Translate this page into:
Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions
⁎Corresponding author. pc.singh@nbri.res.in (Poonam C. Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
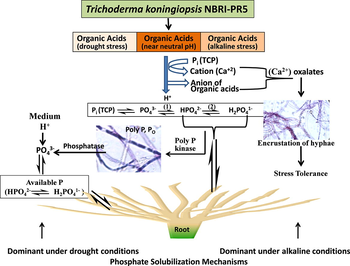
Schematic representation of phosphate solubilization mechanisms present in T. koningiopsis NBRI-PR5 under alkaline and drought stress conditions. Organic acid produced by NBRI-PR5 gets dissociated and provide H+ ions which shifts the equilibrium towards HPO42− and provide soluble HPO42− available to plants. Different shades of the colour bar representing organic acids shows the concentration of the organic acids produced in the conditions mentioned in the parenthesis. The anions released from the organic acids form salts with the metal cations (Ca+2) resulting in crystalline encrustation on fungal hypha. Under drought conditions NBRI-PR5 accumulates P in the form of polyphosphate granules, which releases soluble P (PO43−) through enzymatic activity. This PO43− is made available to plants in the form of HPO4−2 and H2PO4−1 through different physico-chemical alterations in soil.
Abstract
Phosphate (P) solubilizing fungi contribute considerably in microbial phosphate mobilization. However, effects of different abiotic stresses on P solubilization mechanisms in Trichoderma are largely unexplored. In the present study we selected a P solubilizing Trichoderma to study the mechanism of P solubilization under alkaline and drought conditions. Among 33 Trichoderma isolates (NBRI-PR1–NBRI-PR33), NBRI-PR5 was selected after screening for stress tolerance, antagonistic activity against phyto-pathogens and P solubilization. The selected strain, Trichoderma koningiopsis (NBRI-PR5) was characterized and identified using ITS and tef1 sequencing (Accession no. JN375992). Results show that NBRI-PR5 uses different mechanisms of P solubilization under in-vitro alkaline and drought conditions. NBRI-PR5 produced organic acids for solubilizing insoluble tri-calcium phosphate (TCP) at high pH stress. In drought conditions NBRI-PR5 accumulated poly-phosphate in its mycelia and produced alkaline phosphatase enzyme for P solubilization. The study concludes that T. koningiopsis employs different mechanisms of P solubilization in different stress conditions and therefore, it can be used in management of stressed soils.
Keywords
Phosphate solubilization
Trichoderma koningiopsis
Drought stress
Alkaline stress
1 Introduction
Trichoderma species are ubiquitous soil fungi, known for their rapid growth, capability of utilizing diverse substrates, and resistance to noxious chemicals. They are predominant components of the soil mycoflora in all climatic zones, where they are important decomposers of woody and herbaceous materials, and are also necrotrophic against the primary wood decomposers (Mukherjee et al., 2013; Sharma and Gothalwal 2017). Many species of Trichoderma are of economic importance because of their production of enzymes and antibiotics, or use as biocontrol agents (Harman et al., 2004; Schuster and Schmoll, 2010). They intimately associate with plant root systems and provide biocontrol against soil-borne fungal pathogens, induce plant immunity and promote plant growth (Harman et al., 2004; Druzhinina et al., 2011; Zhang et al., 2018). Trichoderma koningiopsis is a potential species being reported for use in agriculture and industry (Moreno et al., 2009; Saxena et al., 2015; Bordin et al., 2018). The strain NBRI-PR5 used in the present study was probably the first report of T. koningiopsis from India which has been bio prospected for different PGPR attributes including P solubilization (Tandon et al., 2018). Phosphate solubilization by Trichoderma species is reported in-vitro as well as in rhizosphere (Altomare et al., 1999; Kapri and Tewari, 2010; Tandon et al., 2018). There are very few reports showing effect of alkalinity and drought on P solubilization ability of Trichoderma (Halvorson et al., 1990; Rawat and Tewari, 2011). However, P solubilization and the mechanism/s of P solubilization under abiotic stress conditions are previously unreported in T. koningiopsis. Since the available pool of P tends to be extremely low in high pH conditions favouring the formation of insoluble metal PO4−3 such as tricalcium phosphate (TCP), a stress tolerant P solubilizing Trichoderma could ensure P availability in alkaline soils besides providing biocontrol against phyto-pathogens.
Frequent occurrence of alkalinity, sodicity and dry soil conditions in agricultural lands of arid and semi-arid regions threaten agriculture sustainability (Squires and Glenn, 2011; Wheaton and Kulshreshtha, 2017). The area of agricultural lands having salinity, alkalinity and water stress problems is increasing continuously with intensification of agriculture and other anthropogenic activities (Squires and Glenn, 2011; Amanullah et al., 2017). These abiotic stresses affect nutrient availability and their uptake in plants besides making the soil deficient in N, Zn, Fe, Ca, and available P (Hu and Schmidhalter, 2005). Phosphorus (P) is the second most limiting nutrient in soil after nitrogen which is added in the form of phosphatidic fertilizers, a part of which is utilized by plants and the remainder is converted into insoluble fixed forms (Narsian and Patel, 2000). The form and availability of soil phosphorus (P) is highly pH dependent, therefore, even if the total soil P is high or applied regularly as fertilizer, it is rapidly fixed to unavailable forms and accounts for low P use efficiency. In acidic soils (pH < 6.5) phosphorus is fixed mainly by aluminum and iron while in alkaline soils (pH > 7.2) phosphorus is fixed mainly by calcium and magnesium. The available P is also fixed in the microbial biomass which is available to plants only after the death and degradation of the biomass. Thus, the availability of P in soil is largely controlled by biologically mediated processes such as P solubilization, gross mineralization and immobilization rates (Alori et al., 2017). These processes employ different mechanisms which produce mineral solubilizing organic/inorganic compounds such as organic acids, siderophores, protons, hydroxyl ions, CO2, and organic P mineralization through enzymes such as phytases and phosphatases (Alori et al., 2017; Balemi and Negisho, 2012; Gaind, 2016). However, effect of abiotic stresses on the mechanisms of P solubilization is not known. Therefore, the present study was taken up with an objective to identify a Trichoderma strain for phosphate solubilization under unfavorable environmental conditions, such as high pH and low water potential which are common in soils of arid and semi arid regions, and explore the mechanisms of P solubilization under these stress conditions. The results show promising P solubilization ability of T. koningiopsis NBRI-PR5 under stress conditions for its potential future application in sodic soils.
