Translate this page into:
Phenolic profile, nutritional potential and biological activities of wildly grown accessions of Cucumis melo var. Agrestis
⁎Corresponding author. hrathore@qu.edu.qa (Hassaan Anwer Rathore)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The aim of the present research work was to investigate the variation in nutritional potential, phenolic profile, antioxidant, and free radical scavenging activities of four wildly grown accessions of Cucumis melo var. Agrestis. The accessions of Cucumis melo var. Agrestis were collected from different areas of the district Faisalabad and Layyah of Punjab province (Pakistan). Mineral profile and amino acid analysis were examined using inductively coupled plasma optical emission spectroscopy (ICP-OES) and an amino acid analyzer. High-pressure liquid chromatography (HPLC) was used for the separation and quantification of polyphenols from chloroform and methanol fractions of Cucumis melo. ICP-OES was used for quantitative and qualitative evaluation of elements, and results showed that magnesium (533.6–663.2 ppm) and iron (308.1–590.8 ppm) were the most abundant minerals. Amino acids analyzer showed that valine (0.03–45.58 mmol/L) and taurine (0.03–2.34 mmol/L) were present in the highest concentration. The methanol extract yield of the accessions was in the range of 14.00–17.03 g/100 g of plant material. HPLC analysis showed the detection of eleven phenolic compounds, and the most abundant compound was chlorogenic acid (1120.53–1237.52 µg/g), followed by gallic acid (188.14–930.74 µg/g) and vanillic acid (6.35–298.81 µg/g). Chloroform and methanol fractions were analyzed for antioxidant activity, and results showed that all the fractions were rich in total phenolic content (TPC) (26.6–112.00 mg/100 g) and total flavonoid content (TFC) (20.5–77.9 mg/100 g). Methanol fraction of accession-1 showed the best free radical scavenging capacity (82.2 %) and % inhibition of linoleic acid peroxidation activity (84.4 %) than other fractions. Statistical analysis revealed significant variation among the nutritional potential and antioxidant activity of all the accessions.
Keywords
Wild melon
Chlorogenic acid
Amino acids
Mineral composition
Polyphenols
Antioxidant potential
1 Introduction
Food, as well as nutritional security, has also lately been identified as one of the world's most persistent problems. Especially in developing nations, low food consumption and limited availability of food remain unsolved concerns (Ercisli and Sagbas, 2017). Moreover, the rapid expansion in the world's population (expected to be 9.6 billion in 2050) caused significant increases in food prices and boosted the land degradation (Meena et al., 2022) that further complicated the food insecurity conditions. So, in view of the present estimates the use of conventional food crops will not be enough to fulfill the world food requirements. Therefore, there is an intense need to explore other non-conventional food sources to accomplish the world food demand and to reduce pressure on conventional food crops (Falade et al., 2020).
The study of underutilized wild plants produced in natural populations can bring new insights that could lead to the introduction of new populations with better composition of secondary metabolites, which are advantageous for various food and pharmaceutical applications (Fallah et al., 2020, Scarano et al., 2021). Furthermore, utilization of mineral, amino acids, and polyphenols-rich food, also called functional food, can provide strength to combat oxidative stress (Iftikhar et al., 2022). Thus, there is a significant scientific interest in the study of underutilized wild plants that are rich in polyphenols to fulfil food insecurity requirements and might be employed in the food and pharmaceutical industries (Stojiljković et al., 2016).
Cucumis melo var. Agrestis, often known as wild melon, is an underutilized vine plant that may grow up to 1.5 m in height. It is an annual or perennial plant and belongs to the Cucurbitaceous family (Hui et al., 2020; Memon et al., 2018). Cucumis melo var. Agrestis exhibits a versatile capacity to grow under various environmental conditions. The cultivation occurs under a consistent temperature range of 25–40 °C (Tanveer et al., 2012). Cucumis melo var. Agrestis fruit are rich in tannins, flavonoids, alkaloids, phenolic components, steroids, resins, terpenoids, glycosides, and saponins (Arunachalam and Chinnaraju, 1970, Memon et al., 2018, Kapoor et al., 2020; Gopalasatheeskumar and Kalaichelvan, 2021). Fruit is also source of important minerals like sodium, potassium, zinc, copper, and iron, as well as some important amino acids (Gopalakrishnan and Thangaraj, 2014). The traditional medicinal uses of Cucumis melo var. Agrestis have inspired many pharmacological studies including anti-inflammatory, diuretic, antioxidant (Naik et al., 1980, Salahuddin and Jalalpure, 2010, Arunachalam and Chinnaraju, 2012, Gopalasatheeskumar et al., 2019, Pratima, 2019). Although few reports on the nutritional potential of Cucumis melo var. Agrestis are available in literature but to the best of our knowledge, no data regarding the variations in phenolic profile, nutritional composition, and biological activities among different accessions of the plant, native to Pakistan is reported. Therefore, the aim of the present study was to investigate the phenolic profile, mineral contents, and amino acid composition along with the antioxidant potential of various accessions of wildly grown Cucumis melo var. Agrestis. Native to Pakistan.
2 Materials and methods
All the chemicals and reagents used in this study were of analytical quality and purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) and Merck (Darmstadt, Germany), respectively, unless otherwise stated.
2.1 Collection, identification, and pretreatment of plant materials
Various accessions of wildly grown Cucumis melo var. Agrestis (fully ripened) were collected from Layyah Desert (30–45 to 31–24 degrees North latitudes and 70–44 to 71–50 degrees East longitudes), South Punjab, Pakistan (Fig. 1). The species were identified and authenticated by a Taxonomist, Department of Botany, Government College University Faisalabad, Pakistan. The fruits were shade-dried for almost a week, ground into fine powder (80-mesh) with a grinder (TSK-949, Westpoint, France), and stored in sealed polythene containers at room temperature for subsequent study.
