Translate this page into:
Phosphatidylethanolamine impregnated Zn-HA coated on titanium for enhanced bone growth with antibacterial properties
⁎Corresponding author. tapash.rautray@gmail.com (Tapash Ranjan Rautray)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the current study, 10% zinc substituted hydroxyapatite coating was formed on titanium surface and subsequently phosphatidylethanolamine was incorporated into the coating to study the osteogenic and antibacterial behavior of the coated surface. X-ray diffraction analysis showed the formation of zinc substituted hydroxyapatite on titanium while energy dispersive X-ray spectrometry substantiated the coating to be calcium deficient. Phosphatidylethanolamine incorporated coating on titanium showed higher cell viability and antibacterial property as compared to the control. The superior cell activities on this surface may be attributed to the presence of phosphatidylethanolamine in the coating.
Keywords
Phosphatidylethanolamine
Zinc
Hydroxyapatite
Titanium
1 Introduction
Infections at the implant sites are caused due to bacteria formation on the surface of bioimplants and subsequent biofilm formation. Biofilms are highly resistant to both immune system and antibiotics. It is critical to inhibit bacterial adhesion in order to prevent infection at implant sites (Kaviyarasu et al., 2017). To prevent infection during surgery, applying prophylactic antibiotics has been shown to be most effective. Titanium (Ti) has been extensively used as medical implants (Rautray et al., 2010) because of its biocompatible properties. To further improve its biocompatibility to be used as an osseointegrative implant material, it is necessary to coat a hydroxyapatite (HA) layer on Ti surface (Qiu et al., 2010; Qiu et al., 2011; Bir et al., 2012). Because of its slow rate of interactions with the bone tissues, application of HA on implant materials seek some biocompatible elemental substitution in the calcium (Ca) site of HA (Rautray et al., 2011).
It has been found that HA shows great flexibility in accepting divalent ions such as Mg2+, Mn2+, Co2+, Cu2+, Zn2+, Sr2+, monovalent ions such as Na+, K+, Ag+ and trivalent ions such as Bi3+, Ln3+ in the Ca site (Pereiro et al., 2012; Bodhak et al., 2011). From among the divalent ions, Zn has been proven to be beneficial in bone growth for which an attempt to substitute Zn in the Ca site of HA was undertaken (Qiu et al., 2010, 2011). The most important property of Zn for which it has been used by researchers, is its anti bacterial property (Kaviyarasu et al., 2017a,b). But it has also been found that Zn shows other properties such as its function in synthesis of DNA, enzymatic and hormonal activities and moreover it shows anabolic effects on bone mineralization (Nagata et al., 2011; Miao et al., 2011), helps in promoting osteoblast marker gene expression and deposition of Ca on mesenchymal stem cells (MSCs). For all these properties and also for inhibiting bone resorption and anti-inflammatory properties (Kaviyarasu et al., 2017c), Zn is used by biological community (Laquerriere et al., 2006; Rutkovskiy et al., 2016).
Because of the lack of better mechanical properties, biocompatible and bioactive HA is coated on metallicimplants to provide strength to human bone. Various techniques have been used to coat HA on implant surface that include cathodic deposition, plasma spraying, chemical vapor deposition and ion implantation. But when compared with other techniques, cathodic deposition method displays better control over crystallinity with short reaction times. Moreover, a film thickness of about 1 µm is achieved that is beneficial for resistance to delamination (Narayanan et al., 2006).
A biomimetic approach to strengthen the bone-to-implant contact is by incorporating phospholipids in the implants. Although eukaryotic membranes have many compositions, 40% of their dry weight is lipids and from among these lipids, phospholipids are the most abundant and are heterogeneously spread in the plasma membranes. The major phospholipids responsible for this asymmetric distribution are phosphatidylserine (PS) and phosphatidylethaolamine (PE) which are located in the phosphatidylcholine (PC), glycosphingolipids and sphingomyelin. Lipids are generally involved in cell signaling and have major role in cell compartmentalisation and required curvature for mitosis. However, PE has been shown to inhibit bacteria cells and increase osteoblast cells in biomedicine (Irie et al., 2017).
The cell viability and antibacterial properties of PE impregnated Zn-HA on Ti surface were studied in this work on the basis of PE incorporation in Zn substituted HA.
2 Materials and methods
All the biological and chemical reagents used in this study were procured commercially.
