Translate this page into:
Pharmacological assessment of delphinidin in counteracting polystyrene microplastic induced renal dysfunction in rats
⁎Corresponding author. zaidsalar1@gmail.com (Muhammad Zaid Salar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polystyrene microplastics (PSMP) are toxic environmental contaminants which can damage various body organs including kidneys. Delphinidin (DEL) is a potential anthocyanidin flavonoid with significant pharmacological benefits. This research was conducted to analyze the protective effect of DEL to avert PSMP prompted renal dysfunction. Rats (n = 24) were divided into 4 separate groups: Control, PSMP (0.01 mgkg−1), PSMP (0.01 mgkg−1) + DEL (25 mgkg−1) and only DEL (25 mgkg−1). Our results showed that PSMP exposure reduced the expressions of Nrf-2 and antioxidant genes while increasing the expression of Keap1. Besides, PSMP intoxication escalated the level of kidney injury markers (urea, KIM-1, creatinine and NGAL) while inducing substantial reduction in the levels of creatinine clearance. Moreover, PSMP significantly reduced the levels of GSH, GST, SOD, HO-1, CAT, GSR, GPx while escalating MDA and ROS. Conversely, inflammatory biomarkers including IL-1β, TNF-α, NF-kB, IL-6 and COX-2 activity were increased due to PSMP intoxication. Our results showed that PSMP administration increased the expressions of Bax and caspase-3 while decreasing the expression of Bcl-2. However, DEL treatment significantly restored the PSMP-induced renal impairments. Therefore, it is suggested that DEL could be used as a therapeutic compound to alleviate PSMP-induced kidney damage in rats, possibly due to its strong pharmacological properties.
Keywords
Protective effect
Delphinidin
Polystyrene microplastics
Renal toxicity
Inflammation
Oxidative stress
Apoptosis
1 Introduction
The increasing environmental pollution caused by plastics has garnered significant global attention (Vanapalli et al., 2019). Polystyrene microplastics (PSMP) are among the most harmful plastic contaminants commonly found in the environment. Mammals are exposed to PSMP through ingestion of contaminated food and water, skin contact, and inhalation (Hwang et al., 2020). Once ingested, these microplastics enter the bloodstream and circulate throughout the body, potentially leading to severe toxic effects. The liver, kidneys, and heart are reported to be particularly vulnerable to microplastic-induced damage, as they are responsible for filtering waste and toxins from the body (Wu et al., 2022; Zhang et al., 2022).
Previous studies have demonstrated that exposure to PSMP disrupts the cellular and biochemical profiles of the body tissues by triggering inflammatory responses and generating oxidative stress (OS) (Akbar and Ijaz, 2024). Furthermore, PSMP exposure has been reported to interfere with lipid and energy metabolism, contributing to OS in body tissues (Lu et al., 2018). Recent studies have documented that PSMP exposure impairs the normal physiological function of renal tissues by elevating the levels of kidney injury marker, altering antioxidant enzyme levels, and significantly dysregulating inflammatory and apoptotic biomarker activities in kidney tissues (Ahmad et al., 2023; Ijaz et al., 2024).
Plant-derived therapeutic compounds provide a safer and more effective form of treatment as compared to synthetic chemical drugs (Tiwari and Mishra, 2023). In recent years, numerous studies have highlighted the therapeutic benefits of natural compounds against contemporary disorders and organ toxicities (Tiwari and Mishra, 2023). Flavonoids have also gained global recognition due to their biological potential (Tiwari and Mishra, 2023). Delphinidin (DEL), a plant-based therapeutic compound initially isolated from Delphinium grandiflorum, has been found to possess potent antioxidative, anticancer, and anti-inflammatory properties (Hussain et al., 2022). Therefore, this study aims to evaluate the effectiveness of DEL in mitigating kidney damage induced by PSMP exposure in albino rats.