Fruits of various accessions of Cucumis melo var. Agrestis.
2.2 Nutritional potential
2.2.1 Mineral composition
The crude powdered sample was digested according to the procedure described by (Wolf, 1982) with slight modifications. Briefly, 0.2 g of powdered material in 2 mL of digestion mixture was left overnight. The solution was subjected to heating at 110–200 °C until the vapors generated at that temperature had completely evaporated. Perchloric acid (HClO4) was added until the color of the liquid changed from black to dark brown and then to colorless. The digestion flasks were then allowed to cool, contents were filtered and at the end make the volume up to 50 ml. Then, the samples were analyzed by ICP-OES (Prodigy, Teledyne, USA). Metal solutions (0.5–1000 mg/mL) were prepared, and calibration curves of each metal were drawn.
2.2.2 Amino acid analysis
Amino acid analysis was performed on an amino acid analyzer following the reported method by Iriti et al. (2009). Briefly, 1 g of sample was added to 5 mL of chilled 80 % ethanol and centrifuged (14,000 rpm) for 10 min at 4 °C. Separate the supernatant and mix with 1 mL of 3 % sulfosalicylic acid (SSA) and vortexed. Kept the mixture overnight and centrifuged it under the same conditions as mentioned above. Dried the sample with nitrogen reflux and then added a buffer in the known quantity of the sample. The sample was analyzed on a Biochrom 30 + amino acid analyzer using a lithium-ion exchange column and ninhydrin post-derivatization method. The quantification was done using the standard addition method.
2.3 Preparation of extracts and fractionation
The extraction of the shade-dried and pulverized material (20 g) was subjected to a 500 mL Soxhlet unit for 4 h. A rotary evaporator was used to remove the solvents from sample extracts under low pressure (Hussain et al., 2013). The dried plant extracts were subjected to fractionation sequentially using three different polarity solvents: n-hexane, chloroform, and methanol, using the flow sheet diagram given below (Fig. 2). The yield of the fractions was calculated, and fractions were stored at −4 °C in glass vials for further analysis.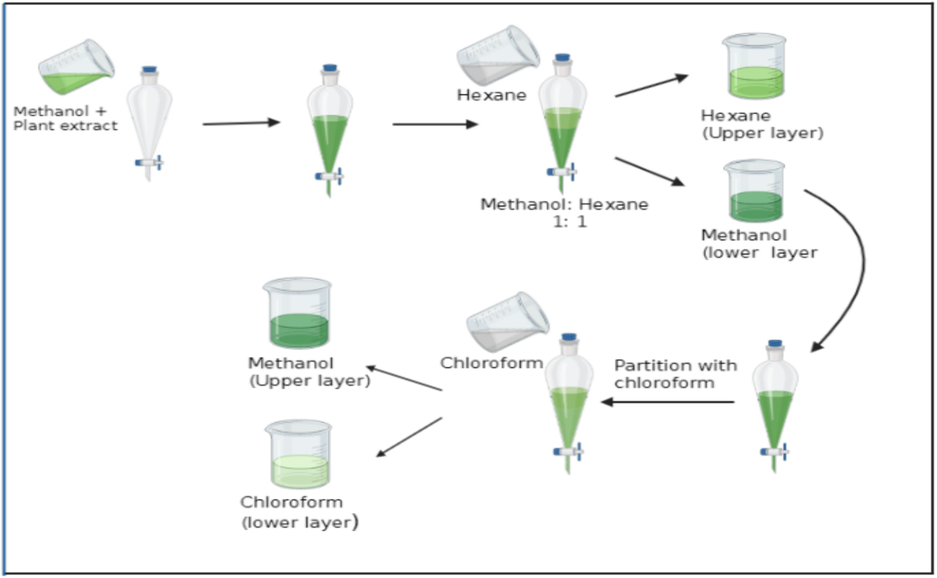
Protocol for the fractionation of extracts of various accessions of Cucumis melo var. Agretis.
2.4 RP-HPLC analysis of phenolic compounds
The analysis of polyphenols from plant extracts was conducted using HPLC with a C18 column, as reported previously by Hussain et al. (2013). The column had dimensions of 250 × 4.6 mm and a particle size of 5 µm. The Flexer Chromera HPLC system from Perkin Elmer, USA, was utilized. It consisted of a binary LC pump and a UV–visible LC Detector from Shelton CT, 06484 USA. System was managed by the software V. 4.2. 6410. The experiment employed a gradient system consisting of solvent A, which was a mixture of acetonitrile and methanol at a ratio of 70:30, and solvent B, which was double distilled water containing 0.5 % glacial acetic acid. Column over temperature was set at 30 °C and UV spectra were taken at 275 nm. The analytes were detected by spiking samples with standards and matching retention times, and further their quantifications were done by using an external standard method.