2.1 Preparation of Zn-HA on Ti substrate
The divalent ion Zn was substituted by 10 atom. % in the Ca site of HA by cathodic deposition on commercially pure Grade 2 Ti (2 × 1 cm2). The Ti substrate was polished with 600 and 1200 grit-sized SiC papers. The Ti substrate was then washed with acetone and deionized water and ultrasonicated. An etchant with a composition of 1 part 50% HF, 4 parts 1 N HCl and 5 parts deionized water was used to eliminate the air-formed TiO2 layer. The electrolyte contained (NH4)2HPO4, Ca(NO3)2·4H2O and Zn(NO3)2·6H2O in a composition such that the molar ratio of (Zn:Ca) was 1:9 and (Ca + Zn)/P ratio was 1.67. The electrolyte maintained at pH 4.2 was continuously stirred using a magnetic stirrer kept at room temperature. The coating was performed using a two electrode system taking Ti as cathode and Pt as anode (Swain et al., 2016). The Zn substituted HA coating of Ti was carried out for 30 min (El-Wassefy et al., 2017; Zhong et al., 2017) and after the cathodic deposition the substrate was rinsed again with deionized water and oven dried till incorporation of PE.
2.2 Incorporation of PE in Zn-HA coating
The Zn-HA coated Ti substrate was dipped in a solution of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE, Avanti Polar Lipids, USA) dissolved in chloroform: methanol (80:20 v/v) for 2 h. After the incorporation of POPE in Zn-HA coated Ti, the substrate was dried at room temperature and taken for further analysis (Zhu et al., 2016).
2.3 Surface characterization
X-ray diffractometer (XRD; X’pert-APD, Philips, Netherlands) was employed to determine the structure of coatings by using Cu Kα radiation in the 2θ range of 15−80° (Gibson et al., 2002). Field emission scanning electron microscopy (FE-SEM; JSM-6700, JEOL, Tokyo, Japan) was employed to observe the surface morphology and thickness of coating layer and Energy dispersive X-ray (EDX) spectrometry was used for the elemental analysis of the coatings (Rautray et al., 2010a). To verify the presence of PE in the PE-Zn-HA coating, High Performance Liquid Chromatography (HPLC) technique was employed as described elsewhere (Brouwers et al., 1999). Zn-HA being the undissolved inorganic component was not involved in the proves of PE estimation. Surface roughness measuring instrument (SURFTEST, Mitutoyo, Japan) was used to measure the roughness (Ra) of the coating. Coating wettability or hydrophilicity was measured by using a contact angle detection system (OCA15 Plus, Dataphysics, Germany), and it was measured by using drops of ultrapure distilled water (0.6 μl) and by capturing the image with delivery span of 10 s. By using a universal testing machine (Instron; 4202) under tension mode in accordance with ASTM C633-79, tensile pull-off adhesion strength was estimated (Narayanan et al., 2009). Ti (diameter 3 mm) was used as a stub and was pasted to the coating side.
2.4 Cell viability study using MTT assay
The cell viability study was performed by culturing the cells on the Zn-HA and PE-Zn-HA samples for eight days. MTT assay test [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] depicted a measure of the activity of mitochondria present in the cells which was that could be associated to the viability and count of cells during culture. Tetrazolium salt transformed its color to a dark-blue insoluble form that is the formazan product by mitochondrial succinic dehydrogenase of viable cells. MTT solution (5 mg/mL, Sigma Aldrich) was added to each well, and cells were incubated at 37 °C for 4 h. Following total removal of the culture medium, dimethylsulfoxide (DMSO) was supplemented to each well to dissolve the cells. Then cell viability was measured based on the optical density of the sample in the solution using a UV–VIS spectrophotometer (Mettler Toledo) set at 540 nm. The blank reference was taken from wells without cells. The collected data were analyzed using SPSS 24.0. The cell viability between these two specimens were performed three times in duplicate and the data were statistically analyzed using a t-test (α = 0.05, p < 0.05). MTT cytotoxicity assays showed statistically significant difference between the Zn-HA and PE-Zn-HA samples.