2 Materials and methods
2.1 Chemicals
PSMP and DEL were acquired from Sigma-Aldrich, Germany.
2.2 Animals
24 male albino rats (age, 8–12 weeks; weight, 250 ± 20 g) were selected for the experiment. The trial was executed in UAF. Rats were kept in standard environmental conditions (Temp. 22–25 °C, relative humidity, 45 ± 5 % &12-hour light–dark cycle). To ensure their nutritional needs, the rats were given with the ad libitum access to tap water and food chaw. Rats were treated in accordance with the approved protocol of the european union for animal care and experimentation (CEE council 86/609)
2.3 Experimental layout
24 rats were apportioned into following groups i.e., Control, PSMP (0.01 mgkg–1), PSMP (0.01 mgkg–1) + DEL (20 mgkg–1) and only DEL (20 mgkg–1) administrated group. The dosage of PSMP, 0.01 mgkg−1 was administered in accordance with the previous study of Akbar and Ijaz (2024), whereas the dose of DEL (25 mgkg−1) was selected in accordance with the study of Alvi et al. (2022). At the end of treatment period rat were decapitated. Heparinized tubes were used to collect blood samples. Blood was centrifuged at 3000 rpm for 15 min. Until further analysis, the resulting plasma was stored at −20 °C. Kidney tissues were dissected, homogenized using Na3PO4 buffer (12,000 rpm for 15 min) at 4 °C, centrifuged and the resulting supernatant was kept at −20 °C which was used for further analysis
2.4 Ribonucleic acid (RNA) extraction & real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
To detect changes in gene expression of Nrf-2, Keap1, Bax, Bcl-2, and caspase-3, 95 mg of tissues from kidneys were used. TRIzol (Invitrogen) reagent (Life Technologies, New York, USA) was used to isolate total RNA, while RNA concentrations were evaluated by Nano-Drop 2000c spectrophotometer. Total RNA with A260/A280 ratio between 1.8 and 2.0 was used in reverse transcriptase PCR. Reverse transcription of RNA transformed it into complementary DNA (cDNA) by using total RNA by Fast Quant RT kit (Takara, China). The qRT-PCR was performed in 25 µL of reaction volume using the SYBR Green. β-actin was used as a standard. Relative expression levels were analyzed by 2-ΔΔCT (Livak and Schmittgen, 2001). The specific primer sequence used in this study are listed in Table 1 as previously reported by Ijaz et al. (2021).
Gene
Primers 5′ − 3′
Accession number
Nrf-2
F: ACCTTGAACACAGATTTCGGTGR: TGTGTTCAGTGAAATGCCGGA
NM_031789.1
Keap-1
F: ACCGAACCTTCAGTTACACACTR: ACCACTTTGTGGGCCATGAA
NM_057152.1
CAT
F: TGCAGATGTGAAGCGCTTCAAR: TGGGAGTTGTACTGGTCCAGAA
NM_012520.2
SOD
F: AGGAGAAACTGACAGCTGTGTCTR: AAGATAGTAAGCGTGCTCCCAC
NM_017051.2
GPx
F: TGCTCATTGAGAATGTCGCGTCR: ACCATTCACCTCGCACTTCTCA
NM_030826.4
GSR
F: ACCAAGTCCCACATCGAAGTCR: ATCACTGGTTATCCCCAGGCT
NM_053906.2
HO-1
F: AGGCTTTAAGCTGGTGATGGCR: ACGCTTTACGTAGTGCTGTGT
NM_012580.2
Bax
F: GGC CTT TTT GCT ACA GGG TTR: AGC TCC ATG TTG TTG TCC AG
NM_017059.2
Bcl-2
F: ACA ACA TCG CTC TGT GGA TR: TCA GAG ACA GCC AGG AGA A
NM_016993.1
Caspase-3
F: ATC CAT GGA AGC AAG TCG ATR: CCT TTT GCT GTG ATC TTC CT
NM_012922.2
β-actin
F: TACAGCTTCACCACCACAGCR: GGAACCGCTCATTGCCGATA
NM_031144
2.5 Determination of biochemical profile
The CAT activity was measured through the Aebi (1984) technique. The SOD activity was calculated by following the technique documented by Kakkar et al. (1984). For the quantification of GPx Rotruck et al. (1973) technique was employed. Carlberg and Mannervik (1975) along with Jollow et al. (1974) protocol was followed for the measurement of GSR along with GSH. Younis et al. (2018) protocol was followed for the measurement of GST. The ROS and MDA level was ascertained using the approaches of Hayashi et al. (2007) and Ohkawa et al. (1979), respectively.
