1968;125:189-198.
- [Google Scholar]
- Pest and diseases of chilli crop in Pakistan: A review. Int. J. Biol. Biotech.. 2011;8:325-332.
- [Google Scholar]
- Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep.. 2021;11(18416)
- [Google Scholar]
- Alterations in lipid peroxidation electrolyte leakage, and proline metabolism in Catharanthus roseus under treatment with triadimefon, a systemic fungicide. C. R. Biol.. 2006;330:905-912.
- [Google Scholar]
- Approaches to Heavy Metal Tolerance In Plants. Singapore: Springer Singapore; 2016.
- Antioxidant modulation in response to gamma induced oxidative stress in developing seedlings of Psoralea corylifolia L. J. Environ. Radioac.. 2012;113:142-149.
- [Google Scholar]
- Enzymatic antioxidant defense in resistant plant: Pennisetum americanum (L.) K. Schum during long-term atrazine exposure. Pestic. Biochem. Physiol.. 2016;133:59-66.
- [Google Scholar]
- Estrogenic activities of two synthetic pyrethroids and their metabolites. J. Environ. Sci.. 2010;22:290-296.
- [Google Scholar]
- Differential antioxidant responses in catalase-deficient maize mutants exposed to norflurazon. Biol. Plant.. 2006;50:383-388.
- [Google Scholar]
- An empirical analysis of pesticide marketing in Pakistan. Pak. Eco. Soc. Rev.. 2008;46:57-74.
- [Google Scholar]
- An empirical analysis of pesticide marketing in Pakistan. Pakistan Econ. Soc. Rev.. 2008;46(1):57-74.
- [Google Scholar]
- Pesticide use and risk reduction in European farming system with IPM: An introduction to the special issue. Crop Prot.. 2017;97:1-6.
- [Google Scholar]
- Atrazine accumulation and toxic responses in maize Zea mays. J. Environ. Sci.. 2012;24(2):203-208.
- [Google Scholar]
- Changes in plant-response to Nacl during development of rice (Oryza sativa L) varieties differing in salinity resistance. J. Exp. Bot.. 1995;46:1843-1852.
- [Google Scholar]
- Diversity and abundance of Lepidopteran populations from selected crops of district Faisalabad, Pakistan. Pakistan J. Agric. Sci.. 2013;50:95-101.
- [Google Scholar]
- Biological responses of maize (Zea mays) plants exposed to chlorobenzenes. Case study of monochloro-, 1, 4-dichloro-and 1, 2, 4-trichloro-benzenes. Ecotoxicology. 2012;21(2):315-324.
- [Google Scholar]
- Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci. Hortic.. 2009;120:373-378.
- [Google Scholar]
- Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: An incubation study. PLoS ONE. 2017;12(7):e0180663.
- [Google Scholar]
- Biochemical responses of glyphosate resistant and susceptible soybean plants exposed to glyphosate. Acta Physiol. Plant.. 2008;30(4):469-479.
- [Google Scholar]
- An observational study of 127 preschool children at their homes and daycare centers in Ohio: Environmental pathways to cis- and trans-permethrin exposure. Environ. Res.. 2007;104:266-274.
- [CrossRef] [Google Scholar]
- Presence of ascorbate peroxidizing enzymes in roots of Brassica campestris L. cv Komatsuna. Plant Sci.. 1996;117:55-63.
- [Google Scholar]
- Cornus macrophylla the antibacterial activity of organic leaf extracts and the characterization of the more lipophilic components by GC/MS. Molecules. 2020;25(10):2395.
- [Google Scholar]
- Toxicity of different imbibitions periods of dimethoate on germination, chlorophyll a/b, and dry matter of Glycine max (L) Merrill. Cv. KHSB-2, during early seedlings growth. J. Physiol. Res.. 2005;18:199-201.
- [Google Scholar]
- Agriculture development, pesticide application and its impact on the environment. Environ. Res. Public Health.. 2021;18:1112.
- [Google Scholar]
- Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol.. 1981;22:867-880.
- [Google Scholar]
- Effect of some herbicide on seed germination and seedling vigour in mungbean. Crop Res.. 1999;17:425-426.
- [Google Scholar]
- Differential influence of herbicide treatments on activity and kinetic parameters of C4 photosynthetic enzymes from Zea mays. Pestic Biochem. Physio.. 2007;89:198-205.
- [Google Scholar]
- Smallholder vegetable farmers in Northern Tanzania: Pesticides use practices, perceptions, cost and health effects. Crop Prot.. 2007;26:1617-1624.
- [CrossRef] [Google Scholar]
- Farmers’ knowledge, perceptions and management of vegetable pests and diseases in Botswana. Crop Prot.. 2008;27:1220-1224.
- [CrossRef] [Google Scholar]
- Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol.. 2012;9(4):605-612.
- [Google Scholar]
- Classical and biochemical endpoints in the evaluation of phytotoxic effects caused by the herbicide trichloroacetate. Environ. Exp. Bot.. 2000;44:221-229.
- [Google Scholar]
- Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pest Biochem. Physiol.. 2012;102:182-188.