2 Materials and methods
2.1 Isolation, screening and growth media
Endophytic Trichoderma were isolated from rice plants collected from fields in Lucknow and Barabanki as described earlier by Rubini et al. (2005). Standard culture, Trichoderma harzianum NBRI-1055 (MTCC3841) was used as the reference strain at the time of screening (Singh et al., 2007). Trichoderma isolates and pathogenic fungi were grown and maintained on potato dextrose agar (PDA) at 28 °C and at 4 °C respectively. Standard Trichoderma strain and pathogenic fungi Sclerotium rolfsii, Sclerotinia sclerotiorum, Fusarium oxysporum and Alternaria solani were obtained from culture collection of Plant Pathology lab of CSIR-NBRI, Lucknow.
2.2 Screening for biocontrol activity
Biocontrol efficacy of NBRI-PR5 was determined on PDA plates inoculated against different soil-borne fungal plant pathogens following dual culture technique (Dennis and Webster, 1971). Per cent inhibition = [(C-T)/T] * 100, where C and T are radial growth of pathogen in control and dual culture plate resp.
2.3 Phosphate solubilization
All P solubilization experiments were carried out in broth modified from Trichoderma selective media (TSM) (Elad et al., 1981) and NBRIP-BPB broth (Mehta and Nautiyal, 2001), and named as NBRI-Trichoderma selective broth (NBRI-MTSB). NBRI-MTSB contained (per liter): glucose, 4 g; MgSO4·7H2O, 0.2 g; K2HPO4 1.0 g; KCl, 0.15 g; (NH4)NO3, 1.0 g; Ca3 (PO4)2, 5 g. For qualitative screening 0.025 g bromo phenol blue BPB dye was added to NBRI-MTSB broth (Mehta and Nautiyal (2001). An 8 mm bit of Trichoderma isolates were inoculated in 50 ml NBRI-MTSB-BPB broth and incubated at 30 °C with 150 rpm shaking on rotary shaker for qualitative screening. Change in color of the dye indicated lowering of pH and was recorded daily for 7 days. For quantitative P solubilization estimations NBRI-PR5 spore suspension adjusted to 6 log10 units was inoculated at 1% in 50 ml NBRI-MTSB and flasks were incubated at 30 °C at 150 rpm on rotary shaker. Phosphate in culture supernatant was estimated on 3rd, 5th, 7th, 10th, and 15th day of inoculation and values were expressed as equivalent phosphate (µg ml−1) (Fiske and Subbarow, 1925). For stress experiments the pH of the NBRI-MTSB was adjusted with 1 M NaOH before autoclaving to create alkaline conditions. For water stress NBRI-MTSB broth was supplemented with PEG-6000 at 2.5, 5.0, 10, 15 and 20% to induce matric potential (Steuter et al., 1981) and glycerol at 2, 5, 10, 15 and 20% to induce osmotic effect (Dallyn and Fox, 1980). The water potential (ψ) of one set of NBRI-MTSB with different treatments was determined after autoclaving the media at 25 °C using Wescor’s Vapour Pressure Osmometer model no. 5520. Interfering absorbance due to the additives, PEG and glycerol were nullified by substracting their respective blank readings taken against plain NBRI-MTSB broth. The P solubilization was calculated against a standard curve prepared with KH2PO4 (10–100 μg ml−1 concentration). The experiments were conducted in triplicates and values are expressed as their means.
2.4 Morphological and molecular characterization of NBRI-PR5
Selected isolate NBRI-PR5 was morphologically characterized and identified by sequencing rDNA Internal transcribed spacer (ITS) and translation-elongation factor 1 alpha (tef1). Morphological and microscopic characterization of NBRI-PR5 cultures was carried out from cultures grown on PDA, corn meal dextrose agar (CMD) and synthetic low-nutrient Agar (SNA) as described earlier by Samuels et al. (2002). For mycoparasitic interaction study of NBRI-PR5 and Rhizoctonia solani, mycelia from the interaction zone of a dual culture plate was stained with cotton blue dye. Photographs were taken on an Olympus microscope at 10 and 40X magnification. Nessiers staining of the NBRI-PR5 mycelia harvested from the P solubilization experiments at different stress conditions was carried out using Modified Neisser’s metachromatic stain kit from Hi-media following the manufacturer’s instructions.
For ITS based identification PCR amplifications were done in a PTC200 thermocycler (MJ Research, Waltham, MA, USA) with primers ITS1 and ITS4 as described by White et al., (1990). Conditions for PCR amplification were initial denaturation of 1 cycle at 94 °C for 3 min; 30 cycles composed of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s and extension at 72 °C for 1 min; final extension at 72 °C for 10 min. The amplified products were sequenced with same primer pairs by Big Dye sequencing kit (Applied Biosystems, Foster City, USA). Two independent sequencing reactions generated data were examined on the web based molecular biology software Bioedit® (http://www.mbio.ncsu.edu/BioEdit/bioedit/). A final set of ITS sequence data and among vouched sequence data of Trichoderma was subjected to global search in BLASTn in TrichoBLAST for closest homology. The strain was further reconfirmed through the tef1 sequencing which can differentiate the members of Trichoderma koningii aggregate species using the primers EF1-728F and TEF1 rev and PCR conditions as described by Samuels et al. (2006).
2.5 Alkaline phosphatase assay
For determination of intracellular alkaline phosphatase enzyme activity, fungal mats were grown in NBRI-P medium at different pH and drought conditions as described in ‘Phosphate solubilization’ section. Fungal mat was washed at least 10 times with ice-cold distilled water to remove traces of extracellular enzymes and weighed after lightly pressing them between sheets of filter paper. A fine powder of the mat was prepared by crushing in liquid nitrogen and suspended in ice-cold sterilized distilled water. The extract obtained was centrifuged at 12,000 rpm for 20 min to settle the fungal debris. A clear supernatant containing the intracellular enzymes was obtained and made up to a known volume and used for assaying the intracellular alkaline phosphatase and total protein following the method of Tabatabai and Bremner (1969). Total protein in the enzyme extract was determined using method of Bradfords (1976) to express specific enzyme activity.