2.5 Evaluation of antioxidant activity
Antioxidant activities of chloroform and methanol fractions (1 mg/ml) of various accessions of Cucumis melo var. Agrestis were evaluated through various antioxidant activities. The quantity of total phenolic content (TPC) of fractions (10 mg/mL) were figured out using FC reagent as reported by Hussain et al. (2012). The absorbance values at different concentrations of gallic acid were used to construct the calibration curve, and the regression equation (y = 2.7974x + 0.5155; R2 = 0.9826) from the calibration curve was used to figure out the TPC from the extracts. Total flavonoid content (TFC) of chloroform and methanol fractions (20 µg/ml) was studied by using the method reported by Hussain et al. (2012). The calibration curve regression equation, y = 0.7242x − 0.0466; R2 = 0.998, was used to estimate the amount of total flavonoid content in the extracts. This was shown as milligrams of quercetin equivalents (QE) per 100 g weight of dry fruit (mg/100 g). To measure the DPPH radical scavenging activity of extracts (50 µg/mL), the protocol given by Hussain et al. (2011), was followed, and BHA was employed as positive control. Bleachability of β-carotene in the linoleic acid system was evaluated to measure the inhibition using the protocol reported by Hussain et al. (2011). Evaluation of reducing power assay of the fractions was done using a method reported by Hussain et al. (2013).
2.6 Statistical analysis
Three samples were taken from each accession and individually evaluated in a triplicate manner. The information was presented as mean values ± standard deviation. The STATISTICA 5.5 software was used to perform the Analysis of Variance (ANOVA) test. The difference was considered statistically significant if the probability (p) value is ≤ 0.05.
3 Result and discussion
3.1 Nutritional potential
3.1.1 Mineral composition
Comparative mineral composition of the various accessions of Cucumis melo var. Agrestis analyzed in this study are shown in Table 1. ICP-OES analysis revealed that Mg, Fe, Cu, and Cd were the major minerals while Si, Co, Ni, Pb, Cr, Mn, and Zn were the minor in the Cucumis melo var. Agrestis (Table 1). Mg and Fe were the most abundant and major minerals detected from all the accessions. Mg and Fe showed concentration ranges from 533.6–663.2 ppm and 308.1–590.8 ppm, respectively. Co and Cd were also found in major amounts from 39.10–40.16 ppm and 11.27–14.21 ppm, respectively. Other elements, i.e., Si, Co, Ni, and Pb, were present in minor amounts in the accessions. Zn, Cr, and Mn were found in traces and below the limit of detection (<0.12 ppm). Overall, accession-1 showed a significant concentration of essential minerals. Statistical analysis showed significant (p ≤ 0.05) variations in the concentration of elements among various accessions of Cucumis melo. According to numerous studies, magnesium intake from the daily diet is insufficient, and magnesium plays a vital role in our body, especially at the cellular level. While iron is involved in many metabolic processes, and iron deficiency is a serious issue for adults, particularly women (Abbaspour et al., 2014, Fiorentini et al., 2021). So, Cucumis melo var. Agrestis can be the cheapest source of magnesium and iron. In the literature, few reports are available on the mineral content of Cucumis melo var. Agrestis. Fruit contains (mg per total ash): Mg, 15.4; Cu, 1.03; Ca, 19.6; Na, 308; Co, 0.47; P, 10; Fe, 0.28; and Ni, 0.37 (Dahot et al., 1999, Memon et al., 2018). In another report, the major mineral metals component in Cucumis melo var. Agrestis fruit analyzed was Zinc (17.6 ppm), Ferrous (141 ppm), Iron (14.3 ppm), and Copper (13.4 ppm) (Memon et al., 2018). Hence, our results are consistent with prior findings. Various accessions of Cucumis melo var. Agrestis have mineral concentrations even higher than values reported from some of the important Cucurbitaceae plants. The iron concentration is higher than Lagenaria siceraria (118.7 ppm), Citrullus lanatus (211.3 ppm), and Citrullus colocynthis (191.3 ppm). Magnesium concentration is also higher than Citrullus lanatus (123.9 ppm) and Citrullus colocynthis (79.75 ppm), but lower than Lagenaria siceraria (1623.3 ppm). The concentration of copper is also higher than Lagenaria siceraria (1.90 ppm), Citrullus lanatus (31.6 ppm) and, Citrullus colocynthis (20.12 ppm) (Shah et al., 2010, Falade et al., 2020). The values are reported as mean ± SD of three independent experiments. Different superscript letters show significant (p ≤ 0.05) difference among accessions.
Minerals
Concentration (ppm)
Accession-1
Accession-2
Accession-3
Accession-4
Mg
663.2 ± 18.0b
587.2 ± 20.0a
533.6 ± 16.2a
653.1 ± 16.3b
Fe
590.8 ± 13.0c
415.0 ± 18.6b
308.1 ± 12.0a
432.2 ± 20.9b
Cu
39.10 ± 1.06a
40.16 ± 1.02a
39.44 ± 1.84a
39.23 ± 0.98a
Cd
14.21 ± 1.01b
12.34 ± 1.07ab
11.27 ± 0.93b
13.13 ± 0.85ab
Si
3.51 ± 0.23a
3.63 ± 0.20a
4.47 ± 0.31b
3.55 ± 0.25a
Co
1.49 ± 0.91a
1.70 ± 0.15a
1.93 ± 0.10a
1.71 ± 0.13a
Ni
0.68 ± 0.04a
0.69 ± 0.03a
0.66 ± 0.03a
0.72 ± 0.05a
Pb
0.92 ± 0.24b
0.90 ± 0.03b
0.75 ± 0.05a
0.75 ± 0.03a
Cr
< 0.12
< 0.12
< 0.12
0.21 ± 0.02a
Mn
< 0.12
< 0.12
< 0.12
< 0.12
Zn
< 0.12
< 0.12
< 0.12
< 0.12
3.1.2 Amino acid analysis
The amino acid compositions of the accessions of Cucumis melo var. Agrestis were expressed as mmol/L and are presented in Table 2. In total, 17 amino acids were identified, including essential and non-essential ones. The highest values among amino acids were valine (0.03–45.58 mmol/L) and taurine (0.03–2.34 mmol/L) followed by isoleucine (0.03–2.26 mmol/L) and leucine (0.01–2.27 mmol/L). Citrulline (0.01–0.29 mmol) and 4-aminobutyric acid (0.01–0.19 mmol/L) were found in minor concentrations. Among all accessions, sarcosine, threonine, cysteine, and tyrosine amino acids were also identified in small concentrations. Statistical analysis showed significant variation (p ≤ 0.05) in the amino acid composition of all the accessions of Cucumis melo var. Agrestis.Table 3 The values are reported as mean ± SD of three independent experiments. Different letters in superscript represented significant different among the different accessions. The results represent the average of three independent experiments with standard deviations. Different superscript alphabet letters show significant (p ≤ 0.05) difference among accessions.