2.6 Determination of renal injury biomarkers
The levels of renal injury biomarkers (urea, KIM-1, creatinine, NGAL and creatinine clearance) were determined by using standard ELISA kits in accordance with the instruction of County Antrim, UK.
2.7 Inflammatory markers analysis
The determination of inflammatory parameters (IL-1β, TNF-α, NF-kB, IL-6 and COX-2) was undertaken using standard kits (ELISA).
2.8 Statistic evaluation
The collected data were presented as Mean ± SEM. Using Minitab software one-way ANOVA was employed as the primary test for comparing means among different groups following Tukey's test that helps to identify which specific groups differ significantly from each other. P<0.05 were set as a level of significance.
3 Results
3.1 Results of PSMP and DEL on Nrf-2/keap1 pathway
Exposure to PSMP led to a significant increase in Keap1, while decreasing the expression of Nrf-2 and antioxidant genes (CAT, SOD, GPx, GSR and HO-1) as compared to the control group. Co-administration of PSMP and DEL together effectively regulated the expression Keap1, Nrf-2 and antioxidant gene (CAT, SOD, GPx, GSR and HO-1) as compared to PSMP only treated group. Nonetheless, the expressions of aforementioned biomarkers in the DEL-supplemented group and control group were approximately close to each other, as shown in Fig. 1.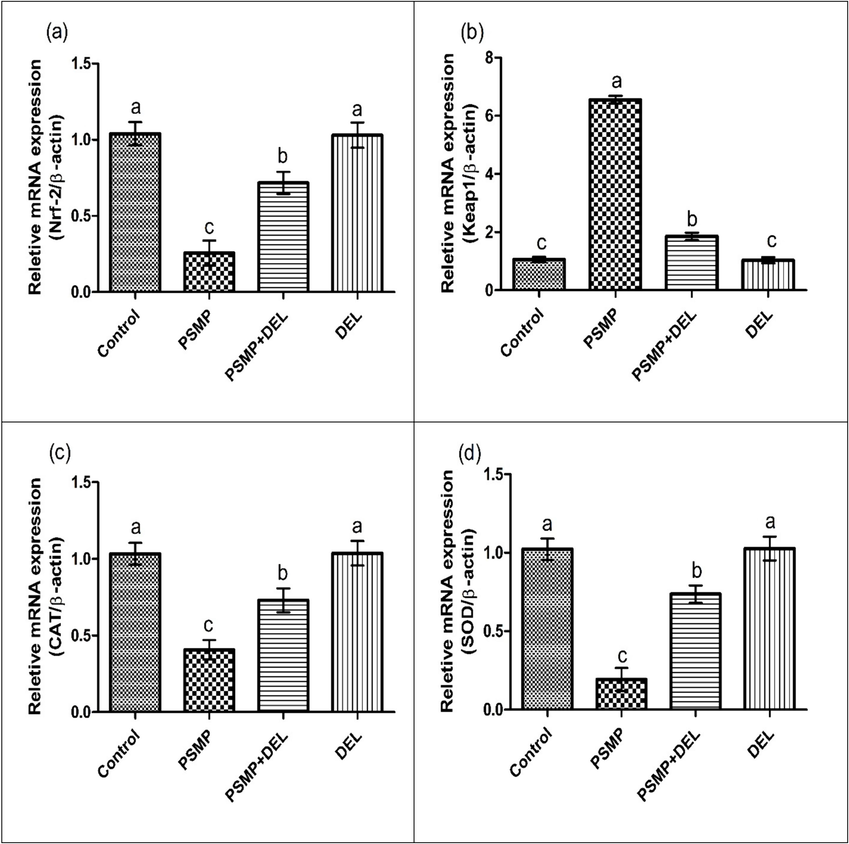
Effect of PSMP and DEL on the expression of (a) Nrf-2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1. Data were shown as Mean ± SEM. Dissimilar letters on graph bars denoting substantial distinctions at P<0.05.
