Phenotypic description and prevalence of Fasciola species in Qena Governorate, Egypt with special reference to a new strain of Fasciola hepatica
*Corresponding author. Address: Biology Department, Faculty of Science, King Khalid University, 9004 Abha, Saudi Arabia abdelnasser@lycos.com (Abdel-Nasser A. Hussein)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
During the present study morphological description was done for the adult flukes and their eggs and information on the prevalence and worm burden per liver were given. From 11 local cows, five imported cows, one local buffalo, and one local sheep, liver have been examined for the abundance. Overall, we collected 254 Fasciola worms from different slaughterhouses in Qena Governorate: 68.89% were morphologically identified as Fasciola gigantica, 30.32% as Fasciola hepatica and two worms (0.79%) were designated as representing a new strain of F. hepatica. The abundance (= worm burden) of F. gigantica in cows was 12 worms/liver, 60 worms/liver in buffalo and 30 worms/liver in sheep. Worm burden of F. hepatica in cows were 9.7 in average. F. hepatica has not encountered in buffaloes or sheep. The F. hepatica new strain was collected from one imported cow. Only one imported cow harboured F. gigantica (five worms), where F. hepatica worms were encountered in three imported cows (worm burden 5, 14, 28) and from five local cows (6 worms/liver). This study documents the existence of two common liver flukes species in addition to a new strain of F. hepatica. Also the size of eggs is not suitable for species identification and the ratio between the size of the egg and the size of the mother worm is not proportional.
Keywords
Phenotype
Fasciola gigantica
Fasciola hepatica
Abundance
Qena
Egypt
1 Introduction
Trematodes of the genus Fasciola have been identified and known since the fourteenth century. The species of Fasciola in Egypt are mainly Fasciola gigantica and Fasciola hepatica (Lotfy and Hillyer, 2003). However, with a large number of imported live animals, which sometimes infected with different Fasciola species, new strains or species may be introduced to Egypt. Many authors have studied the morphology of Fasciola spp. (Beaver et al., 1984; Manson-Bahr and Bell, 1987; Schmidt and Roberts, 1989). Kimura et al. (1984) tried to clarify the species of liver flukes collected from five carabaos in the Philippines according to the shape and size of the adult, but they found that it is very difficult to distinguish the species exactly. A morphological study of adult liver flukes and eggs from Spanish and Bolivian sheeps revealed only slight allometric differences in worms (Valero et al., 1999). Another study done by Valero et al. (2001) in the northern Bolivian Altiplano, revealed that the definitive host species clearly influences the size of F. hepatica adults and eggs, these influences do not persist in a rodent definitive host model. The abundance (= worm burden) is 25.75 fluke per liver which is collected from three sites in Mexico (Rangel-Ruiz et al., 1999).
In Egypt many previous Egyptian investigators have studied Fasciola spp. in Lower Egypt (Ezzat, 1960; El-Sayed, 1977; El-Magdoub et al., 1980). Many new species have been reported in different areas all over the world.
Sinitsin (1933) described Fasciola californica and Fasciola halli from American ruminants. However, Price (1953) examined these forms and found that F. californica indistinguishable from F. hepatica, and F. halli close to F. gigantica in morphology. Sarwar (1957) considered F. indica that described by Varma (1953) in oriental countries, a synonym of F. gigantica, F. tragelaphi in Rhodesia (Pike and Condy, 1966); Gariev (1970) has made the first report of F. indica in USSR. Ali (1993) reported a new species in Assiut Governorate, Egypt.
Lotfy and Hillyer (2003) attempted to identify the species of Fasciola in locally bred animals. Morphologic, morphoanatomic, morphometric and chemotaxonomic criteria of the flukes were studied. Species identity based on morphologic and morphometric data was not decisive due to overlap in the values of most measurements. Morphoanatomic data proved the presence of both species, and isoelectric focusing (IEF) of fluke soluble protein confirmed the presence of both F. gigantica and F. hepatica in Egypt. Periago et al. (2008) had studied the species of Fasciola in Nile Delta, Egypt, and results indicate the presence of F. hepatica, F. gigantica and intermediate forms (Fasciola sp.) in Egypt. The interspecific cross-hybridization between F. hepatica and F. gigantica has been well known for many years and documented by molecular evidence by Agatsuma et al. (2000).
This study aimed to identify and describe the liver fluke (Fasciola) parasitizing buffaloes, cows and sheep in Qena Governorate, Egypt. Also renewing the present information on the prevalence and worm burden per liver.