Amino acids
Retention time (min)
Concentration (mmol/L)
Accession-1
Accession-2
Accession-3
Accession-4
Taurine
4.43
2.34 ± 0.13d
1.85 ± 0.11c
0.60 ± 0.05b
0.03 ± 0.01a
Aspartic acid
16.23
1.29 ± 0.08b
−
0.06 ± 0.01a
−
Threonine
19.50
0.13 ± 0.01c
0.11 ± 0.01bc
0.02 ± 0.00a
0.09 ± 0.01b
Serine
20.83
0.55 ± 0.03c
0.03 ± 0.01a
0.04 ± 0.01a
0.22 ± 0.02b
Asparagine
24.36
0.08 ± 0.01a
0.14 ± 0.01b
0.16 ± 0.01b
1.41 ± 0.08c
Glutamic acid
26.60
0.29 ± 0.01d
0.20 ± 0.10c
0.15 ± 0.01b
0.01 ± 0.00a
Sarcosine
31.76
0.01 ± 0.00a
0.02 ± 0.01a
0.08 ± 0.01b
0.27 ± 0.01c
Citruline
39.40
−
0.29 ± 0.01c
0.01 ± 0.00a
0.07 ± 0.01b
Valine
42.13
8.54 ± 1.02b
10.68 ± 1.06b
0.03 ± 0.01a
45.58 ± 1.27c
Cysteine
51.00
0.01 ± 0.00a
0.01 ± 0.00a
−
0.01 ± 0.00a
Methionine
51.23
−
−
−
0.04 ± 0.01
Isoleucine
54.60
1.86 ± 0.07c
2.26 ± 0.09d
0.03 ± 0.01a
0.20 ± 0.02b
Leucine
55.76
1.85 ± 0.08c
2.27 ± 0.11d
0.01 ± 0.01a
0.20 ± 0.01b
Tyrosine
59.43
0.11 ± 0.01b
0.02 ± 0.01a
0.01 ± 0.01a
0.02 ± 0.01a
β-Alanine
60.46
1.75 ± 0.07b
3.68 ± 0.21c
−
0.30 ± 0.02a
4-Aminobutyric acid
68.33
−
0.19 ± 0.01c
0.01 ± 0.00a
0.04 ± 0.01b
Lysine
81.20
0.02 ± 0.01a
−
−
0.05 ± 0.01b
Fruit Accession
Extract Yield
(g/100 g)
Fractions (g/100 g)
Hexane
Chloroform
Methanol
Accession-1
17.03 ± 1.10b
0.96 ± 0.06b
4.53 ± 0.12c
11.45 ± 0.69c
Accession-2
14.00 ± 1.11a
0.83 ± 0.10a
3.15 ± 0.13a
10.04 ± 0.76bc
Accession-3
15.40 ± 0.81ab
1.84 ± 0.12c
3.92 ± 0.13b
9.65 ± 0.28b
Accession-4
14.70 ± 0.84a
2.35 ± 0.10d
3.24 ± 0.09a
9.09 ± 0.19a
Amino acid analyzer allows far faster and more dedicated analysis of amino acids than prior technologies that used UV or fluorescence detection (Shafaei et al., 2011). Both essential and non-essential amino acids play a crucial function in the human body such as in endothelial cells and non-vascular tissues e.g. arginine, glutamine, alanine, taurine, and glycine enhance CO production through heme oxygenase, also arginine, glutamate, and proline participate in many of the cell signaling pathways (Takahashi et al., 2011). Hence, Cucumis melo var. Agrestis can be the cheapest source of many of the essential and non-essential amino acids. There is limited literature available that reports the identification and quantification of amino acids in Cucumis melo var. Agrestis. To the best of our knowledge, no data has been published that reports the free amino acid content in various accessions of Cucumis melo var. Agrestis.
3.2 Yield of fruit extract
The extracts yield from the various accessions of Cucumis melo fruits was 14.00–17.03 g/100 g of dry plant material (Table 1). Yields of hexane, chloroform, and methanol fractions were in the range of 0.96–2.35, 3.15–4.53, and 9.09–11.45 g/100 g of dry plant material, respectively. Overall, the yield of extracts from different fractions increased in the manner of low-polarity to high-polarity solvents. The Cucumis melo fruit extracts exhibited significant concentrations of polar compounds, including flavonoids and phenolics. Gopalasatheeskumar and Kalaichelvan, (2021), reported the percentage yield of Cucumis melo var. Agrestis was 18.22 % with the cold maceration extraction method. There is currently no literature that provides information on the yield of distinct solvent-based fractions from various accessions of Cucumis melo var. Agrestis. A minimal difference in extract yield could be attributed to the availability of various extractable components using different solvents and extraction techniques (Hussain et al., 2012, Iqbal et al., 2013).