2.6 Organic acid analysis in broth
Crude culture filtrates were analyzed directly for the presence of organic acids after filtering through 0.22 μm nylon filters as described earlier, Cawthray (2003). All HPLC analyses were conducted by reversed phase chromatography on a 250 mm × 4.6 mm i.d., 5 µm particle C-18 Coloumn, having a UV detector from Cyberlab (Salo Terracce, Millsbury, USA) UV detector. All the chemicals and standards used were of HPLC grade. The mobile phase consisted of 25 mM KH2PO4 adjusted to pH 2.5 with concentrated orthophosphoric acid, and methanol (7%). The flow rate of the mobile phase was 0.7 ml min−1 for all the chromatographic separations. The volume injected was 20 μl of the prepared sample or standard solution.
2.7 Data analysis
Data analyses were carried out using the descriptive statistical analysis tools of windows office excel 2007.
3 Results
3.1 Isolation and screening
Total 33 endophytic Trichoderma isolates were obtained from surface sterilized tissues of rice plants and were named as NBRI-PR1-NBRI-PR33. Based on qualitative method for phosphate solubilization 22 isolates were selected which were further quantified for P solubilization and antagonistic activity against phytopathogens. Results for antagonistic activity, P solubilization, broth pH and mycelia dry weight 7 days post inoculation (dpi) is given in Table 1. Maximum P solubilization (70.79 µg ml−1) was observed by Trichoderma isolate NBRI-PR5 which also showed 100% inhibition of various phyto-pathogens viz S. rolfsii, S. sclerotiorum, F. oxysporum, and A. solani Table 1. Based on the two screening results NBRI-PR5 was selected for further study and characterization. Microscopic study shows mycoparasitic colonization of Rhizoctonia solani by NBRI-PR5 as the mode of biocontrol activity (Fig. 1A). S.r: Sclerotium rolfsii; S.sc: Sclerotinia sclerotiorum; F.o: Fusarium oxysporum; A.s: Alternaria solani. Values are average of three replicates ± standard deviation.
S. No.
Isolate No.
Antagonistic activity (% inhibition)
Phosphate solubilization (7 dpi)
S. r
S. sc
F. o
A. s
Soluble P (μg ml−1)
Broth pH
Mycelia dry wt. (g)
1
PR-1
25.5 ± 1.3
72.72
97.5
100.00
11.2 ± 1.4
4.8 ± 0.2
0.20 ± 0.03
2
PR-2
57.4 ± 1.4
59.09
75.0
44.44
3.0 ± 1.2
5.4 ± 0.1
0.18 ± 0.02
3
PR-5
100.00 ± 0
100.0 ± 0
80.0 ± 1
100.0 ± 0
70.8 ± 1.6
3.6 ± 0.2
0.17 ± 0.04
4
PR-16
68.0 ± 0.8
45.4 ± 0.5
87.5 ± 1.0
100.0 ± 0
1.6 ± 0.9
4.4 ± 0.2
0.22 ± 0.09
5
PR-17
97.8 ± 0.7
77.2 ± 1.1
80.0 ± 0.9
44.4 ± 1.4
7.47 ± 0.8
4.4 ± 0.1
0.20 ± 0.02
6
PR-18
76.5 ± 0.9
54.5 ± 2.1
95.0 ± 1.7
11.1 ± 0.7
17.2 ± 1.1
4.4 ± 0.1
0.20 ± 0.06
7
PR-19
45.2 ± 3.0
61.3 ± 1.5
52.0 ± 2.1
38.5 ± 1.6
6.2 ± 1.3
4.2 ± 0.1
0.23 ± 0.02
8
PR-20
52.5 ± 1.8
48.5 ± 1.2
51.2 ± 2.5
56.2 ± 1.5
5.7 ± 0.9
4.4 ± 0.2
0.23 ± 0.04
9
PR-21
25.5 ± 2.5
70.2 ± 0.5
73.9 ± 1.3
54.3 ± 0.9
9.4 ± 0.8
4.4 ± 0.1
0.20 ± 0.05
10
PR-22
70.2 ± 1.0
50.0 ± 1.7
75.0 ± 0.8
22.2 ± 1.2
9.2 ± 1.2
4.3 ± 0.1
0.20 ± 0.06
11
PR-23
68.0 ± 0.8
27.2 ± 1.7
87.5 ± 1.1
55.5 ± 0.7
10.0 ± 1.4
4.4 ± 0.1
0.21 ± 0.07
12
PR-24
76.5 ± 1.9
45.4 ± 1.5
95.0 ± 1.3
55.5 ± 1.5
5.9 ± 1.1
4.3 ± 0.2
0.14 ± 0.02
13
PR-25
74.4 ± 0.6
31.8 ± 1.1
90.0 ± 2.0
33.3 ± 1.3
3.0 ± 0.9
4.5 ± 0.2
0.24 ± 0.03
14
PR-26
52.4 ± 2.4
43.2 ± 1.6
68.2 ± 1.5
52.2 ± 1.3
1.9 ± 1.5
4.3 ± 0.1
0.28 ± 0.05
15
PR-27
44.0 ± 1.0
48.3 ± 2.5
66.5 ± 1.0
36.5 ± 1.2