2 Materials and methods
2.1 Collection of flukes
The adult flukes were collected from naturally infected animals (buffaloes, cows and sheep) that slaughtered in different slaughterhauses in Qena Governorate. Twelve slaughterhouses in Qena Governorate were visited. During the present study, livers from 11 local cows, five imported cows, one local buffalo, and one local sheep were collected (Table 1). Infected livers were identified by their abnormal appearance and then excised using a knife. Liver flukes were removed from bile ducts and gall bladder and washed in saline solution (0.9% NaCl). The flukes from each infected liver were preserved (in 0.9% NaCl) in a labelled plastic container (slaughterhouse, date, slaughtered animal and origin, and number of collected flukes).
| No. | Host | Number collected | Specification |
|---|---|---|---|
| 1 | Cow | 25 | F. gigantica |
| 2 | Sheep | 30 | F. gigantica |
| 3 | Buffalo | 60 | F. gigantica |
| 4 | Cow | 26 | F. gigantica |
| 5 | Cow | 7 | F. gigantica |
| 6 | Cow | 6 | F. hepatica |
| 7 | Cow | 7 | F. gigantica |
| 8 | Cow | 6 | F. hepatica |
| 9 | Cow | 8 | F. gigantica |
| 10 | Cow | 6 | F. hepatica |
| 11 | Cow | 7 | F. gigantica |
| 12 | Imported cow | 28 | F. hepatica |
| 13 | Imported cow | 2 | F. hepatica (new strain) |
| 14 | Imported cow | 5 | F. gigantica |
| 15 | Imported cow | 5 | F. hepatica |
| 16 | Cow | 6 | F. hepatica |
| 17 | Cow | 6 | F. hepatica |
| 18 | Imported cow | 14 | F. hepatica |
| Total | 254 |
2.2 Fixation, staining and examination of flukes
The collected flukes were washed in the saline solution. Fixation and staining were done according to Drury and Wallingten (1980). The parasite was gently flattened between two glass slides. The slides were clamped using a rubber band over each end of slides to maintain the pressure. The slides were then dropped into a beaker containing 10% neutral formalin fixative for about 4 h. The fixed specimens were washed several times with tap water to remove the traces of formalin and to be able to free fixed worms from the slides. Selected numbers of fixed specimens were stained using Kirk Patrick's Carmalum staining method. The stained specimen was transferred to a clear slide and mounted in Canada balsam. Examination of stained flukes was done and the characters of each sample were studied, the length and width of flattened stained Fasciola flukes were measured using a transparent ruler. Other internal structures were measured using an eyepiece micrometer, and were documented by photography.
2.3 Collection, preservation and measurement of eggs
After washing adults with saline solution, this solution contained several eggs, which were preserved in aqueous media “glycerol-jelly”. Intrautrine eggs were extracted from infected livers using a dissecting needle. From each fluke, 50–100 unbroken eggs measured using eyepiece micrometer. These intrauterine eggs were mounted on slides according to Drury and Wallingten (1980). Then, eggs were fixed in 10% neutral formalin. By using a pipette, one drop of the fixative containing eggs was transferred onto a glass slide. The slide was left until the formalin evaporated. Then the eggs were mounted in Kaiser's glycerol-jelly (aqueous media).
The study was carried out after informed consent was obtained from the local authorities in the villages, as well as all participants.
3 Results
3.1 Fasciola species
During the present study different Fasciola species were collected from cows, buffaloes and sheep (Table 1). According to the morphometreic data, out of 254 worms collected, 175 (68.89%) were F. gigantica, 77 (30.32%) F. hepatica and 2 (0.79%) were identified as a new strain of F. hepatica (Table 2). Worm burden of F. gigantica in buffaloes and cows ranged between 5 and 26 (average 12) worms/liver, while in one buffalo it was 60 worms/liver and in one sheep it was 30 worms/liver. Worm burden of F. hepatica in buffaloes and cows ranged between 5 and 28 (average 9.7) worms/liver. Neither in buffalo nor in sheep was F. hepatica encountered. The new strain was encountered only in one cow, which harboured two worms (one immature and one mature) in the biliary tracts (Table 1). Out of 175 F. gigantica collected, 170 (97.1%) were from local hosts and 5 (2.9%) were from imported hosts. While for F. hepatica, 30 (39%) were from local hosts and 47 (61%) were from imported hosts. The new strain was found in 2 imported hosts. Thus, local worms were 200 (78.7%) and imported worms were 54 (21.3) of the total 254 worms collected (Table 2). No mixed infections were encountered.