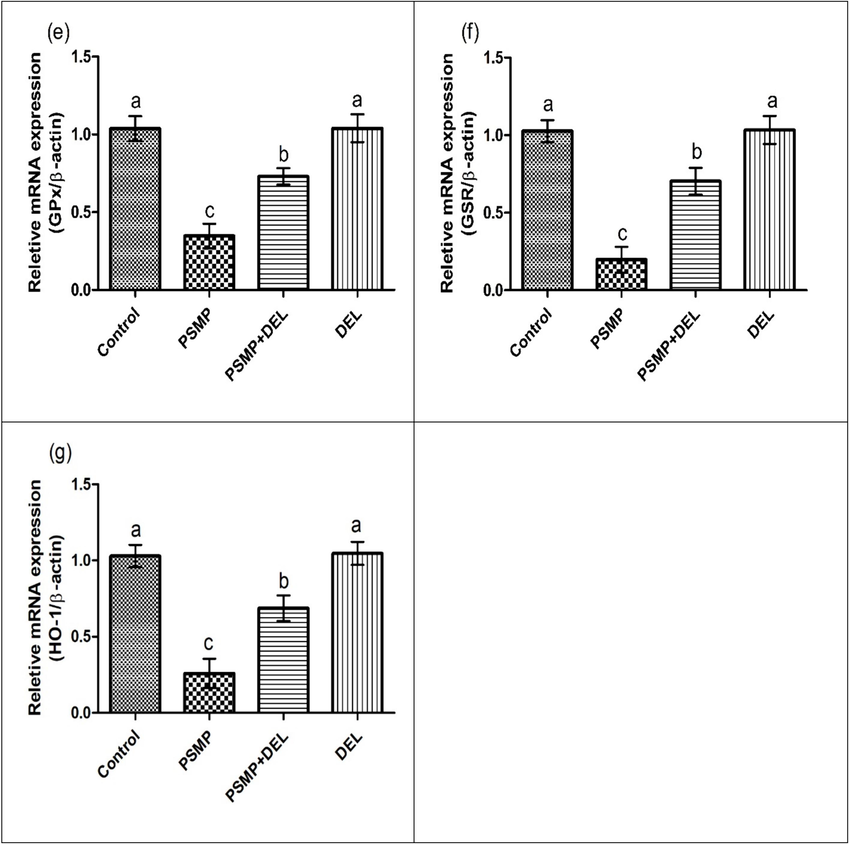
Effect of PSMP and DEL on the expression of (a) Nrf-2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1. Data were shown as Mean ± SEM. Dissimilar letters on graph bars denoting substantial distinctions at P<0.05.
3.2 Results of PSMP and DEL on biochemical parameters
Exposure to PSMP increased the levels of MDA and ROS, while reducing the levels of antioxidant enzymes as compared to the control group. However, co-administration of PSMP and DEL decreased the levels of MDA and ROS while escalating the levels of antioxidant enzymes as compared to the PSMP treated group. Nonetheless, only DEL-supplemented group showed values of abovementioned biochemical markers close to the control group, as shown in Table 2. Distinct letters next to specific values indicate significant differences., which highlights considerable variations between the groups.