6.1 ± 1.1
4.4 ± 0.2
0.22 ± 0.04
16
PR-28
39.2 ± 2.5
58.2 ± 1.3
69.3 ± 2.4
51.3 ± 1.5
1.6 ± 1.2
4.1 ± 0.1
0.23 ± 0.01
17
PR-29
59.6 ± 1.3
38.6 ± 1.5
48.5 ± 1.2
69.5 ± 2.2
3.5 ± 0.7
4.3 ± 0.1
0.26 ± 0.01
18
PR-30
57.5 ± 0.6
69.5 ± 1.1
64.3 ± 0.5
39.6 ± 1.0
4.9 ± 1.4
4.4 ± 0.3
0.24 ± 0.03
19
PR-31
68.8 ± 1.5
77.2 ± 1.5
29.3 ± 2.3
25.5 ± 1.3
7.4 ± 0.7
4.3 ± 0.2
0.20 ± 0.02
20
PR-32
63.2 ± 0.5
58.5 ± 1.2
45.5 ± 1.0
68.5 ± 2.3
7.0 ± 1.4
4.0 ± 0.2
0.17 ± 0.07
21
PR-33
58.9 ± 0.9
45.5 ± 1.1
55.5 ± 0.9
53.5 ± 1.3
1.6 ± 1.2
4.2 ± 0.1
0.12 ± 0.01
22
MTCC3841
62.5 ± 1.1
65.3 ± 1.5
38.5 ± 1.0
39.5 ± 1.1
3.8 ± 1.0
5.4 ± 0.1
0.24 ± 0.04
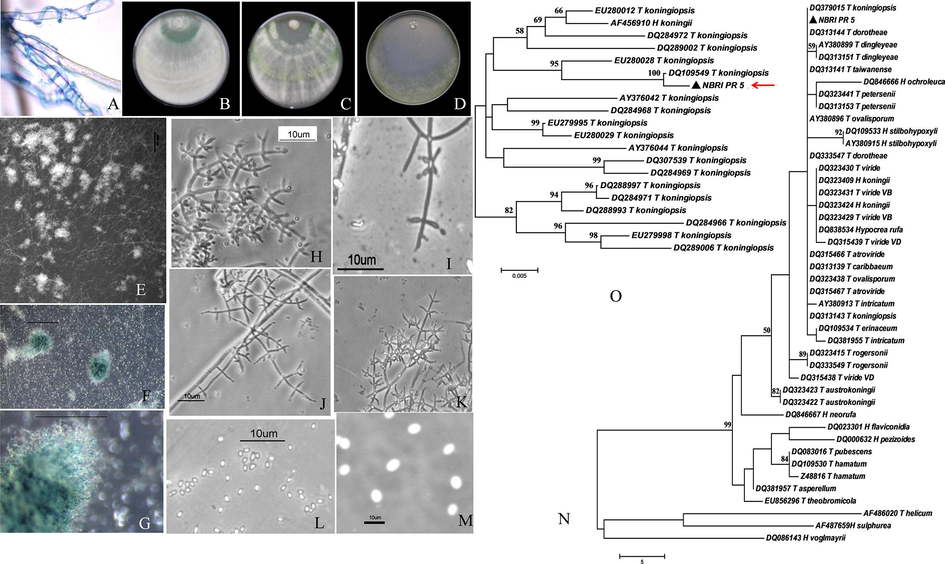
Biocontrol and morphological characterization of T. koningiopsis-NBRI-PR5; Mycoparasitism against Rhizoctonia solani (A); Aerial growth with conidiation pattern on PDA (B), CMD (C) and SNA (D); Hemispherical to spherical pustules (E-G); Gross morphology with primary branch arrangement (H), intercalary phialide (I), developing conidiophores (J), phialides arise in whorls (K); conidia morphology (L-M). ITS based MP tree of T. koningiopsis NBRI-PR5 (N). tef1 based NJ tree of T. koningiopsis NBRI-PR5 (O) Pictures of pustules were taken using Stereomicroscope from Leica (E-G); Pictures of branching arrangement, phialide structure and spores were taken using Phase contrast microscope form Nikon (H-M).
3.2 Morphological characterization and ITS sequencing of Trichoderma isolate NBRI-PR5
Puffy aerial growth with conidiation was observed on PDA after 96 h with 12 h dark and light conditions at 30 °C (Fig. 1B). Appressed aerial growth with conidiation in 2–3 concentric rings having light sweet, coconut odour was observed on CMD (Fig. 1C). Little aerial mycilium, conidia aggregated at margins was observed on SNA (Fig. 1D). Radial growth of 67.5 ± 3.5 mm on PDA 51 ± 4.2 mm on CMD 47 ± 2.8 mm on SNA in dark conditions was observed. No pigmentation diffusing in the agar was observed. Hemispherical to spherical pustules with rough surface less than 1 mm size on CMD was observed (Fig. 1E–G). The primary branch arrangement is pachybasium like that arise at acute angles. The phialides are laginiform mainly with pointed neck, rarely ampuliform and sinuous forms are seen. The phialides arise in the whorls of 3–5 directly on main axis and on supporting cell on primary branches (Fig. 1H, J–K). Despite T. koningi like colony morphology and conidiophore ornamentation, intercalary phialides as diagnostic characteristics of T. koningiopsis were observed (Fig. 1I). Light green conidia elliptical to ellipsoid, rarely sub-globose with smooth surface are present (Fig. 1L–M).
The ITS and tef1 region were nearly 600 and 900 bp single amplicon respectively and any other non specific amplifications were not detected on gel. The BLASTn and TrichoBLAST results on ITS sequence showed 99–100% homology to T. koningiopsis accessions and in particular to vouchered culture T. koningiopsis GenBank accession DQ379015. The tef1 showed 99–100% homology to T. koningiopsis accession DQ109549. Therefore the isolate under present study was designated as Trichoderma koningiopsis NBRI-PR5. The GenBank accession for ITS sequence of T. koningiopsis NBRI-PR5 is JN375992. ITS and tef1 based MP tree of T. koningiopsis NBRI-PR5 is given in supplementry Fig. 1N and O respectively.