| Species | Local | Imported | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| F. gigantica | 170 | 97.1 | 5 | 2.9 | 175 | 68.9 |
| F. hepatica | 30 | 39 | 47 | 61 | 77 | 30.3 |
| F. hepatica (new strain) | – | – | 2 | 100 | 2 | 0.8 |
| N | Body size (mm) | Suckers (mm) | Intrauterine eggs (μm) | Host | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | W | V | V | L | W | ||||||||||||||
| Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | ||
| 25 | 36 | 56 | 44.4 | 8 | 12 | 10.1 | 0.7 | 1.19 | 1.12 | 1.54 | 2.24 | 1.83 | 112 | 182 | 145.3 | 56 | 98 | 81.7 | Male cow |
| 30 | 34 | 47 | 41.8 | 9 | 13 | 11 | 0.91 | 1.19 | 1.07 | 1.7 | 2.24 | 1.95 | 112 | 182 | 144.9 | 56 | 98 | 80.1 | Female sheep |
| 60 | 23 | 37 | 28.8 | 9 | 11 | 10 | 0.98 | 1.12 | 1.04 | 1.6 | 1.96 | 1.81 | 126 | 182 | 151.5 | 70 | 84 | 80.1 | Male buffalo |
| 26 | 20 | 39 | 30.4 | 11 | 13 | 11.9 | 0.98 | 1.19 | 1.1 | 1.54 | 2.1 | 1.84 | 140 | 182 | 151.2 | 70 | 98 | 76.9 | Male cow |
| 7 | 25 | 38 | 30 | 9.5 | 11.8 | 11 | 0.7 | 1.19 | 0.98 | 1.6 | 2.1 | 1.68 | 140 | 154 | 150 | 70 | 84 | 74 | Male cow |
| 7 | 35 | 44 | 41 | 10 | 12.8 | 11 | 0.98 | 1.19 | 1.1 | 1.6 | 2.1 | 1.96 | 140 | 168 | 148.4 | 70 | 98 | 77.9 | Male cow |
| 8 | 24 | 32 | 27 | 8 | 9.3 | 8.5 | 0.98 | 1.19 | 1.12 | 1.7 | 2.24 | 2.1 | 112 | 140 | 124 | 70 | 84 | 78 | Male cow |
| 7 | 30 | 36.5 | 33 | 10.2 | 11.8 | 11 | 0.91 | 1.12 | 1.05 | 1.6 | 2.24 | 2.1 | 126 | 154 | 142.8 | 70 | 84 | 75.6 | Male cow |
| 5 | 43.5 | 50 | 47 | 8.8 | 10 | 9 | 0.91 | 1.04 | 0.98 | 1.54 | 2.1 | 1.89 | 140 | 154 | 147 | 70 | 84 | 75.6 | Male imported cow |
| N | Body size (mm) | Suckers (mm) | Intrauterine eggs (μm) | Host | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | W | O | V | L | W | ||||||||||||||
| Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | Mn | Mx | Av | ||
| 6 | 26 | 29 | 27.8 | 11 | 12 | 11.3 | 0.84 | 1.19 | 1.05 | 1.26 | 1.68 | 1.42 | 126 | 168 | 142.7 | 70 | 98 | 79.8 | Male cow |
| 6 | 21 | 28 | 24 | 9 | 12 | 11.5 | 0.98 | 1.19 | 0.98 | 1.19 | 1.4 | 1.26 | 126 | 140 | 129.5 | 70 | 84 | 72.9 | Male cow |
| 6 | 20 | 29 | 24 | 10 | 11.6 | 10.8 | 0.84 | 1.05 | 0.98 | 1.19 | 1.68 | 1.4 | 140 | 154 | 142 | 56 | 70 | 62 | Male cow |
| 28 | 20.5 | 26 | 22 | 10 | 11 | 11.3 | 0.98 | 1.12 | 1.02 | 1.19 | 1.26 | 1.21 | 112 | 140 | 129.5 | 56 | 70 | 65.6 | Male imported cow |
| 5 | 20 | 28.5 | 24.3 | 10.5 | 12 | 11.3 | 0.59 | 0.98 | 0.84 | 1.12 | 1.68 | 1.4 | 126 | 140 | 128.8 | 56 | 70 | 61.6 | Male imported cow |
| 6 | 16 | 20.6 | 18.5 | 9.2 | 11.4 | 10.5 | 0.59 | 1.05 | 0.84 | 1.19 | 1.68 | 1.26 | 112 | 140 | 124.6 | 56 | 84 | 72.8 | Male cow |
| 6 | 20 | 25.3 | 22 | 10 | 12 | 11.6 | 0.98 | 1.19 | 1.05 | 1.12 | 1.68 | 1.4 | 126 | 140 | 131.6 | 56 | 70 | 64.4 | Male cow |
| 14 | 17 | 21.2 | 19 | 9 | 11.6 | 10.8 | 0.84 | 1.19 | 0.98 | 1.12 | 1.4 | 1.2 | 140 | 154 | 144.2 | 56 | 70 | 63 | Male imported cow |
According to the measurements and characters of the different Fasciola spp., the collected flukes were specified as follows.
3.1.1 F. gigantica Cobbold (1856) (Table 3 and Fig. 1)