2.5 Antibacterial activities in solid agar medium
The antibacterial test was performed in Petri-dishes with 90 mm diameter that contained Nutrient agar medium (15 ml). Subsequent to the renewal of culture of bacterial for 24 h., freshly prepared bacterial inoculums were mopped over the whole surface of the medium three times following rotation of the agar plate by 60° after each mopping by using cotton swab to uniformly spread bacteria over the plate surfaces. Wells of 6 mm diameter each was bored in the medium for individual plates and filled with 50 μl of the suspension of the sample. Plates were left for 45 min at 25 °C to allow proper spreading of the extract. The plates were then incubated at 37 °C for 24 h after which the diameters of zones of inhibition were measured. Inhibition zones of bacterial growth were measured. All the measurements were performed in triplicate and the reported data corresponds to average values ± SD. Nine Gram negative and one Gram positive bacterial strains were chosen to study the anti-bacterial activities.
3 Results and discussion
XRD of the coatings is shown in Fig. 1. Ti peaks from the coated Ti substrate is evident in the XRD pattern. Peaks of Zn were observed in the PE incorporated Zn-HA (PE-Zn-HA) deposited Ti substrate. The lattice cell parameters of the as-formed PE-Zn-HA coating by Rietveld refinement is depicted in Table 1. After the substitution of Zn2+ ions into the HA structure, the lattice parameters were observed. It can be seen that the a- and c-axes decreased with the substitution of Zn2+ ions that agrees with literature values (Thian et al., 2013). The results found in this study are congruent in the fact that the ionic radius of Ca2+ (0.099 nm) is higher than Zn2+ (0.074 nm).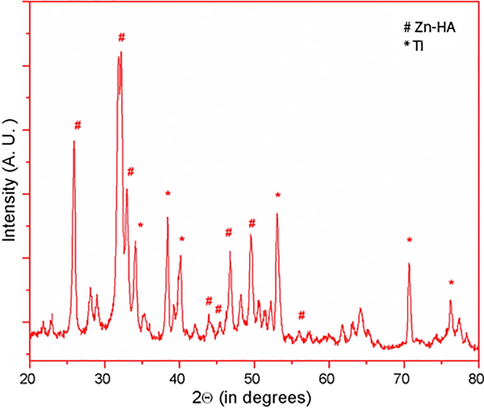
XRD pattern of PE-Zn-HA on Ti substrate.
Sample
Cell parameters
a (nm)
c (nm)
HA
0.9418
0.6884
ZnHA
0.9395 ± 4
0.6870 ± 4
SEM micrographs of the as-formed Zn-HA and PE-Zn-HA are depicted in Figs. 2 and 3 respectively. SEM morphologies of the samples showed bead like particles that were uniformly deposited throughout the Ti substrate having hexagonal structure. EDX spectroscopic technique was used for the elemental analysis that showed the presence of Zn in the coatings. PE was observed at about 5.8 min of HPLC analysis as depicted in Fig. 4. Although many studies in past have been performed to understand the osteogenic and antibacterial properties using either PE in HA or Zn in HA, the current study sheds light on the potential of PE in Zn-HA coated Ti implants.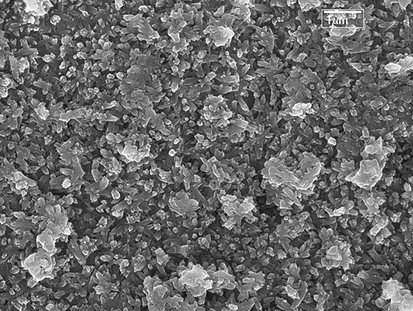
SEM morphology showing Zn-HA on Ti substrate.
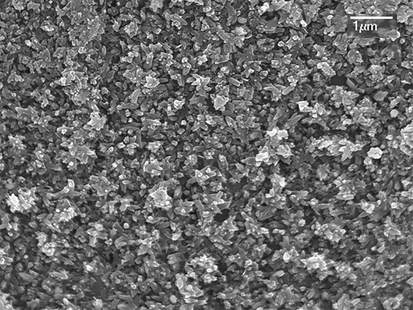
SEM morphology showing PE-Zn-HA on Ti substrate.
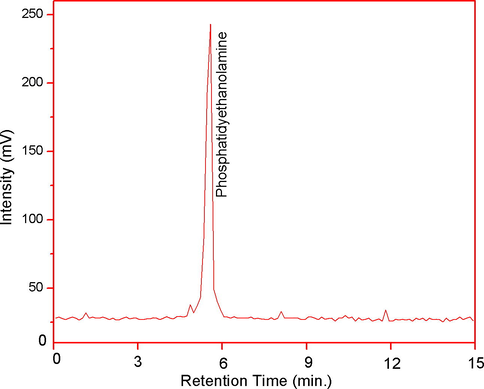
HPLC chromatogram showing presence of PE in PE-Zn-HA samples.