3.3 Phenolic acid and flavonoid profile
Polar fractions of accessions of Cucumis melo var. Agrestis were qualitatively and quantitatively analyzed for phenolic acid and flavonoids using the RP-HPLC and results are presented in Table 4. Phenolic acids include caffeic acid, chlorogenic acid, ferulic acid, gallic acid, hydroxybenzoic acid, kaempferol, p-coumaric acid, quercetin, sinapic acid, salicylic acid, vanillic acid, While Gallic acid (188.14–930.74 µg/g) and vanillic acid (6.35–298.81 µg/g) were the major phenolics in all the accessions. p-coumaric acid (33.28 µg/g), kaempferol (3.86–563.24 ppm) and quercetin (9.77–239.02 µg/g) were present in moderate concentrations. Small amounts of caffeic acid and sinapic acid were detected. In comparison, approximately all the accessions are found with the same number of phenolic compounds, while accession 1 has a comparatively smaller number of phenolics. Sinapic acid was only found in accession 3 and 4. HPLC chromatograms of accession 3 and 4 chloroform, and methanol fractions are shown in Fig. 3. The results represent the average of three independent experiments. The SD was <10 % in all the values. Different superscript letters show significant (p ≤ 0.05) difference among fractions.
Compounds
Concentration (µg/g)
Accession-1
Accession-2
Accession-3
Accession-4
CH3Cl
CH3OH
CH3Cl
CH3OH
CH3Cl
CH3OH
CH3Cl
CH3OH
Chlorogenic acid
−
−
−
1237.52a
−
1120.53a
−
−
p-cumaric acid
−
511.84d
88.12b
−
−
−
138.10c
33.28a
Gallic Acid
308.33c
188.14a
384.33d
217.31b
654.2f
328.45c
422.74e
930.74 g
Hydroxy benzoic acid
−
−
−
33.45
−
−
−
−
Caffeic Acid
7.76b
−
−
−
3.61a
−
3.61a
−
Vanillic acid
27.64c
6.35a
68.25d
−
−
20.49b
298.81e
−-
Kaempferol
−
−
39.96c
12.36b
−
13.40b
563.24d
3.86a
Ferulic acid
12.75a
−
−
−
43.72b
−
−
56.86c
Sinapic acid
−
−
−
−
9.44b
−
−
1.93a
Salicylic acid
1.89a
−
257.88c
−
13.27b
−
−
−
Quercetin
9.77a
−
−
167.32c
−
−
27.73b
239.02d
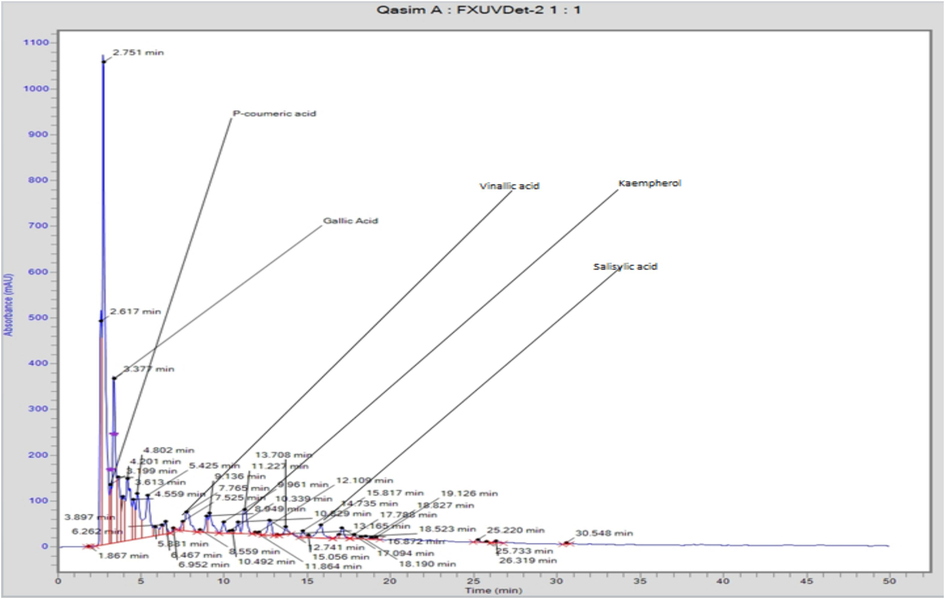
Typical Chromatogram showing the separation of phenolic acids and flavonoids from chloroform fraction of accession 4 of Cucumis melo.
Dietary phenolics are garnering a lot of attention now because of their potential antioxidative and anticarcinogenic effects. Free radical scavengers, reducing agents, and scavengers of singlet oxygen production are other roles played by phenolic compounds (Ghasemzadeh and Ghasemzadeh, 2011). Hence, many phenolic compounds in Cucumis melo var. Agrestis depicts the nutritive value of this cheap fruit. A literature study showed that Cucumis melo var. Agrestis has different phenolic acids in its fruit and seeds. Vanillic acid is the major phenolic compound 2.3–2.9 % in it, followed by callistephin and catechin (Mariod and Matthaeus, 2008). Furthermore, there is a lack of data on the detection and measurement of phenolic compounds in the fractions of various Cucumis melo var. Agrestis accessions.