These flukes are large, leaf-like with parallel margins. The body length varied from 20 to 56 (av 36) mm length and 8 to 3 (av 10) mm width. They have a well-developed cephalic cone followed by indistinct shoulders, which run down into parallel sides ending in rounded posterior end. The whole body surface covered with strong spines which were more concentrated at the cephalic cone. In three medium-sized flukes, papillae were found covering all the body. The cephalic cone measured 2–5 (av 3.7) mm in length. Oral sucker was subterminal and was perforated by the mouth opening. A well-developed muscular ventral sucker was located at the level of the junction of the anterior cephalic region with the rest of the body; it is nearly double the size of the oral sucker. The ventral sucker measured 1.54–2.24 (av 1.19) mm and the oral sucker was 0.7–1.91 (av 1.06) mm in diameter. The ratio between the two suckers was 1.8:1. The alimentary canal begins with the mouth opening surrounded by the oral sucker. It leads to oesophagus, which is usually completely surrounded by a well-developed muscular pharynx, but a short oesophagus rarely follows from the pharynx. The pharynx measures 0.61–0.89 length, 0.58–0.75 width (av 0.74 × 0.65) mm. The intestine bifurcates at the anterior border of the genital atrium leading to two extensively branched intestinal caeca, which extend on the lateral side of the fluke to end blindly near the posterior extremity, the branches nearly at right angles to the main stem. The cephalic portions of the intestinal caeca are characterised by giving four lateral branches. In the cephalic region, medial intestinal branching are either non-evident or rudimentary and they are represented by one or two knob-like secular extensions which start to appear opposite to the posterior two lateral branches. In the body, intestinal caeca are extensively branching laterally into elongated secondary and tertiary branches. On the other hand, medial branches are short, not extensive and inconspicuous. Male genital system begins with two extensively branched testes occurring one above the other obliquely and occupy about middle one-third to two-fourths of the total body length. They are median in position, located nearer to the anterior end than to the posterior end, where the distance between the posterior end of the testes and the posterior end of the body ranging between 7 and 22 (av 12.79) mm. A vas deferens arises from each testis; they unite with each other into a vas deferens a little before entering to the cirrus pouch at the level of ventral sucker, leading to a vesicula seminalis, followed by cirrus and cirrus sac. The cirrus was noticed to be bearing spine-like structures. The male genital system opened through the male genital pore at the left side of genital atrium. Female genital system starts with highly branched ovary at the right side of the ootype; the ovarian branches were less thick than the testicular branches. The ootype may be circular, where its diameter is 0.12 mm. or oval, where its length reaches 0.14 mm and the width is 0.09 mm. However, the ootype was sometimes found just below the ovarian branches. A highly coiled uterus filled with eggs arises from the left anterior border of the ootype and opened with female genital pore in the right side of the genital atrium. The genital atrium is either oval in shape measuring 0.18–0.28 × 0.12–0.14 mm or rounded with a diameter measuring 0.17 mm. The measurements of intrauterine eggs of nine groups of Fasciola worms have done, which were morphologically related to F. gigantica (Table 3). The maximum length of these eggs is 182 μm and the minimum length is 112 μm, at the same time the size of egg has not considered in this study for differentiation between the species, where in the same fluke different size of eggs recorded (Fig. 3). The ratio between the size of eggs and the size of the mother worm was not proportional.
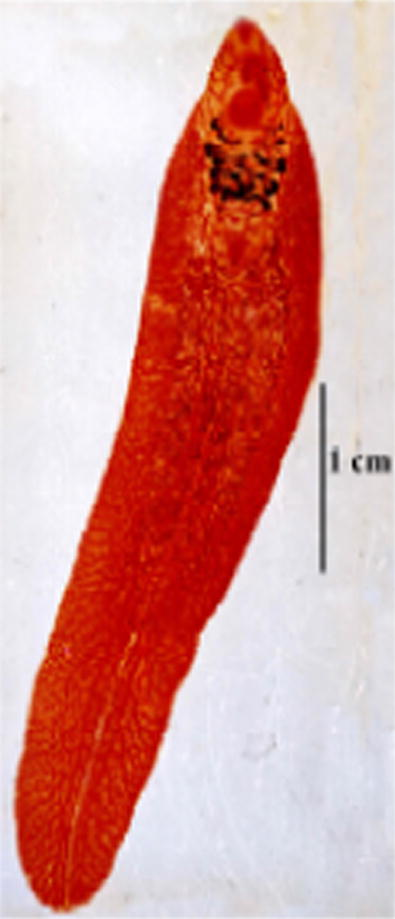
- Photomicrograph of F. gigantica from local animal (male cow).
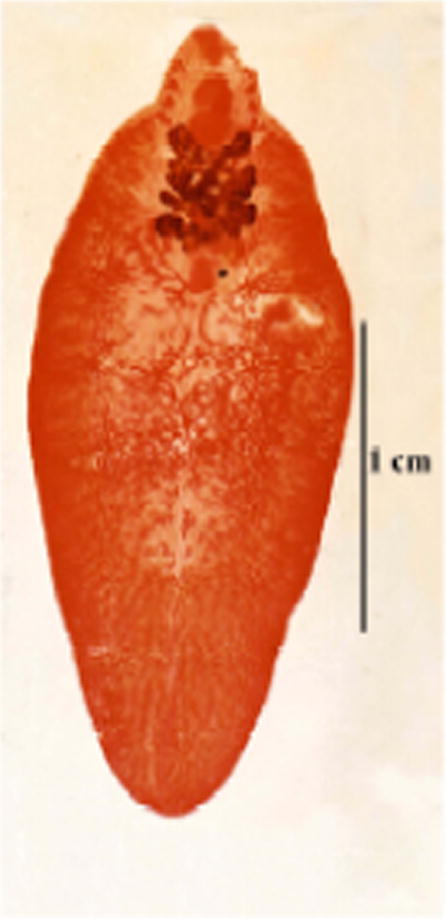
- Photomicrograph of F. hepatica from local animal (male cow).