Parameters
Groups
Control
PSMP
PSMP+DEL
DEL
CAT (U/mg protein)
1.03 ± 0.12a
0.40 ± 0.11c
0.73 ± 0.13b
1.03 ± 0.13a
GSR (nM NADPH oxidized/min/mg tissue
1.02 ± 0.12a
0.19 ± 0.14c
0.70 ± 0.15b
1.03 ± 0.15a
SOD (U/mg protein)
1.02 ± 0.11a
0.19 ± 0.12c
0.73 ± 0.09b
1.02 ± 0.13a
GSH (U/mg protein)
21.27 ± 1.48a
7.73 ± 0.64c
16.62 ± 0.89b
22.21 ± 1.55a
GPx (U/mg protein)
1.03 ± 0.13a
0.34 ± 0.13c
0.73 ± 0.09b
1.04 ± 0.15a
HO-1 (pmoles bilirubin/ mg protein/h)
1.03 ± 0.12a
0.26 ± 0.16c
0.68 ± 0.14b
1.04 ± 0.13a
GST (U/mg protein)
38.49 ± 2.39a
15.03 ± 2.64c
28.06 ± 2.17b
39.52 ± 2.91a
MDA (nmol/g)
0.63 ± 0.25a
4.35 ± 0.39c
1.35 ± 0.29b
0.59 ± 0.26a
ROS (nmol/g)
1.35 ± 0.28a
7.34 ± 0.49c
2.76 ± 0.34b
1.29 ± 0.30a
3.3 Results of PSMP and DEL administration on renal parameters
PSMP intoxication significantly increased the levels of urea, KIM-1, creatinine and NGAL, while causing a considerable reduction in the level of creatinine clearance as compared to the control group. However, PSMP+DEL co-administration restored the levels of these biomarkers as compared to the control group. Nonetheless, the levels of these biomarkers in the DEL-dosed group and the control group were approximately close to each other, as shown in Table 3. Distinct letters next to specific values indicate significant differences., which highlights considerable variations between the groups.
Parameters
Groups
Control
PSMP
PSMP+DEL
DEL
NGAL (ng/day)
0.68 ± 0.19c
5.58 ± 0.55a
1.62 ± 0.41b
0.64 ± 0.17c
Urea (mg/dl)
16.77 ± 3.86c
41.46 ± 2.83a
28.21 ± 1.16b
16.59 ± 3.62c
Creatinine Clearance (ml/min)
1.97 ± 0.42a
0.27 ± 0.23c
1.31 ± 0.38b
2.32 ± 0.30a
KIM-1 (mg/dl)
0.32 ± 0.23c
3.26 ± 0.28a
1.36 ± 0.17b
0.27 ± 0.21c
Creatinine (mg/dl)
1.52 ± 0.33c
5.88 ± 0.49a
2.41 ± 0.20b
1.44 ± 0.28c
3.4 Results of PSMP and DEL administration on inflammatory parameters
Exposure to PSMP increased the levels of IL-1β, TNF-α, NF-kB, IL-6 and COX-2 activity as compared to the control group. However, co-administration of PSMP and DEL decreased the levels IL-1β, TNF-α, NF-kB, IL-6 and COX-2 activity as compared to PSMP treated group. Nonetheless, only DEL-supplemented group showed values of abovementioned biochemical markers close to the control group, as shown in Table 4. Distinct letters next to specific values indicate significant differences., which highlights considerable variations between the groups.