3.4 Antioxidant activity
3.4.1 Total phenolic and total flavonoid contents
The total phenolic contents of the extracts were estimated using a calibration curve and represented as mg of gallic acid equivalents (GAE) per 100 g of dry weight of fruit (mg/g), as shown in Table 5. TPC of chloroform (CH3Cl) and methanol fractions (CH3OH) ranges from 26.6–112.0 mg/100 g of dry fruit material measured as GAE. Accession 1 showed highest while accession 2 showed the lowest TPC. Total flavonoid contents (TFC) in chloroform and methanol fractions of various accessions of Cucumis melo var. Agrestis were determined and reported in Table 5 as mg/100 g of dry material, measured as QE. TFC of chloroform (CH3Cl) and methanol fractions (CH3OH) ranges from 20.5–77.9 mg/100 g of dry fruit materials, measured as QE. Total flavonoid contents were highest in accession 1, while lowest in accession 3. Our results showed variation in the TPC and TFC of different fractions of Cucumis melo var. Agrestis accessions were significant (p ≤ 0.05). The values are reported as mean ± SD of three independent experiments. Different superscript alphabet letters show significant (p ≤ 0.05) difference among fractions.
Antioxidant Assays
Accession-1
Accession-2
Accession-3
Accession-4
Standard
CH3Cl
CH3OH
CH3Cl
CH3OH
CH3Cl
CH3OH
CH3Cl
CH3OH
BHA
TPC (mg/100 g)α
80.3 ± 2.1d
112.0 ± 4.8f
26.6 ± 1.1a
68.2 ± 1.9c
72.2 ± 2.8c
98.4 ± 3.1e
62.3 ± 2.2b
105.3 ± 2.5f
−
TFC (mg/100 g)β
47.4 ± 1.4d
77.9 ± 1.6f
34.2 ± 1.0b
52.2 ± 1.6e
20.5 ± 1.0a
48.5 ± 1.5d
38.2 ± 1.0c
75.2 ± 2.1f
−
DPPH (% RSA)γ
75.2 ± 2.2bc
82.2 ± 2.6d
62.3 ± 2.6a
74.3 ± 2.8b
58.5 ± 1.9a
76.4 ± 2.8bc
70.2 ± 2.7b
78.2 ± 1.1c
97.1 ± 2.5e
Inhibition of linoleic acid peroxidation (%)δ
77.2 ± 2.0bc
84.4 ± 3.0c
65.0 ± 2.1a
71.2 ± 2.9ab
68.4 ± 1.9a
80.2 ± 2.2bc
70.1 ± 1.8a
75.2 ± 2.6b
94.3 ± 2.0d
Phenolics are present in nearly all plants, and their antioxidant property has important physiological and morphological benefits. As a result, quantifying phenolic content and assessing its contribution to oxidative stress is important (Iftikhar et al., 2022). Limited literature exists regarding the TPC and TFC of Cucumis melo var. Agrestis fruit; however, no report has yet been compiled that conducts a comparative analysis of the fractions from different accessions (Arora et al., 2011, Gill et al., 2011, Sahithi et al., 2015, Memon et al., 2018). According to the literature, the fruit of Cucumis melo var. Agrestis contained 19.82 mg/g TPC, calculated as GAE, and 18.39 mg/g TFC, calculated as QE (Gopalasatheeskumar and Kalaichelvan, 2021).
3.4.2 DPPH radical scavenging assay and inhibition of linoleic acid peroxidation
The DPPH radical scavenging antioxidant assay was used to assess the antioxidant activity of chloroform and methanol fractions (50 µg/mL) of various accessions of Cucumis melo var. Agrestis in vitro, while the results are reported as % radical scavenging activity (% RSA) in Table 5. All the extracts were able to quench 58.5–82.2 % DPPH free radicals. Accession 1 showed the best radical scavenging capacity (82.2 %). Methanol (CH3OH) fraction generally showed significantly higher RSA than Chloroform (CH3Cl) fractions. Antioxidant properties are basically influenced by phenolic components, and the highest activity of accession 1 methanol extract may be correlated with the highest TPC and TFC of the extracts. Due to an increase in the concentration of phenolic components, the DPPH free radical scavenging ability of extracts rises as its concentration rises (Iftikhar et al., 2022).
Literature review showed that there is no data available on DPPH free radical scavenging activity for fractions of various accessions of Cucumis melo var. Agrestis. According to Arora et al., 2011, seed extract had a strong DPPH scavenging action of 75.59 % at 300 µg/mL and 69.86 % at 400 µg/mL. The percent inhibition of linoleic acid peroxidation by different fractions of Cucumis melo var. Agrestis accessions (10 mg/mL) are shown in Table 5. Accessions 1 and 3 showed the highest while accession 2 showed the lowest percent inhibition. Percent inhibition in linoleic acid peroxidation by various accessions fractions of Cucumis melo var. Agrestis ranged from 65.01–84.35 %. Methanol (CH3OH) fractions of all the accessions showed significantly higher percent inhibition of linoleic acid peroxidation than Chloroform (CH3Cl) fractions. The result showed that fractions rich in TPC and TFC inhibited linoleic acid peroxidation to a greater extent. No earlier reports are available regarding the data availability on percent inhibition in the linoleic acid system assay for Cucumis melo var. Agrestis fractions.