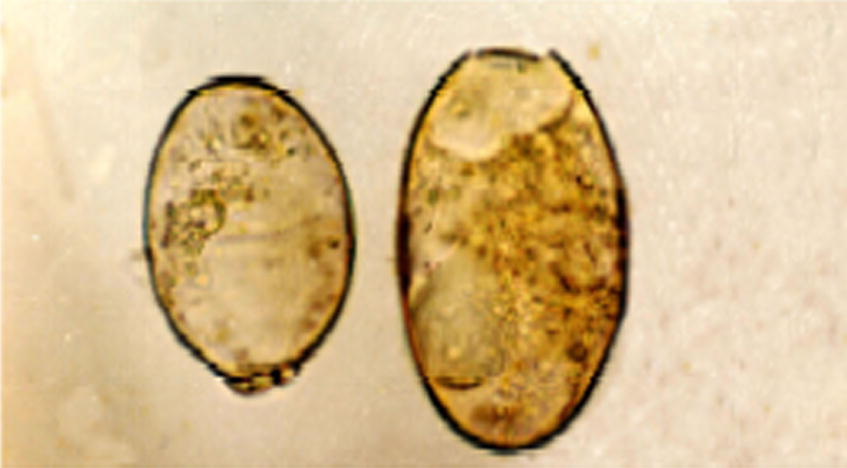
- Photomicrograph of different eggs size from the same fluke 100×.
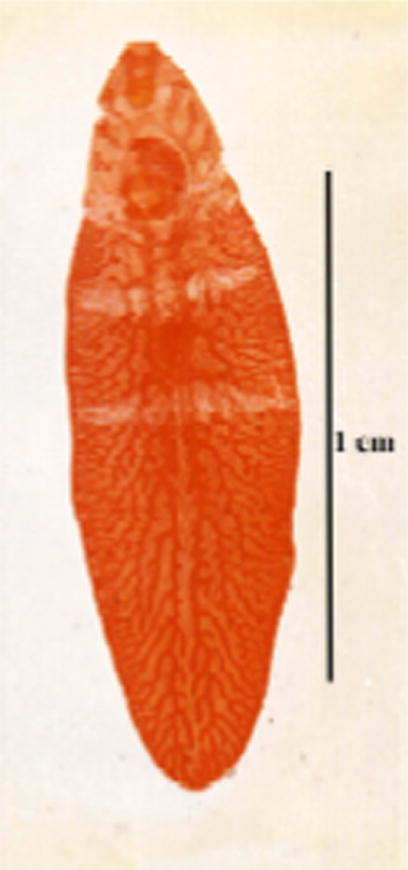
- Photomicrograph of the immature worm of new strain of F. hepatica.
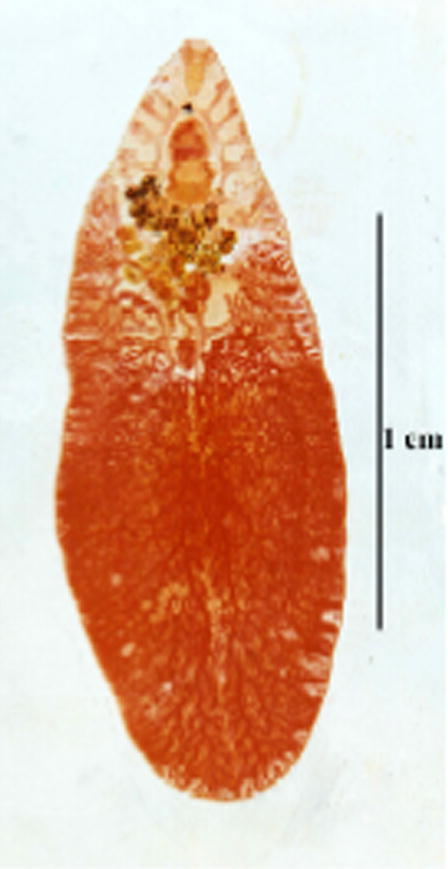
- Photomicrograph of the mature worm of new strain of F. hepatica.
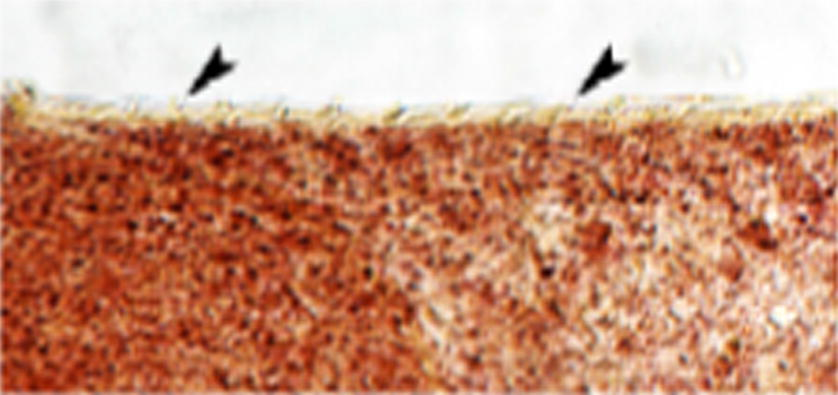
- Photomicrograph of fine spines (arrowheads) on the cephalic cone of F. hepatica (new strain) 100×.