3.3 Effect of pH and water stress on P solubilization ability of NBRI-PR5
Under ambient conditions (pH 7.0) NBRI-PR5 was found to solubilize TCP 40 times more efficiently than the standard culture NBRI-1055 (MTCC-3841) (Table 1). P solubilization under stress conditions was carried out in-vitro by using stress inducers such as salt, PEG and glycerol. The osmotic potential (OP) of the NBRIP broth in different stress treatments ranged from −0.78 ± 0.01 Mpa at control conditions to −11.91 ± 0.03 Mpa at 20% glycerol. Addition of PEG in the medium increased the osmotic potential to −1.88 ± 0.02 at 20% concentration while 20% glycerol increased the OP to −11.91 MPa ± 0.03 (Table 2). NBRI-PR5 optimally solubilized P at the pH range of 7 to 9. Decrease of 44% P solubilization was observed at pH 10.0 (47.64 μg ml−1) which sharply declined to 0.37 μg ml−1 at pH 11.0 (Fig. 2) Maximum P solubilization was observed at pH 8.0 which was 17% higher than in control (pH 7.0) (Fig. 2A). The P solubilization by NBRI-PR5 reduced with the increasing concentration of PEG and glycerol (Fig. 2A). Based on the osmotic potential developed by addition of PEG and glycerol, PEG was found to be more potent inhibitor for P solubilization. Correlation of P solubilization with mycelial dry mass (r2 = 0.99) in alkaline condition showed a direct effect of pH on mycelial growth and P solubilization (Fig. 2B). On the contrary, although a progressive decrease in P solubilization was observed with increasing concentration of PEG, fungal biomass remained nearly constant in all the concentrations of PEG with a correlation coefficient of r2 = 0.49 (Fig. 2B). Similarly in presence of lower concentrations of glycerol (2.5 and 5%) P solubilization was considerably reduced while the biomass remained close to the control. These results suggest that different mechanisms of P solubilization may be involved in different stress conditions in the fungi. Under drought stress conditions production of alkaline phosphatase enzyme was observed (Fig. 2A). This is further supported by the correlation observations of alkaline phosphatase activity with P solubilization in alkaline and drought stress conditions where a correlation of the enzyme with P solubilization is observed in presence of PEG (r2 = 0.87) and glycerol (r2 = 0.98) as compared to alkaline condition where r2 = −0.07 (Fig. 2B). These observations are further strengthened from frequency distribution of P solubilization with respect to final pH of the broth, Fig. 3. The Fig. 3A shows the frequency distribution of the P solubized and its corresponding final pH (initial pH 7–11). The hightest P solubilization point (94 µg ml−1) coincides with the lowest pH (3.5 pH) with a correlation coefficient of r2 = 0.968 showing that in alkaline conditions the P solubilization is mainly achieved through acidification of the medium. However, under water stress conditions acidification of the medium does not show any correlation with the soluble P (r2 = 0.543) (Fig. 3B). Besides, the final broth pH is 1–2 units higher in drought stress than in the alkaline stress.
Osmotic potential (OP) of NBRI-modified Trichoderma broth
pH
OP (Mpa)
PEG (%)
OP (Mpa)
Glycerol (%)
OP (Mpa)
7
−0.78 ± 0.01
2.5
−0.89 ± 0.03
2.5
−1.91 ± 0.05
8
−1.02 ± 0.05
5
−0.99 ± 0.02
5
−2.94 ± 0.06
9
−0.98 ± 0.04
10
−1.16 ± 0.01
10
−4.78 ± 0.06
10
−0.87 ± 0.02
15
−1.49 ± 0.04
15
−8.47 ± 0.04
–
–
20
−1.88 ± 0.02
20
−11.91 ± 0.03
–
–
30
−3.59 ± 0.06
30
−15.03 ± 0.05
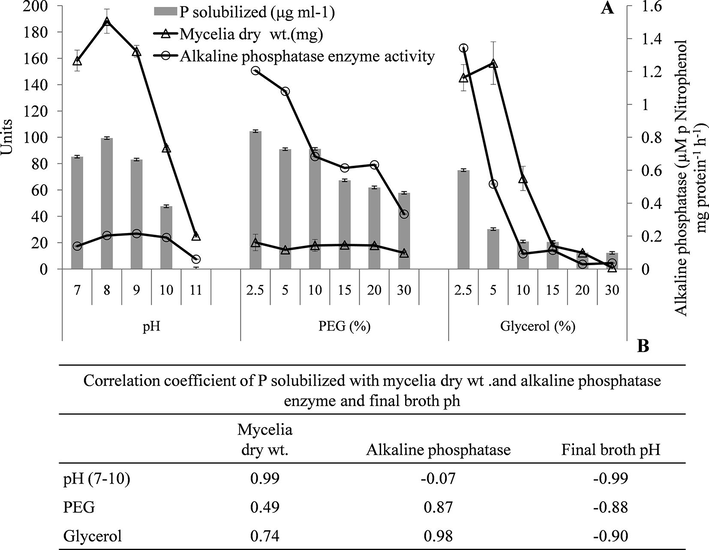
Effect of pH and water stress on Phosphate solubilization (■), fungal biomass (Δ) and alkaline phosphatase enzyme (ο) (A). Correlation coefficient of P solubilized with different parameters of phosphate solubilization in broth conditions (B).
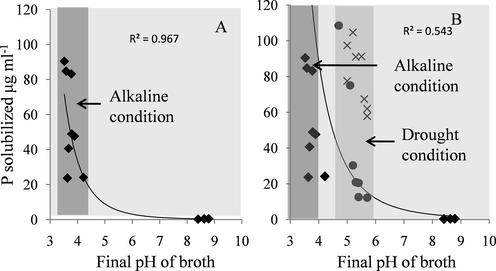
Role of pH in P solubilization under different stress conditions. Frequency distribution of P solubilized with respect to final pH of the broth at an initial pH range of 7.0–11.0 (◆) (A); Distribution of P solubilized with respect to final pH of the broth at a pH range of 7.0–11.0 (◆), PEG concentration 0–30% (×) and glycerol concentration 0–30% (●) (B).Dark vertical strip in A and B shows lower final pH (2.5–4.5) achieved for P solubilization under alkaline conditions. Light vertical strip in B shows higher pH (4.5–6.0) achieved for P solubilization under drought stress indicating presence of alternative mechanisms in alkaline and drought stress for equivalent amounts of soluble P.