3.4.3 Evaluation of reducing power assay
The trends of reducing potentials of different factions of Cucumis melo var. Agrestis accessions are presented in Fig. 4. The figure illustrates a positive correlation between absorbance and the antioxidant activity and reduction potential of the samples. At concentrations of up to 10 mg/ml, the reducing power of various fractions of Cucumis melo var. Agrestis accessions are evaluated. The reducing potential of samples was proportional to their extract concentration. The reducing power of different fractions of extracts lowered in the order: Standard (Ascorbic acid) ˃ accession 1 methanol ˃ accession 1 chloroform ˃ accession 3 chloroform ˃ accession 2 methanol ˃ accession 2 chloroform ˃ accession 4 methanol ˃ accession 3 methanol ˃ accession 4 chloroform. When compared with ascorbic acid (standard), all the fractions showed significantly (p ≤ 0.05) less reducing power.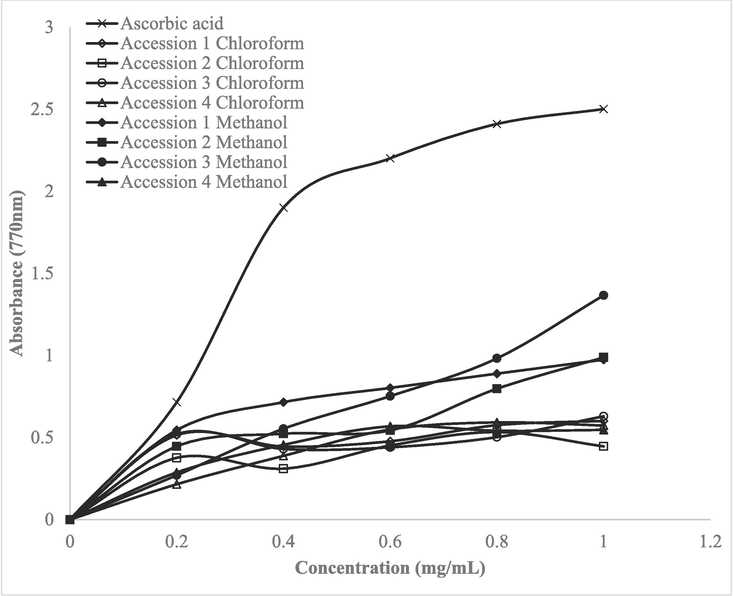
Evaluation of reducing power of different fractions of Cucumis melo var. Agrestis accessions.
Limited data are available on the ferric reducing power of Cucumis melo var. Agrestis. In a previous study, it was reported that the ferric reducing power of fruit extract was 30.77 µg/mL, while for seeds, 28.13 µg/mL was calculated in terms of ascorbic acid equivalent (Gopalasatheeskumar and Kalaichelvan, 2021). However, there is no report available for ferric reducing power on different fractions of various accessions of Cucumis melo var. Agrestis.
3.5 Conclusion
The present study highlighted the untapped potential of Cucumis melo var. Agrestis, a wild fruit rich in minerals, amino acids, and phenolic compounds. It can be concluded that all the accessions were rich in important minerals and amino acids. Magnesium and iron were the most abundant minerals found in all the accessions. Other minerals detected in high concentration were copper, cadmium, silicon, and cobalt. A range of both essential and non-essential amino acids were detected from all the accessions. The major amino acid was valine and accession 4 has maximum concentration of valine followed by accession 2 and 1. The amino acids found in Cucumis melo var. Agrestis are essential for protein synthesis, metabolic activities, and overall good health, highlighting the promising potential of this fruit as a functional food ingredient. Moreover, HPLC study revealed presence of 11 phenolic compounds. Qualitative and quantitative variations in the phenolic acid and flavonoid contents of all the accessions were recorded. Chlorogenic acid was the most abundant phenolic acid identified in accession 2 and 3. Gallic acid was detected in all the accessions. Kaempferol and quercetin were highest in accession 4 followed by accession 2 of Cucumis melo. All the accessions exhibited antioxidant activity in terms of TPC, TFC, DPPH radical scavenging, inhibition of linoleic acid peroxidation and reducing potential. Methanol fraction of accession 1 showed the highest TPC and TFC and thus best DPPH radical scavenging and percent inhibition of linoleic acid peroxidation activities. extracts, as compared to the chloroform fractions. These results provide good evidence to explore Cucumis melo var. Agrestis, as potent agents for the antioxidant and nutritional properties and can be utilized for food products. Further research can be conducted to investigate the antioxidant potential of Cucumis melo extracts in the various food systems.
Funding
None.
CRediT authorship contribution statement
Hira Zulfiqar: Writing – original draft, Methodology, Investigation, Formal analysis. Abdullah Ijaz Hussain: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization. Qasim Ali: Writing – original draft, Software, Conceptualization. Hassaan A. Rathore: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization. Iqbal Ahmed: Writing – original draft, Supervision, Software, Methodology.
Acknowledgments
The authors express their gratitude for the support provided by the Central Hi-Tech Lab at Government College University Faisalabad, Pakistan, in the characterization of extracts. Resources made available by College of Pharmacy, QU Health, Qatar University are greatly acknowledged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Review on iron and its importance for human health. J. Res. Med. Sci.. 2014;19(2):164.
- [Google Scholar]
- Antioxidant activity and pharmacological evaluation of Cucumis melo var. Agrestis methanolic seed extract. R. J. Phytochemistry. 2011;5(3):146-155.
- [Google Scholar]
- Preliminary phytochemical analysis and cytotoxic potential of Cucumis trigonus Roxb. J. Complement. Med. Res.. 1970;1(1):13.
- [Google Scholar]
- Preliminary phytochemical analysis and cytotoxic potential of Cucumis trigonus Roxb. J. Intercultural Ethnopharmacol.. 2012;1:13-18.
- [Google Scholar]
- Nutrient composition of chibber fruit. Commun. Soil Sci. Plant Anal.. 1999;30(1–2):75-82.