3.1.2 F. hepatica Linnaeus (1758) (Table 4 and Fig. 2)
Adult F. hepatica encountered during the present study measures 17–29 × 9–12 (average 22.7 × 11.6) mm. Its body is leaf-like with well-defined cephalic cone followed by distinct shoulders, which run down into converging sidewalls and ending in a pointed posterior extremity. The tegument is covered by large spines, which were more abundant on the anterior cone. The prominent cephalic cone measured 2–4 mm in length (av 3.39). Below the cone the body is characterised by having its maximum width at a region a little below the shoulders, then the width becomes progressively decreased posteriorly until the pointed end. Suckers are unequal; the oral being relatively smaller than the ventral, the former measures 0.59–1.19 (av 0.97) mm in diameter, while the diameter of the latter varied between 1.12 and 1.68 (av 1.3) mm. Ratio between the two sucker was 1:1.3. The oral sucker is followed by a well-developed muscular pharynx, it measures 1.1–1.7 × 0.8–1.3 (av 1.58 × 1.14) mm. Usually the pharynx surrounds the whole length of the oesophagus, but sometimes a small part of the lower end of the oesophagus was left unsurrounded. Intestinal bifurcation occurred just below or a little below the pharynx, into two branched intestinal caeca that end blindly near the posterior extremity of the body, the branches have backward direction In the cephalic cone, there are usually four lateral branches while in that region, medial intestinal branches could not be seen. In the body, lateral intestinal branches are more pronounced showing secondary and tertiary side branches while medial intestinal branches were rudimentary and were actually difficult to detect. Male genitalia appeared in the form of two highly branched testes, occurring one above the other obliquely and roughly occupying the middle body third. The distance between the posterior end of the posterior testis and the posterior end of the body was 3–8.63 (av 5.56) mm. Vasa efferentia ending in a single vas deferens that enters into the cirrus pouch. The latter is pear-shaped well-developed structure, lying between the upper border of the ventral sucker and the intestinal bifurcation. It opens into the male genital pore occurring at the left side of the genital atrium. Female genitalia start by dendritic branched ovary lying on the right side, which is pretesticular in position. Ovarian branches are usually thicker than testicular ones. A roughly rounded ootype (measuring 1.2–1.6 × 0.9–1.2 (av 1.44 × 1.02) mm is located in the middle line just behind the median ovarian branches or just beside them. A highly coiled uterus full of numerous eggs is usually located between the ootype and the ventral sucker. Female genital pore lies in the right side of the genital atrium just besides the male opening in the genital atrium, which is located just at the upper border of the ventral sucker. Eggs are thin-shelled, yellowish green in colour (bile-stained), operculated and contain one-cell stage embryos surrounded by clusters of yolk cells. Size of eggs varied between 112 and 168 (av 134.1) × 56–84 (av 67.7) μm. The measurements of intrauterine eggs of eight groups of Fasciola worms were done, which were morphologically related to F. hepatica (Table 4). The maximum length of these eggs in five groups of worms does not exceed 140 μm. In two groups it was 154 μm and in only one group, the length reached 168 μm. There was no a direct relationship between the length of eggs and the species of Fasciola, while the ratio between the size of the eggs and the size of the mother worm was not proportional.
3.1.3 F. hepatica (new strain) (Figs. 4–6)
From an imported Friesian cow, one immature (Fig. 4) and one mature worm (Fig. 5) were removed from the biliary tracts. The immature worm was thick and measured 15 mm in length by 4.4 mm in width, and showed a characteristic intestinal branching, a well formed cephalic cone measuring 3 mm in length, oral and ventral suckers, and an ootype and cirrus pouch. However, the body was rather lanceolate-shaped; not showing the characteristic shoulders that usually follow the cephalic cone. The mature worm measured 19 mm in length and 6.5 mm in width (maximum thickness in the testicular region). It has inconspicuous shoulders, sidewalls neither parallel nor converging and posterior end definitely blunt. The tegument was covered with minute spines. Oral sucker was smaller than the ventral sucker measuring 0.91 and 1.19 mm, respectively (ratio between suckers 1:2.1). The pharynx surrounds nearly all the oesophagus except the more posterior part; it measures 1 mm in length and 0.05 mm in width. The intestine bifurcates anterior to the genital atrium into two caeca that run parallel and close to end blindly at the end of the body. The first two branches of the intestinal caecum were less branched than the two branches in the cephalic cone. The medial branches begin behind ventral sucker (ventral sucker found at the end of cephalic cone). The intestinal caeca have secondary and tertiary branches and forming complicated form of branches. Female genital system starts by highly branched ovary at the right side of the body, in pre-testicular position. Ovarian branches have similar thickness with testicular ones. An oval ootype lies in the median line in the beginning of the second third of the body. It measures 0.08 in length and 0.05 mm in width. A long and coiled uterus full of eggs arises from the ootype and ends with female genital opening in the right side of the genital atrium. Male genital system begins with highly branched testes, which occupy a distance of 5 mm (about 1/2 of the total body length). The testes occurring one above the other obliquely and the distance between the posterior end of the testes and the posterior end of the body is 3 mm. From each testis arises vas deferens, united with the other forming vas deferens which enters into the vesicula seminalis that ended with cirrus surrounded with cirrus pouch, the male genital system opened in the left side of the genital atrium with male genital pore. The genital atrium is situated above the anterior border of ventral sucker. Eggs are oval, operculated and bile stained. Size of the intrauterine eggs varied between 126 and 154 (av 141.8) μm in length by 70–84 (av 68.6) μm in width.