3.4 Effect of pH and water stress on organic acid and alkaline phosphatase enzyme production by NBRI-PR5 in broth
HPLC analysis of the organic acids produced by NBRI-PR5 in NBRIP broth showed qualitative and quantitative effects of alkaline conditions. Fig. 4 show chromatograms of blank media, control without TCP (-TCP), control with TCP (+TCP) (pH 7) (blue) and different treatments of pH (8–11), PEG (2.5–20%) and glycerol (2.5–20). Peaks corresponding to oxalic acid (RT 2.70) at pH 8 and two unknown peaks at RT 9.75 and 11.33 at pH 9 were observed which were not detected in other treatments. Organic acid production in presence of PEG and glycerol were highly reduced (Fig. 4B-C). The results show that role of organic acid production in P solubilization is more prominent in alkaline stress than in drought stress.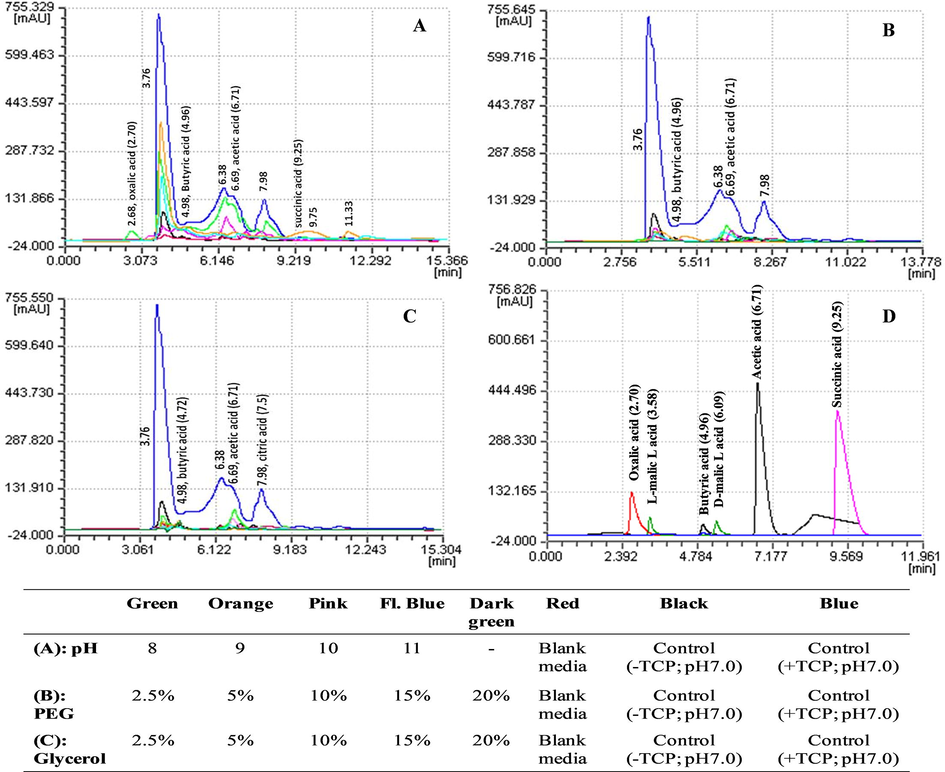
Comparative HPLC chromatograms of organic acids produced by T. koningiopsis NBRI-PR5 at different pH (A), PEG (B) and glycerol (C) treatments. Chromatograms of standard organic acids D. Concentration of standards: 100 mM of oxalic, formic, acetic, citric and succinic acid and 10 mM of d and l malic acid).
Further on, a microscopic examination of the Neisser’s stained mycelia grown in presence of the stress conditions show polyphosphate accumulation in the mycelia (Fig. 5). Neisser’s staining differentially stains hyphae with accumulated polyphosphates in blue-black and the negative hyphae as brown or red. Positive staining was observed with blue to blue-black regions in hyphae in presence of TCP at various pH (inset Fig. 5B-D) and drought conditions (Fig. 5E-K). No staining in the blank (-TCP) treatment (Fig. 5A), and negative staining in presence of high concentrations of glycerol (Fig. 5L) was observed. Encrustation of NBRI-PR5 hyphae with salts was also observed under alkaline conditions (Fig. 5B-D). The P solubilization mechanisms in stress conditions, as infered from the present study in NBRI-PR5 is summurized in graphical abstract.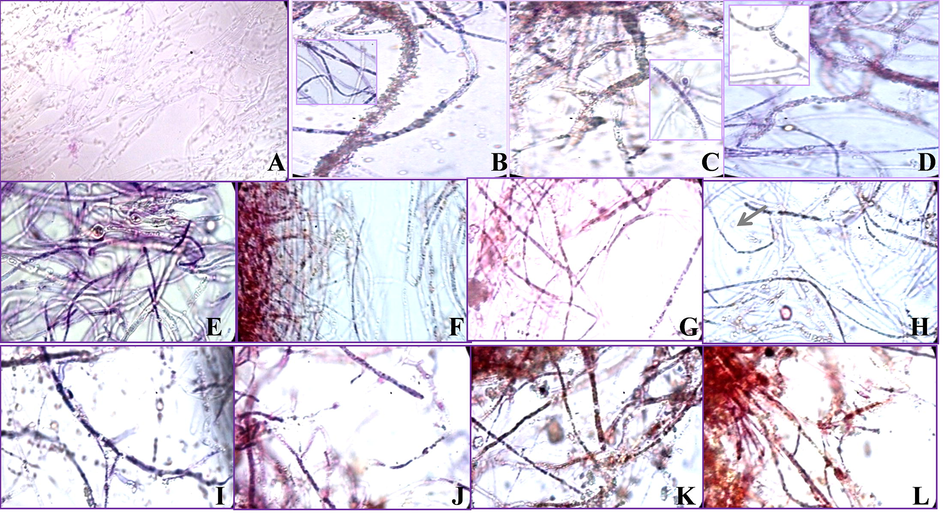
Polyphosphate granules (dark blue stains in hyphae) are absent in the negative control (-TCP) having P starvation conditions (A). Formation of polyphosphate granules was observed in presence of TCP at pH 7–9 (inset of B-D), glycerol 2.5% (E), 5% (F), 10% (G) and PEG 2.5% (I), 5% (J), 15% (K), 25% (K). Polyphosphate accumulation was absent in glycerol concentrations above 15% (H) showing brown staining all the treatments except at higher concentrations of glycerol (H). Encrustation of TCP granules was observed on the mycelia at pH 7.0, pH 8.0 and at pH 9.0 (B-D). Formation of chlamydospore was prominent in the presence of glycerol. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4 Discussion
In the present scenario of changing climate, drought, salinity, alkalinity and sodicity have become important limiting factors to crop productivity (Lobell et al., 2011). Drought and alkalinity affects the microbiome and its functions in the rhizosphere (Santos-Medellín et al., 2017; Walter et al., 2017). Therefore, application of microbes which are functional at abiotic stress conditions is of importance. Phosphate solubilization is one such important property which has commercial importance and can be exploited in the genus Trichoderma for sustainable agriculture. Only few reports show P solubilization under different abiotic stress conditions. Phosphate solubilization by Trichoderma viride in cadmium stress and at a temperature range of 0–45 °C has been earlier reported (Rawat and Tewari, 2011). Phosphate solubilization ability of NBRI-PR5 was optimum over a pH range of 7–9 and nearly 50% ability was retained between pH 9 and 10. A decrease in mycelia biomass observed at pH 10 and above is of major concern as it affects the growth and P solubilization properties of NBRI-PR5. This is applicable for all the P solubilizing bacteria or fungi and emphasises on the importance of screening microbes that can not only survive but also grow and express their functions at these pH conditions.