- [Google Scholar]
- Insight into metal immobilization and microbial community structure in soil from a steel disposal dump phytostabilized with composted, pyrolyzed or gasified wastes. Chemosphere. 2021;272:129576
- [Google Scholar]
- Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf.. 2017;135:165-172.
- [Google Scholar]
- Mitigation of bacterial spot disease induced biotic stress in Capsicum annuum L. cultivars via antioxidant enzymes and isoforms. Sci. Rep.. 2021;11:9445.
- [Google Scholar]
- Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi J. Biol. Sci.. 2021;28(11):6339-6351.
- [Google Scholar]
- Pesticides induced alterations in physiological responses in Solanum melongena L. Int. J Pharm. Bio. Sci.. 2011;2:374-384.
- [Google Scholar]
- Amalgamation of farmers’ bio-priming knowledge in integrated nutrient management for sustainable management of red cabbage soil under Middle Gangetic Plains, India. Environ. Manage. 2022
- [CrossRef] [Google Scholar]
- Organic interventions conferring stress tolerance and crop quality in agroecosystems during the UN Decade on Ecosystem Restoration. Land Degrad. Dev.. 2021;32(17):4797-4816.
- [Google Scholar]
- Safeguarding the fragile rice–wheat ecosystem of the Indo-Gangetic Plains through bio-priming and bioaugmentation interventions. FEMS Microbiol. Ecol.. 2020;96(12):fiaa221.
- [Google Scholar]
- Management of increasing soil pollution in the ecosystem. Adv. Res.. 2017;12(2):1-9.
- [Google Scholar]
- Synergistic effect of Bacillus thuringiensis IAGS 199 and putrescine on alleviating cadmium-induced phytotoxicity in capsicum annum. Plants. 2020;9(11):1512.
- [Google Scholar]
- Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum) Ecotoxicology. 2016;25:329-341.
- [Google Scholar]
- Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology. 2018;27:919-935.
- [Google Scholar]
- Phytoremediation: role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environ. Sci. Pollut. Res.. 2015;22:946-962.
- [Google Scholar]
- Growth and photosynthetic characteristics of two biotypes of the weed black-grass (Alopecurus myosuroides Huds) resistant and susceptible to the herbicide chlorotoluron. Ann. Bot.. 1997;79:455-461.
- [Google Scholar]
- Effect of systemic fungicide on germination, seedling growth and phenolic contents of Vigna radiata L. Pak. J. Bot.. 1996;28:191-193.
- [Google Scholar]
- A current review of cypermethrin induced neurotoxicity and nigrostriatal dopaminergic neurodegeneration. Curr. Neuropharmacol.. 2012;10(1):64-71.
- [Google Scholar]
- Herbicide induced oxidative stress in lettuce, beans, pea seeds and leaves. Biol. Plant.. 2003;47(4):575-579.
- [Google Scholar]
- Pesticide residues in fruits and vegetables from Pakistan: a review of the occurrence and associated human health risks. Environ. Sci. Pollut. Res.. 2014;21:13367-13393.
- [Google Scholar]
- Photosynthesis and growth responses of grapevine to acetochlor and fluoroglycofen. Pestic. Biochem. Physiol.. 2012;103:210-218.
- [Google Scholar]
- Effect of some pesticides on plant growth, root nodulation and chlorophyll content of chickpea. Arch. Agron. Soil Sci.. 2004;50:529-553.
- [Google Scholar]
- Determination of chlorinated pesticide in vegetables, cereals and pulses by gas chromatography in east national capital region, Delhi, India. Res. J. Agric. For. Sci.. 2013;1:27-28.
- [Google Scholar]
- Determination of chlorinated pesticide in vegetables, cereals and pulses by gas chromatography in east national capital region, Delhi, India. Res. J. Agric. For. Sci.. 2013;1:27-28.
- [Google Scholar]
- Physiological effects of captan fungicide on pepper (Capsicum annuum L.) Pak. J. Biol. Sci.. 2003;6(24):2026-2029.
- [Google Scholar]
- QTLs underlying natural variation in stele and xylem structures of rice root. Breed. Sci.. 2008;58(1):7-14.
- [Google Scholar]
- Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res.. 2015;22(13):9646-9657.
- [Google Scholar]
- Residual phytotoxicity effects of paraquat, glyphosate and glufosinate-ammonium herbicides in soils from field treated plots. Int. J. Agric. Biol.. 2009;11:214-216.
- [Google Scholar]
- Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut.. 2002;116:37-47.
- [Google Scholar]
- Effect of herbicide mefenacet on response of active oxygen scavenging system in rice plant. Agro. Environ. Protect.. 2001;20:411-413.
- [Google Scholar]
- Yang H Accumulation and toxicological response of atrazine in rice crops. Ecotoxicol. Environ. Safe.. 2014;102:105-112.
- [Google Scholar]
- Protective effect of exogenous polyamines against atrazine in pea plants. J. Plant Growth Regul.. 1994;13:203-211.
- [Google Scholar]
- Pesticides regulations and their malpractice implications on food and environment safety. Congent. Food Agric.. 2019;5:1601544.
- [Google Scholar]