4 Discussion
Both F. hepatica and F. gigantica are present in Lower Egypt based on different parameters for species identification (Lotfy et al., 2002; Lotfy and Hillyer, 2003). In Alexandria, Lower Egypt based on the size of the eggs, 51.3% of infections are caused by F. hepatica and 32.65% by F. gigantica but 16.32% have mixed infection (Allam, 1992). The present study agrees with Inoue et al. (2007) that the size of eggs is not a suitable base for species identification, so all worm individual are identified as 68.89% F. gigantica and 30.3% F. hepatica and 0.79% was identified as a new strain. Jansen (1972) and Duewel (1982) reported unusually large-sized F. hepatica eggs in a Dutch strain from sheep, and did not accepted to use egg size in identification of Fasciola species. In the present study, F. hepatica was encountered in three and five imported and local animals, respectively. Worm burden was 5–28 in imported animals and 6 worms/liver in local animals.
Thus the present work recorded – for the first time – the presence of F. hepatica in local animals in Qena Governorate. Differentiation of the two species is accepted because; F. gigantica is longer (Belding, 1965), less prominent shoulders (Kendall, 1965), shorter cephalic cone (Beaver et al., 1984), has caecal branching more branched especially towards the midline of the body (the centipedal branches) (Bhalero, 1935), testes situated more anteriorly (Malek, 1980), ovary was more branched (Looss, 1896), ventral sucker is markedly bigger than oral sucker (Beaver et al., 1984), eggs, bigger than 150 μm and less than 150 μm in F. hepatica (Belding, 1965), moreover, Sahba et al. (1972) pointed out a very important difference that in F. gigantica, the ovarian branches are longer and more numerous, giving a more complicated appearance, but they are smaller and club-shaped in F. hepatica. In differentiating the two species, Varma (1953) has accepted the opinion of Jackson (1921) that, the shape and size cannot count for a great deal in the differentiation due to changes produced by maceration and fixation, unless coupled with distinct anatomical differences. The present work agrees that the length of the eggs should not be considered as an important criterion in differentiation. Each species also showed characteristic distinction regarding the size of cephalic cone, shoulders and sidewalls as well as posterior end. Regarding intestinal branching, Varma (1953) remarked that lateral branches of intestinal caeca in F. hepatica tend to have a pronounced backward direction whereas in F. gigantica they were more or less at right angles to the main stem. Although this character was observed to be true in some specimens of both species collected during the present study, yet it was not confirmed in other specimens. Thus, this character was not a constant one and it needs further studies to be confirmed. Sinitsin (1933) and Varma (1953) felt that the cuticular armature of F. hepatica is formed of scales and not spines, as in F. gigantica. Varma (1953) indicated that, these scales are more elongated and thinner in F. hepatica and stouter and broader at the root in F. gigantica. However, spines on F. hepatica adults were thinner and comparatively smaller than those on F. gigantica during the present work. Papillae on some properly flattened local F. gigantica adults, as well, a special kind of spination is described during the present study on the extruded cirrus of F. gigantica. It seems that these two characters are described for the first time. Regarding suckers, the oral sucker is equal to the ventral sucker in F. hepatica while it is smaller than the ventral sucker in F. gigantica (Brown and Neva, 1983; Schmidt and Roberts, 1989) Actually, in the specimens of the present study, the ratio between oral suckers to ventral sucker was found to be 1:1.3 in F. hepatica but 1:1.8 in F. gigantica. Sinitsin (1933) described F. californica and F. halli from American ruminants, based on distribution and form of cuticular armature and some features in the life cycle. Price (1953) examined these forms and found F. californica to be indistinguishable from F. hepatica, and F. halli and approach F. gigantica morphologically. In 1953, Varma erected F. indica from Indian flukes with intermediate characters. Sarwar (1957) considered F. indica a synonym of F. gigantica and confirmed by Kendall and Parfitt (1959). Pike and Condy (1966) erected F. tragelaphi as a new species in the bile ducts of a Sitatunga (Tragelaphus spekei) from Rhodesia depending on morphology. Ali (1993) described one Fasciola worm which was having mixed morphological characters from both F. hepatica and F. gigantica. Lotfy and Hillyer (2003) did not mention about new species of Fasciola in Egypt.
During the present work, the new strain was obtained from infected liver of an imported cow, those worms have most characteristic morphological features of F. hepatica. However, they showed the following differences: no prominent shoulders, the immature worm was rather lanceolate in shape. Maximum thickness of the mature worm was at the level of testicular region, ventral sucker was much larger than the oral sucker. Ratio between oral to ventral suckers was 1:2.1, and the position of the ootype, situated a short distance below the ovary. One of the present worms was obviously immature while the other was in the process of maturation. This cannot explain the deviation of the position of the ootype and the extraordinary large size of ventral sucker. Also, these deviations cannot be due to the possibility of cross-fertilization between F. hepatica and F. gigantica as was proposed by Price (1953), Stundkard (1957), Ali (1993). Therefore, these worms considered as a new strain of F. hepatica.
In conclusion, there are two valid species; F. hepatica and F. gigantica were present. The first being in Qena Governorate, introduced to Upper Egypt with imported living animals. Moreover, there is no doubt that because of geographic isolation, various species of the parasite might have been developed. So the present study recorded a new strain that is closely related to F. hepatica, that waiting for further investigations and life cycle studies, in addition to PCR Technique.
Acknowledgement
This work is dedicated to the memory of Prof. Dr. Ismael M. Hassan, Zoology Department, Faculty of Science, South Valley University, Qena, Egypt.