Since soil alkalinity and sodicity are often related to drought stress the Trichoderma strain NBRI-PR5 was further studied for its P solubilization activity under drought stress conditions induced by PEG and Glycerol. The two solutes were used to induce matric and osmotic potentials respectively which are the most important factors governing water flow and availability for physiological processes in soil (Cook and Duniway, 1980). Osmotic potential is due to solutes in soil water and is important in saline soils or soils amended with fertilisers and organic waste. Matric potential includes both adsorption and capillary effects and is most relevant to growth in soil or on root surfaces. They have been shown to impart significant effects on the activities and germination of various fungi (Jones et al., 2011). Differences in the osmotic (glycerol induced) and matric (PEG induced) treatments were found at the same water potential. The water potential developed by 2.5% of glycerol and 20% PEG were nearly same showing that water potential arising due to matric stress was more deleterious for NBRI-PR5 growth and P solubilization. A progressive decrease in P solubilization inspite of the abundant mycelial growth in presence of PEG and lower concentrations of glycerol suggest physiological and metabolic modifications with respect to P solubilization. However, a progressive decrease in mycelia dry weight and P solubilization with increasing alkalinity shows its direct influence on the growth and organic acid production by NBRI-PR5. Present study elucidates that P solubilization is a complex phenomenon and cannot be explained with one simple parameter of pH lowering. However, it was found to be the major mechanism employed under alkaline condition as evident from the frequency distribution of P solubilized against final pH in different stress conditions. Lowering of pH and organic acid production by Trichoderma has been reported in various studies (Promwee, 2014; Li et al., 2015; Gaind, 2016; Alori et al., 2017). The observation that phosphatase enzyme remained lower in alkaline conditions as compared to water stress condition further confirms that lowering of pH is the dominant mechanism of P solubilization at alkaline stress condition. Different P solubilization mechanisms from organic acid production in small amounts to mechanisms like production of alkaline phosphatase enzymes take part in presence of glycerol and PEG. Phosphate solubilization mediated through redox, chelation (siderophore production) and hydrolysis (phytase and ferric reductase activity) by Trichoderma has been reported by Li et al. (2015). Trichoderma asperellum Q1 has been reported to produce phosphatase enzymes for P solubilization under salt stress which also helped in salt stress tolerance in Arabidopsis (Zhao et al., 2017).
Based on the current study the P solubilization mechanisms present in NBRI-PR5 under alkaline and drought conditions is summarized and elucidated in the graphical abstract. The figure shows the major Pi dynamic equilibrium existing in soil solution in the vicinity of the root surface: A = HPO4−2 (soil solution) ⇌ HPO4−2 (root surface); B = HPO4−2 ⇌ H2PO4−1; C = HPO4−2 ⇌ PO4−3. The organic acid produced by NBRI-PR5 (pH ≥ 6.8) gets dissociated and provides H+ (which disturbs the equilibrium). This H+ shifts the equilibrium towards HPO42− to maintain the magnitude of equilibrium constant (Le Chatelier’s principle) at a given temperature, ie., it provides more and more HPO42− for plant roots. Thus, under alkaline conditions NBRI-PR5 makes P available in the form of HPO42− for plants and helps in the plant growth. As a byproduct the anions released from the organic acids formed salts with the metal cations (Ca+2) which were deposited as crystalline encrustation on some of the NBRI-PR5 hyphae under alkaline conditions. Such type of hypha showing encrustations are reported to provide microenvironment for chemical reactions, crystal deposition, stress tolerance and growth to the fungi (Arocena et al., 2001; Fomina et al., 2005). Microorganisms are known to accumulate polyphosphates in P limiting conditions as an alternative source of energy and P which are further made available through phosphatases like alkaline phosphatase and release orthophosphate (Omelon and Grynpas, 2008). This is evident from the microscopic observations showing NBRI-PR5 to accumulate P in the form of polyphosphate granules, which was probably the source for soluble P released (as orthophosphate) through enzymatic activity. This was found to be prominent under water stress conditions as evident from highest the alkaline phosphatase enzyme activity and maximum soluble P value by NBRI-PR5 at 2.5–10% PEG (23–7% higher than control). Therefore, considering the P solubilization ability of NBRI-PR5 under stress conditions the strain shows promising potential for application in reclamation of degraded soils where such high pH and drought conditions are dominant. Trichoderma has been used as P solubilizer in soil nutrient deficient grasslands for soil management and increasing grassland plant biomass (Zhang et al., 2018)
5 Conclusion
It is concluded from the study that alternative mechanisms for P solubilization are present in Trichoderma koningiopsis NBRI-PR5 which are overlapping and subject to selective induction by environmental conditions. A detailed mechanistic, biochemical and molecular studies are required to understand the environmental conditions controlling the induction of P solubilization mechanisms in Trichoderma koningiopsis NBRI-PR5.