Parameters
Groups
Control
PSMP
PSMP+DEL
DEL
NF-kB (ng/g tissue)
16.77 ± 1.63a
72.58 ± 2.46c
28.49 ± 2.41b
16.54 ± 1.55a
TNFα (ng/g tissue)
13.68 ± 1.54a
66.12 ± 2.14c
23.32 ± 1.95b
13.65 ± 1.34a
IL-1ß (ng/g tissue)
8.07 ± 2.22a
47.22 ± 1.83c
15.33 ± 1.90b
7.83 ± 2.07a
IL-6 (ng/g tissue)
4.95 ± 2.15a
59.34 ± 2.80c
23.59 ± 1.92b
4.79 ± 2.22a
COX-2 (ng/g tissue)
17.31 ± 2.89a
87.83 ± 4.01c
35.95 ± 2.42b
16.55 ± 2.62a
3.5 Impact of PSMP and DEL administration on apoptotic biomarkers
PSMP intoxication significantly increased the expression of Bax and Caspase-3 while causing a considerable reduction in the expression of Bcl-2 as compared to the control group. However, PSMP+DEL co-administration restored the expressions of these biomarkers as compared to the control group. Nonetheless, the expressions of these biomarkers in the DEL-dosed group and the control group were approximately close to each other, as shown in Fig. 2.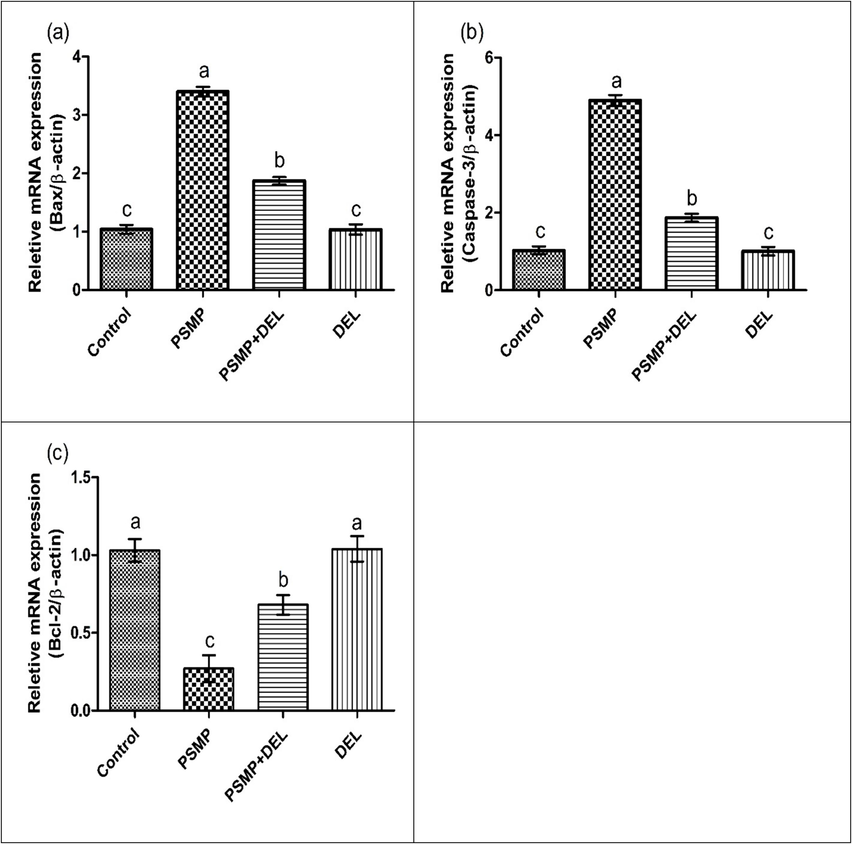
Effect of PSMP and DEL on the expression of (a) Bax, (b) caspase-3 and (c) Bcl-2. Dissimilar letters on graph bars denoting substantial distinctions at P<0.05.
4 Discussion
The rising environmental accumulation of plastics, particularly PSMP, is increasingly recognized as a significant public health concern due to their role in amplifying the production of reactive oxygen species (ROS) (Geyer et al., 2017). The kidneys, which are crucial for the removal of metabolic waste, are particularly vulnerable to oxidative stress-induced damage. Acute kidney injury (AKI) is a critical condition strongly correlated with elevated oxidative stress (OS) in renal tissues. Therefore, this study was conducted to investigate the protective effects of DEL against PSMP induced renal dysfunction.