References
- Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitology International. 2000;49:231-238.
- [Google Scholar]
- Ali, M.A.H., 1993. Studies on some tissue parasites of some animals used for human consumption. M.Sc. Thesis, Faculty of Medicine, Assiut University, Egypt.
- Allam, A.F.M.B., 1992. Studies on the Lymnaea–Fasciola (Host–Parasite) Relationships in Abis area. Ph.D. Thesis, High Institute of Public Health, Alexandria University, Egypt.
- Clinical Parasitology (9th ed.). Philadelphia: Lea and Febiger; 1984.
- Text Book of Clinical Parasitology (3rd ed.). New York: Appelton Century Grofts Inc.; 1965. pp. 625–640
- Bhalero, G.D., 1935. Helminth parasites of the domesticated animals in India. Imperial Council of Agricultural Research, Scientific Monograph 6.
- Basic Clinical Parasitology (5th ed.). Appleton-Century-Crofts; 1983. pp. 205–212
- Carleton's Histological Technique (5th ed.). Oxford University Press; 1980.
- Unusually large eggs of a Fasciola hepatica strain. Zeitschrift fuer Parasitenkunde. 1982;67:121-124.
- [Google Scholar]
- Distribution of endoparasites among livestock in Egypt. Alexandria Journal of Agriculture Research. 1980;28:419-425.
- [Google Scholar]
- El-Sayed, J.A., 1977. Environmental influence on parasitic infections in Egyptian Buffaloes. M.Sc. Thesis, Faculty of Agriculture, Alexandria University, Egypt.
- Ezzat, M.A.E., 1960. A helminthological investigation on some animals in Dakhla and Kharga Oases. Bull Vet Path Labs No. 256, Ministry of Agriculture, Egypt.
- The first report of Fasciola indica (Trematoda, Fasciolidae) in the USSR. Zoologicheskii Zhurnal. 1970;49:570-571.
- [Google Scholar]
- A case of human fasciolosis: discrepancy between egg size and genotype of Fasciola sp. Parasitology Research. 2007;100:665-667.
- [Google Scholar]
- A revision of the genus Fasciola, with particular reference to F. gigantica and F. nyanzi (Leiper) Parasitology. 1921;13:48-56.
- [Google Scholar]
- New cases of Fasciola hepatica with large eggs. International Journal for Parasitol. 1972;2:281-282.
- [Google Scholar]
- Relationships between the species of Fasciola and their molluscan hosts. Advances in Parasitology. 1965;3:59-95.
- [Google Scholar]
- Studies on the susceptibility of some species of Lymnaea to infection with Fasciola gigantica and Fasciola hepatica. Annals of Tropical Medicine and Parasitology. 1959;53:220-227.
- [Google Scholar]
- Morhphological observation on liver fluke detected from naturally infected carabaos in the Philippines. Science Reports of Faculty of Agriculture Kobe University. 1984;16:353-357.
- [Google Scholar]
- Recherches sur la faune parasitaire de l’ Egypte premiere partie. Memoires de l’Institut Egyptien. 1896;3:1-252.
- [Google Scholar]
- Fasciola species in Egypt. Experimental Pathology and Parasitology. 2003;6/11:9-22.
- [Google Scholar]
- Identification of the Egyptian species of Fasciola. Veterinary Parasitology. 2002;103:323-332.
- [Google Scholar]
- Snail Transmitted Parasitic Diseases. Vol vol. II/132. Boca Roton, Florida: CRC Press Inc.; 1980.
- Manson's Tropical Diseases (9th ed.). London: Bailliere Tindall; 1987.
- First phenotypic description of Fasciola hepatica/Fasciola gigantica intermediate forms from the human endemic area of the Nile Delta, Egypt. Infection, Genetics and Evolution. 2008;8:51-58.
- [Google Scholar]
- Fasciola tragelaphi Sp Nov from the Siatunga, Tragelaphus speckei Rothschild, with a note on the prepharyngeal pouch in the genus Fasciola Linnaeus. Parasitolology. 1966;56:511-520.
- [Google Scholar]
- The fluke situation in American ruminanants. Journal of Parasitology. 1953;39:119-122.
- [Google Scholar]
- Animal fascioliasis in khuzestan, South Western Iran. Journal of Parasitology. 1972;58:712-716.
- [Google Scholar]
- Fasciola hepatica Varma, a synonym of F. gigantica Cobbold. Biologia. 1957;3:168-175.
- [Google Scholar]
- Foundation of Parasitology (4th ed.). Times Mirror/Mosby College Publishing; 1989. pp. 282–287
- Studien Ueber Die Phylogenie Der Trematoden. VI. The life history of some American liver flukes. Zeitschrift fuer Parasitenkunde. 1933;6:170-191.
- [Google Scholar]
- Comparison of adult liver flukes from highland and lowland populations of Bolivian and Spanish sheep. Journal of Helminthology. 1999;73:341-345.
- [Google Scholar]
- Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the northern Bolivian Altiplano hyperendemic region. Veterinary Parasitology. 2001;85:100-102.
- [Google Scholar]
- On Fasciola indica N sp with some observations on Fasciola hepatica and Fasciola gigantica. Journal of Helminthology. 1953;27:185-198.
- [Google Scholar]