Properties of the coatings are given in Table 2. It has been known that more the surface roughness, more is the osteoblast adhesion and thus they enhance bioactivity on the surface. Roughened surfaces are more suitable for bone anchoring and biomechanical stability. Surface profile is one of the most important parameters when producing HA coatings. Surface roughness can be described by various measures such as Ra, Rz and Rmax. In biomedical applications, roughness is often described by the parameter Ra (absolute roughness). As compared to control HA surface, surface roughness (Ra) on the Zn-HA surface was higher indicating better osteoblast adhesion on its surface. The water wetting angle measurement showed that the PE-Zn-HA surface is more hydrophilic than the control Zn-HA surface. Literature cites that hydrophilic surfaces favor better bioactivity (Elayaraja et al., 2012; Narayanan et al., 2009). Hence PE-Zn-HA coating is supposed to be a better bioimplant surface as far as hydrophilicity is concerned. The (Ca + Zn)/P ratio of the coatings was found to be calcium deficient and it is 1.62 i.e. lower than the standard stoichiometric value of 1.67 (Ca/P) of HA.
Adhesion strength is a very important characteristic because the low strength coatings delaminate and show micromotion with respect to bone-to-implant contact. PE-Zn-HA with 9.5 MPa showed better adhesion strength than the control HA surface. It has been specified that the pull off strengths of the HA coatings used as implants should have at least 18 MPa but as low as 10 MPa of adhesion strength of HA coatings were also reported using plasma spray technique (Narayanan et al., 2009).
Fig. 5 shows the cell viability of PE-Zn-HA coated Ti with Zn-HA as control. It was found that there was 19% increase in cell numbers as compared to the control Zn-HA coating. Hence it can be suggested that incorporation of PE in the Zn-HA coating resulted in enhancing the osteoblast activities on coated Ti substrate.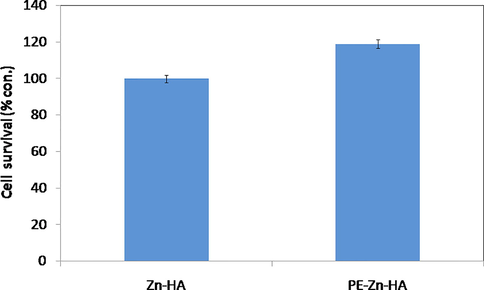
MTT assay of (a) Zn-HA deposited Ti and (b) PE-Zn-HA deposited Ti (p < 0.05).
Based on previous studies, substitution of Ca by Ag, Mg and Zn is limited, which may be attributed to the dissimilar size between these divalent cations and Ca2+ that results in HA lattice distortion and simultaneously altering their solubility and biodegradable properties in physiological conditions.
Pathogenic microorganisms, such as gram-positive bacteria S. aureus and S. epidermidis, have been found to be associated with about 90% of implant failure cases (Irie et al., 2017). Gram-negative bacteria such as E. coli and Pseudomonas aeruginosa and other bacteria such as P. mirabilis and P. vulgaris have also been associated with the infections in implants. From the antimicrobial results, it was found that PE-Zn-HA coated Ti showed higher amount of antibacterial activity of the sample. Fig. 6 depicts the antibacterial activities on the coated Ti substrate. It was found that PE-Zn-HA sample is resistant to bacteria with 27-mm inhibition zone (Rath et al., 2017).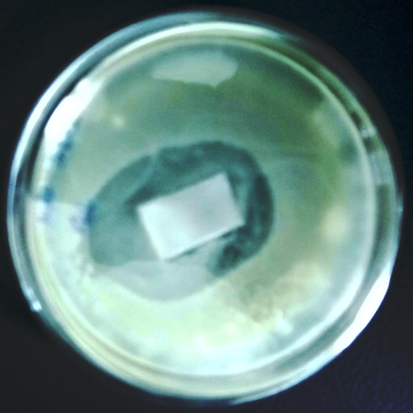
Antibacterial activities on the PE-Zn-HA on Ti substrate.