Current study explored that PSMP supplementation disturbed the expression of antioxidative genes (CAT, SOD, GPx, GSR and HO-1), Nrf-2 and Keap1. The modulation of these gene expressions through the Nrf-2 and keap1 enables the neutralization of OS (Yamamoto et al., 2018; Hou et al., 2022). Furthermore, Nrf-2 and Keap1 are recognized as modulators of immune responses in the body (Bellezza et al., 2018). Nevertheless, DEL dosage successfully recovered the aforementioned dysregulations.
A downregulation of creatinine clearance and an upregulation of urea, NGAL, creatinine, and KIM-1 can indicate impaired kidney function, reflecting renal stress. Besides, it is discovered that excessive levels of urea can cause tissue injury, inappropriate excretion, and renal failure (Yousef et al., 2006). The loss of glomerular function and tubular injury in the renal tissues can cause a significant rise in creatinine concentration (Mansour et al., 2010). Our results demonstrated that administration of PSMP considerably increased the level of urea, creatinine, KIM-1 and NGAL while substantially reducing creatinine clearance. However, co-administration with DEL escalated the levels of creatinine clearance while downregulating the levels of urea, creatine, KIM-1 and NGAL in renal tissues.
PSMP exposure reduced the activities of antioxidant enzymes while escalating the levels of ROS and MDA. A physiological imbalance between oxidants and antioxidants triggers the cellular phenomena referred to as OS. Various sorts of antioxidant enzymes play an important role in countering OS in the body. SOD is the first and foremost enzyme which is involved in the detoxification processes (Saxena et al., 2022). It is documented that ROS disrupts the cellular redox balance, therefore, it is considered as the major culprit underlying renal toxicity (González et al., 2022). However, the supplementation of DEL escalated the activities of aforementioned antioxidant enzymes as well as elevated the levels of ROS and MDA owing to its polyphenolic nature.
PSMP treatment increased the concentrations of renal inflammatory biomarkers (IL-1β, TNF-α, NF-kB, IL-6 and COX-2). NF-kB holds the potential to control numerous phases of inflammation. OS initiates NF-kB activation which prompt the movement of NF-kB into the nucleus and transcription of interleukins (Wang et al., 2018; Hou et al., 2022; Meng et al., 2022). Hou et al. (2022) and Meng et al. (2022) described that Cox-2 has potential to activate NF-kB that elicits inflammatory responses and causes tissue damage in the body. The results of our study were in line with the study of Ijaz et al. (2024), who reported that PSMP intoxication significantly upregulated the levels of inflammatory markers in the renal tissues of rats. However, DEL supplementation considerably reduced the aforesaid biomarkers concentration.
Exposure to PSMP dysregulated the concentration of apoptotic parameters. Caspase-3 and Bax serve as a stimulator of cellular apoptosis while Bcl-2 counteracts the progression of apoptosis in the cells. The overexpression of Bax modulates the permeability of the mitochondrial membrane, thus culminating in the activation of caspases (Robinson et al., 2011; Meng et al., 2022). Caspases, particularly caspase-3, are reported as fundamental mediators of the apoptotic response, initiating the cell death mechanism by activating other enzymes (Sing et al., 2019). Nonetheless, DEL supplementation reinstated the aforesaid biomarkers.
5 Conclusion
In conclusion, PSMP exposure induced kidney impairments in rats by increasing Keap-1, kidney injury markers, inflammatory and pro-apoptotic mediators as well as OS. Additionally, PSMP lessened the expressions of Nrf-2, and antioxidant genes as well as the levels of renal anti-apoptotic markers. Nevertheless, DEL supplementation restored all the impairments that were induced by PSMP intoxication.
6 Limitations
Limitation of this study is that clinical studies are required to evaluate potential pharmacotherapeutic effects of DEL to counteract PSMP prompted kidney toxicity in humans. Furthermore, investigating the detailed underlying molecular mechanism responsible for the protective effects of DEL are also required to be studied.