- [Google Scholar]
- Nutrient composition of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) and egusi melon (Citrullus colocynthis (L.) Schrad.) seeds. Agric. Conspec. Sci.. 2020;85(1):43-49.
- [Google Scholar]
- Chemical compositions and antioxidant activity of essential oil of wild and cultivated Dracocephalum kotschyi grown in different ecosystems: a comparative study. Ind. Crop. Prod.. 2020;143:111885
- [Google Scholar]
- Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrient.. 2021;13(4):1136.
- [Google Scholar]
- Flavonoids and phenolic acids: role and biochemical activity in plants and humans. J. Med. Plant. Res.. 2011;5(31):6697-6703.
- [Google Scholar]
- Evaluation of therapeutic potential of traditionally consumed Cucumis melo seeds. Asian J. Plant Sci.. 2011;10(1):86-91.
- [Google Scholar]
- Comparative phytochemical screening of the fruits of Cucumis trigonus Roxb. and Cucumis sativus Linn. World J. Pharm. Pharma Sci. (WJPPS). 2014;3(4):1455-1468.
- [Google Scholar]
- Confirmation of best method for detection of alpha amylase activity of Cucumis melo Var Agrestis (Wild Musk Melon): in vitro antidiabetic activity of Wild musk melon. J. Ad. Res. Biochem. Pharma.. 2019;2(1):18-20.
- [Google Scholar]
- Antioxidant potential of different parts (leaves, stem, fruit, seed, flower and root) extracts of Cucumis melo var agrestis. Int. J. Pharm. Sci. Res.. 2021;12(1):465-469.
- [Google Scholar]
- Characterization of the complete chloroplast genome of Cucumis mismelo L. var. Agrestis Naud. Mitochondrial DNA Part b.. 2020;5(3):2744-2745.
- [Google Scholar]
- Antioxidant attributes of four Lamiaceae essential oils. Pak. J. Bot.. 2011;43(2):1315-1321.
- [Google Scholar]
- Effect of extraction techniques and solvent systems on the extraction of antioxidant components from peanut (Arachis hypogaea L.) hulls. Food Anal. Methods.. 2012;5:890-896.
- [Google Scholar]
- Phenolic profile and antioxidant activity of various extracts from Citrullus colocynthis (L.) from the Pakistani flora. Ind. Crops Prod.. 2013;45:416-422.
- [Google Scholar]
- Effects of polyphenol-rich traditional herbal teas on obesity and oxidative stress in rats fed a high-fat–sugar diet. Food Sci. Nut.. 2022;10(3):698-711.
- [Google Scholar]
- Iqbal, T., Hussain, A.I., Chatha, S.A.S., Naqvi, S.A.R., Bokhari, T.H., 2013. Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani flora. J. Anal. Method. Chem. 536490.
- Nutritional traits of bean (Phaseolus vulgaris) seeds from plants chronically exposed to ozone pollution. J. Agric. Food Chem.. 2009;57(1):201-208.
- [Google Scholar]
- Phyto-pharmacological aspects of Cucumis melo var. Agrestis: a systematic review. Phcog Rev.. 2020;14(27)
- [Google Scholar]
- Fatty acids, tocopherols, sterols, phenolic profiles, and oxidative stability of Cucumis melo var. Agrestis Oil. J. Food Lipid.. 2008;15(1):56-67.
- [Google Scholar]
- Long-term nutrient management o=in an intensive rice-wheat cropping system improves the quantities, qualities, and availability of soil sulfur. Front. Sustain. Food Syst.. 2022;6
- [CrossRef] [Google Scholar]
- Nutritional profile and medicinal properties of Cucumis melo var Agrestis: a non-conventional vegetable. Sindh Univ. Res. J.-SURJ (sci. Ser.). 2018;50:115-118.
- [Google Scholar]
- Analgesic and anti-inflammatory activity in alcoholic extracts of Cucumis trigonus Roxburghii: a preliminary communication. Pharmacology. 1980;20(1):52-56.
- [Google Scholar]
- Antioxidant and antibacterial activity of alkaloid extract of Cucumis trigonus roxb. Int. J. Pharm. Pharma Sci.. 2019;4:45-48.
- [Google Scholar]
- Study of phytochemical and antioxidant activity of Cucumis melo var. Agrestis Fruit. J. Pharmacogn. Phytochem.. 2015;4(2):303-306.
- [Google Scholar]
- Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced-diabetic rats. J. Ethnopharmacol.. 2010;127(2):565-567.
- [Google Scholar]
- Neglected and underutilized plant species (Nus) from the apulia region worthy of being rescued and re-included in daily diet. Horticulturae. 2021;7(7):177.
- [Google Scholar]
- Evaluation of toxicological and standardization parameters and phytochemical investigation of Ficus deltoidea leaves. Am. J. Biochem. Mol. Biol.. 2011;1:237-243.
- [Google Scholar]
- Phytopharmacological profile of Lagenaria siceraria: A review. Asian J. Plant Sci.. 2010;9(3):152-157.
- [Google Scholar]
- Extracts of wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crops Prod.. 2016;80:165-176.
- [Google Scholar]
- Takahashi, T., Toda, E., B Singh, R., De Meester, F., Wilczynska, A., Wilson, D., R Juneja, L., 2011. Essential and non-essential amino acids in relation to glutamate. The Open Nutraceutical. J. 4 (1).
- Effect of temperature, light, salinity, drought stress and seeding depth on germination of Cucumis melo var. agrestis. Pakistan J. Weed Sci. Res.. 2012;18(4)
- [Google Scholar]
- A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal.. 1982;13(12):1035-1059.
- [Google Scholar]