Three main methodologies used for anti bacterial coatings are (i) contact killing of bacteria, (ii) antibacterial agent release and (iii) anti-adhesion or bacteria-repelling technique. In the current study, Zn acts as the agent for contact killing of bacteria while PE acts as an antibacterial agent by anti-adhesion by increasing osteoblast activities. PE consists of a phosphate group (PO42−) that is bonded to another small molecule. Despite their clinical applications, liposomes exhibit many drawbacks such as their low stability, low solubility and sterilization issues. Natural phospholipids are less stable than the synthetic phospholipids produced from natural lipids (Hirsch et al., 1992). But since the present study aims to decrease bacterial activities by anti-adhesion through increase in osteoblast number, chemical stability of PE was not a serious concern in fulfilling the objective of the study. Delamination of PE was compensated by the presence of Zn-HA coating subsequently providing osteogenic and antibacterial activities.
Zn plays a key role in various body functions such as regulating nucleic acid metabolism, maintenance of membrane structure, hormonal activity and is an enzyme cofactor in more than 300 enzymes involved in bone metabolism. Zn also stimulates bone formation and mineralisation by inhibiting osteoclast differentiation and enhancing osteoblast activity, while it has been also shown that it can stimulate bone resorption at higher concentrations. Zn is well known for its inhibitory effect on HA crystal growth, but it has been shown that it can be quantitatively substituted in the HA lattice at concentrations of up to 20 atom. % (Boanini et al., 2010).
The mechanism underlying the antibacterial effect of Zn substituted HA is yet to be understood fully. However, it is proposed that strong bonds are developed between Zn ions with carboxylic group of proteins, thiolic, imidazole and amine that creates structural deformation by affecting the membrane permeability and transport resulting in cell death. It is also shown that Zn ions can damage the enzymatic activity and DNA and RNA of bacteria with similar results. In addition to its role as an antibacterial agent, Zn-HA ceramics are used to regulate other biological functions. Kawamura et al. (2000) used composite ceramics of Zn-TCP/HA as implants in the femor of New Zealand white rabbits for 4 weeks and reported increasing rates of bone formation at concentrations of 0.316 wt%. Laquerriere et al. (2006) reported, that the addition of Zn-HA decreased the inflammatory reaction following the implantation of HA based prosthesis by increasing the production of cytokines. Li et al. (2008) suggested that Zn-HA can be used as a next generation material in the field of biomedical research.
It was found from antimicrobial studies that PE-Zn-HA reduced bacteria number by more than 50% by enhancing osteoblast cell number. Hence incorporation of PE resulted in increased cell count in the PE-Zn-HA as compared to the Zn-HA control.
4 Conclusions
The cell viability studies showed that there was 19% increase in osteoblast cell numbers as compared to the control Zn-HA coating. It may be inferred that presence of PE in Zn-HA coating enhances the osteoblast activities on coated Ti substrate thereby decreasing the bacterial activities. Having better mechanical strength, surface roughness, hydrophilicity, osteogenic and antibacterial properties, PE-Zn-HA coated Ti may be used as a next generation bioimplants in the field of bone tissue engineering.
Acknowledgment
One of the authors (TRR) is thankful for the financial support to Department of Science and Technology, Govt. of India with grant No. SB/SO/HS-113/2013 (A).
References
- Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci.. 2012;258:7021-7030.
- [CrossRef] [Google Scholar]
- Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater.. 2010;6(6):1882-1894.
- [CrossRef] [Google Scholar]
- Bone cell–material interactions on metal-ion doped polarized hydroxyapatite. Mater. Sci. Eng. C. 2011;31(4):755-761.
- [CrossRef] [Google Scholar]
- Rapid separation and identification of phosphatidylethanolamine molecular species. J. Lipid Res.. 1999;40:164-169.
- [Google Scholar]
- Enhancement of wettability and antibiotic loading/release of hydroxyapatite thin film modified by 100 MeV Ag7+ ion irradiation. Mater. Chem. Phys.. 2012;134:464-477.
- [CrossRef] [Google Scholar]
- Electro-chemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int. J. Implant Dent.. 2017;39(3):1-8.
- [CrossRef] [Google Scholar]
- Preparation and characterization of magnesium/carbonate co-substituted hydroxyapatites. J. Mater. Sci. Mater. Med.. 2002;13:685-693.
- [CrossRef] [Google Scholar]
- Liposome interactions with hydroxyapatite crystals: a possible mechanism in the calcification of atherosclerotic plaques. Calcif. Tissue Int.. 1992;50:261-265.