CRediT authorship contribution statement
Nimra Nazir: Writing – original draft, Methodology, Investigation, Conceptualization. Ali Akbar: Writing – review & editing, Methodology, Investigation. Muhammad Zaid Salar: Writing – original draft, Methodology, Investigation, Conceptualization. Mohammad Z. Ahmed: Writing – review & editing, Resources, Funding acquisition. Ayesha Ishtiaq: Validation, Software, Data curation.
Acknowledgement
The authors are thankful to the Researchers Supporting Project number (RSPD2024R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-Induced Nephrotoxicity in Rats. Pakistan Veterinary Journal. 2023;43(3):623-627.
- [Google Scholar]
- Pharmacotherapeutic potential of ginkgetin against polystyrene microplastics–instigated testicular toxicity in rats: A biochemical, spermatological, and histopathological assessment. ESPR. 2024;31:9031-9044.
- [Google Scholar]
- Nephroprotective effects of delphinidin against bisphenol a induced kidney damage in rats. Anim Vet Sci 2022:22-356.
- [Google Scholar]
- Nrf-2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2018;1865(5):721-733.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Bio Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Study of the dermal anti-inflammatory antioxidant and analgesic activity of Pinostrobin. Heliyon.. 2022;8:10413.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagen. 2007;631:55-61.
- [Google Scholar]
- Polystyrene microplastics induce apoptosis in chicken testis via crosstalk between NF-κB and Nrf2 pathways. Comparative Biochemistry and Physiology Part c: Toxicol and Pharmacol. 2022;262:109444
- [Google Scholar]
- Chemistry and pharmacological actions of delphinidin, a dietary purple pigment in anthocyanidin and anthocyanin forms. Front. Nutr.. 2022;9:746881
- [Google Scholar]
- Evaluation of Possible Ameliorative Role of Robinetin to Counteract Polystyrene Microplastics Instigated Renal Toxicity in Rats. Pakistan Veterinary Journal. 2024;44(2):400-404.
- [Google Scholar]
- Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol.. 2021;40(3):403-416.
- [Google Scholar]
- Narirutin ameliorates polystyrene microplastics induced nephrotoxicity by modulating oxidative stress, inflammation and Nrf-2/Keap1 pathway. Journal of King Saud University-Science. 2024;36(8):103288
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys.. 1984;21:30-32.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402-408.
- [Google Scholar]
- Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio) Chemosphere. 2018;202:514-520.
- [Google Scholar]
- Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol.. 2010;96:14-23.
- [Google Scholar]
- Polystyrene microplastics induced oxidative stress, inflammation and necroptosis via NF-κB and RIP1/RIP3/MLKL pathway in chicken kidney. Toxicology. 2022;478:153296
- [Google Scholar]
- Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem.. 1979;44:276-278.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2022:1-22.
- [Google Scholar]
- Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol.. 2019;20:175-193.
- [Google Scholar]
- Role of plant-derived flavonoids in cancer treatment. Nutr. Cancer. 2023;75(2):430-449.
- [Google Scholar]
- Emissions and environmental burdens associated with plastic solid waste management. In Plastics to Energy.. 2019;2019:313-342.
- [Google Scholar]
- Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf-2-Keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol.. 2018;32(1):22011.
- [CrossRef] [Google Scholar]
- Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: A critical review. Water Res. 2022;221:118825
- [Google Scholar]
- The KEAP1-NRF-2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev.. 2018;98:1169-1203.
- [Google Scholar]
- Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl 4-challanged nephrotoxicity in rats. Pak. J. Pharm. Sci.. 2018;31:1475-1484.
- [Google Scholar]
- Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicology. 2006;227:240-247.
- [Google Scholar]
- Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ.. 2022;840:156727
- [Google Scholar]