Acknowledgements
The study was supported by In-house project (OLP-0105) of CSIR-National Botanical Research Institute, Lucknow, India. AT, acknowledges Academy of Scientific & Innovative Research (AcSIR), Chennai, 600113, India and fellowship grant from UGC-CSIR.
References
- Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017:8.
- [Google Scholar]
- Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai. Appl. Environ. Microbiol.. 1999;65:2926-2933.
- [Google Scholar]
- Amanullah, Darwish, T., Erpul, G., Horn, R., Nkongolo, N., Brajendra, Pierzynski, G., Peter De Ruiter, Taboada, M., 2017. Threats to soils: global trends and perspectives. A contribution from the intergovernmental technical panel on soils (eds: Pierzynski G and Brajendra). Global Soil Partnership Food and Agriculture Organization of the United Nations. September 2017. https://static1.squarespace.com/static/5694c48bd82d5e9597570999/t/5931752920099eabdb9b6a7a/1496413492935/Threats+to+Soils__Pierzynski_Brajendra.pdf.
- Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J. Soil Sci. Plant Nut.. 2012;12:547-562.
- [Google Scholar]
- Non-toxic bioherbicides obtained from Trichoderma Koningiopsis can be applied to the control of weeds in agriculture crops. Indus. Biotech.. 2018;14:157-163.
- [Google Scholar]
- An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J. Chromat. A. 2003;1011:233-240.
- [Google Scholar]
- Water relations in the life cycles of soilborne plant pathogens. In: Parr J.F., Gardner W.R., Elliot L.F., eds. Water potential relations in soil microbiology. Madison: American Society for Agronomy; 1980. p. :119-139.
- [Google Scholar]
- Spoilage of material of reduced water activity by xerophilic fungi. In: Gould G.H., Corry E.L., eds. Microbial growth and survival in extreme environments. London and New York: Academic Press; 1980. p. :129-139.
- [Google Scholar]
- Antagonistic properties of species groups of Trichoderma I. Production of non-volatile antibiotics. Trans. British Mycol. Soc.. 1971;57:25-39.
- [Google Scholar]
- Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol.. 2011;9:749-759.
- [Google Scholar]
- A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica. 1981;9:59-67.
- [Google Scholar]
- Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol.. 2005;71:371-381.
- [Google Scholar]
- Phosphate dissolving fungi: mechanism and application in alleviation of salt stress in wheat. Microbiol. Res.. 2016;193:94-102.
- [Google Scholar]
- Utilization of calcium phosphates for microbial growth at alkaline pH. Soil Biol. Biochem.. 1990;22:887-890.
- [Google Scholar]
- Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol.. 2004;2:43-56.
- [Google Scholar]
- Drought and salinity: a comparison of their effects on mineral nutrition of plants. J. Plant Nut. Soil Sci.. 2005;168:541-549.
- [Google Scholar]
- Water potential affects Coniothyrium minitans growth, germination and parasitismof Sclerotinia sclerotiorum sclerotia. Fungal Biol.. 2011;115:871-881.
- [Google Scholar]
- Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol.. 2010;41:787-795.
- [Google Scholar]
- Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS One. 2015;10(6) e0130081
- [Google Scholar]
- An efficient method for qualitative screening of phosphate solubilizing bacteria. Curr. Microbiol.. 2001;43:51-56.
- [Google Scholar]
- Biological and molecular characterization of the response of tomato plants treated with Trichoderma koningiopsis. Physiol. Mol. Plant Path.. 2009;74(2):111-120.
- [Google Scholar]
- Aspergillus aculeatus as rock phosphate solubilizers. Soil Biol. Biochem.. 2000;32:559-565.
- [Google Scholar]
- Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev.. 2008;108:4694-4715.
- [Google Scholar]
- Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agric. Sci.. 2014;6(9):8.
- [Google Scholar]
- Effect of abiotic stress on phosphate solubilization by biocontrol fungus Trichoderma sp. Curr. Microbiol.. 2011;62:1521-1526.
- [Google Scholar]
- Azevedo diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of witches' broom disease. Internat. J. Biol. Sci.. 2005;24:24-33.
- [Google Scholar]
- Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia. 2002;94:146-170.
- [Google Scholar]
- Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio. 2017;8 pp. e00764-17
- [Google Scholar]
- Trichoderma species mediated differential tolerance against biotic stress of phytopathogens in. Cicer arietinum 2015:195-206.
- [Google Scholar]
- Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotech.. 2010;87:787-799.
- [Google Scholar]
- Trichoderma: A Potent Fungus as Biological Control Agent. In: Agro-Environmental Sustainability. Springer International Publishing; 2017. p. :113-125.
- [Google Scholar]
- Effect of substrates on growth and shelf life of Trichoderma harzianum and its use in biocontrol of diseases. Biores. Technol.. 2007;98:470-473.
- [Google Scholar]
- Squires, V.R., Glenn, E.P., 2011. Salination, desertification, and soil erosion. The Role of Food, Agriculture, Forestry and Fisheries in Human Nutrition-Volume III. p 102.
- Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Boil. Biochem.. 1969;1:301-307.
- [Google Scholar]
- Effect of Trichoderma koningiopsis on Chickpea Rhizosphere Activities under Different Fertilization Regimes. Open J. Soil Sc.. 2018;8(10):261-275.
- [Google Scholar]
- The effect of pH, electrolytes and temperature on the rhizosphere geochemistry of phytosiderophores. Plant Soil. 2017;418:5-23.
- [Google Scholar]
- Environmental sustainability of agriculture stressed by changing extremes of drought and excess moisture: a conceptual review. Sustainability. 2017;9:970.
- [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., eds. PCR Protocols: A Guide to methods and applications. New York: Academic Press, Inclusions; 1990. p. :315-322.
- [Google Scholar]
- Trichoderma biofertilizer links to altered soil chemistry, altered microbial communities, and improved grassland biomass. Front. Microbiol.. 2018;9
- [Google Scholar]
- Effect of acid phosphatase produced by Trichoderma asperellum Q1 on growth of Arabidopsis under salt stress. J. Integ. Agric. 2017:1341-1346.
- [Google Scholar]