- [Google Scholar]
- Phosphatidylethanolamine dynamics are required for osteoclast fusion. Sci. Rep.. 2017;7(46715):1-13.
- [CrossRef] [Google Scholar]
- Antiproliferative effects on human lung cell lines A549 activity of cadmium selenide nanoparticles extracted from cytotoxic effects: investigation of bio-electronic application. Mater. Sci. Eng. C. 2017;76:1012-1025.
- [CrossRef] [Google Scholar]
- Elucidation of photocatalysis, photoluminescence and antibacterial studies of ZnO thin films by spin coating method. J. Photochem. Photobiol. B. 2017;173:466-475.
- [CrossRef] [Google Scholar]
- In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of Zinc oxide doped TiO2 nanocrystals: investigation of bio-medical application by chemical method. Mater. Sci. Eng. C. 2017;74:325-333.
- [CrossRef] [Google Scholar]
- Photocatalytic performance and antimicrobial activities of HAp-TiO2 nanocomposite thin films by sol-gel method. Surf. Interfaces. 2017;6:247-255.
- [CrossRef] [Google Scholar]
- Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res.. 2000;50(2):184-190.
- [Google Scholar]
- Influence of the zinc concentration of sol-gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials. 2006;27:3195-3200.
- [CrossRef] [Google Scholar]
- Structural characterization of zinc-substituted hydroxyapatite prepared by hydrothermal method. J. Mater. Sci. Mater. Med.. 2008;19(2):797-803.
- [CrossRef] [Google Scholar]
- Zn-releasing FHA coating and its enhanced osseointegration ability. J. Am. Ceram. Soc.. 2011;94:255-260.
- [CrossRef] [Google Scholar]
- Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. J. Nutr. Biochem.. 2011;22:172-178.
- [CrossRef] [Google Scholar]
- Hydroxyapatite coatings on Ti-6Al-4V from seashell. Surf. Coat. Technol.. 2006;200:4720-4730.
- [CrossRef] [Google Scholar]
- TiO2 nanotubes from stirred glycerol/NH4F electrolyte: roughness, wetting behavior and adhesion for implant applications. Mater. Chem. Phys.. 2009;117:460-464.
- [CrossRef] [Google Scholar]
- Pulsed laser deposition of strontium-substituted hydroxyapatite coatings. Appl. Surf. Sci.. 2012;258:9192-9197.
- [CrossRef] [Google Scholar]
- Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci.. 2010;257:1774-1778.
- [CrossRef] [Google Scholar]
- Preparation and characterization of hydroxyapatite/titania composite coating on NiTi alloy by electrochemical deposition. Surf. Coat. Technol.. 2011;205:3280-3284.
- [CrossRef] [Google Scholar]
- Surveillance of bacteria Pseudomonas aeruginosa and MRSA associated with chronic suppurative otitis media. Braz. J. Otorhinolaryngol.. 2017;83(2):201-206.
- [CrossRef] [Google Scholar]
- Nanoelectrochemical coatings on titanium for bioimplant applications. Mater. Technol.. 2010;25:143-148.
- [CrossRef] [Google Scholar]
- Surface modification of titanium and titanium alloys by ion implantation. J. Biomed. Mater. Res.. 2010;93B:581-591.
- [CrossRef] [Google Scholar]
- Ion implantation of titanium based biomaterials. Prog. Mater. Sci.. 2011;56:1137-1177.
- [CrossRef] [Google Scholar]
- Osteoblast differentiation at a glance. Med. Sci. Monit. Basic. Res.. 2016;22:95-106.
- [CrossRef] [Google Scholar]
- Sr, Mg, and Co substituted hydroxyapatite coating on TiO2 nanotubes formed by electrochemical methods. Adv. Sci. Lett.. 2016;22:482-487.
- [CrossRef] [Google Scholar]
- Zinc-substituted hydroxyapatite: a biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci: Mater. Med.. 2013;24:437-445.
- [CrossRef] [Google Scholar]
- Fabrication, characterization, and in vitro study of zinc substituted hydroxyapatite/silk fibroin composite coatings on titanium for biomedical applications. J. Biomater. Appl.. 2017;32(3):399-409.
- [CrossRef] [Google Scholar]
- Molecular insight into affinities of gallated and nongallated proanthocyanidins dimers to lipid bilayers. Sci. Rep.. 2016;6:37680.
- [CrossRef] [Google Scholar]







